Abstract
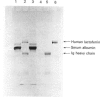
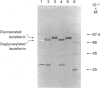
Human lactoferrin was expressed from a cloned cDNA introduced into mammalian cells in tissue culture. Total RNA was extracted from human bone marrow, and lactoferrin cDNA was synthesized by primer-specific polymerase chain reaction after oligo(dT)-primed first-strand synthesis. The cDNA was sequenced to confirm its identity with previously published human lactoferrin sequences and cloned into the eukaryotic expression vector pNUT. Recombinant vector DNA containing the human lactoferrin sequence was introduced into baby-hamster kidney (BHK) cells in culture, and stable transfectants were produced by dominant marker selection. Human lactoferrin was expressed from the metallothionein promoter of pNUT by Zn2+ induction. The protein was secreted into the tissue-culture medium and was subsequently purified to homogeneity in a single step. Initial characterization suggests that the protein expressed by BHK cells is identical with native human lactoferrin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Dodson E. J., Norris G. E., Rumball S. V., Waters J. M., Baker E. N. Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1769–1773. doi: 10.1073/pnas.84.7.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Anderson B. F., Baker H. M., Norris G. E., Rumball S. V., Baker E. N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990 Apr 19;344(6268):784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- Bethell G. S., Ayers J. S., Hancock W. S., Hearn M. T. A novel method of activation of cross-linked agaroses with 1,1'-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J Biol Chem. 1979 Apr 25;254(8):2572–2574. [PubMed] [Google Scholar]
- Brock J. H. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the newborn infant. Arch Dis Child. 1980 Jun;55(6):417–421. doi: 10.1136/adc.55.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasteen N. D. Transferrin: a perspective. Adv Inorg Biochem. 1983;5:201–233. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davidson L. A., Lönnerdal B. Specific binding of lactoferrin to brush-border membrane: ontogeny and effect of glycan chain. Am J Physiol. 1988 Apr;254(4 Pt 1):G580–G585. doi: 10.1152/ajpgi.1988.254.4.G580. [DOI] [PubMed] [Google Scholar]
- Funk W. D., MacGillivray R. T., Mason A. B., Brown S. A., Woodworth R. C. Expression of the amino-terminal half-molecule of human serum transferrin in cultured cells and characterization of the recombinant protein. Biochemistry. 1990 Feb 13;29(6):1654–1660. doi: 10.1021/bi00458a043. [DOI] [PubMed] [Google Scholar]
- Gorinsky B., Horsburgh C., Lindley P. F., Moss D. S., Parkar M., Watson J. L. Evidence for the bilobal nature of diferric rabbit plasma transferrin. Nature. 1979 Sep 13;281(5727):157–158. doi: 10.1038/281157a0. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Hu W. L., Mazurier J., Sawatzki G., Montreuil J., Spik G. Lactotransferrin receptor of mouse small-intestinal brush border. Binding characteristics of membrane-bound and triton X-100-solubilized forms. Biochem J. 1988 Jan 15;249(2):435–441. doi: 10.1042/bj2490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H. A., Finch C. A. The physiology of transferrin and transferrin receptors. Physiol Rev. 1987 Apr;67(2):520–582. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Legrand D., Hu W. L., Montreuil J., Spik G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur J Biochem. 1989 Feb 1;179(2):481–487. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Parry R. M., Jr, Brown E. M. Lactoferrin conformation and metal binding properties. Adv Exp Med Biol. 1974;48(0):141–160. doi: 10.1007/978-1-4684-0943-7_8. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Powell M. J., Ogden J. E. Nucleotide sequence of human lactoferrin cDNA. Nucleic Acids Res. 1990 Jul 11;18(13):4013–4013. doi: 10.1093/nar/18.13.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rado T. A., Wei X. P., Benz E. J., Jr Isolation of lactoferrin cDNA from a human myeloid library and expression of mRNA during normal and leukemic myelopoiesis. Blood. 1987 Oct;70(4):989–993. [PubMed] [Google Scholar]
- Reiter B. The biological significance of lactoferrin. Int J Tissue React. 1983;5(1):87–96. [PubMed] [Google Scholar]
- Rose T. M., Plowman G. D., Teplow D. B., Dreyer W. J., Hellström K. E., Brown J. P. Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1261–1265. doi: 10.1073/pnas.83.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. RNA amplification with transcript sequencing (RAWTS). Nucleic Acids Res. 1988 Jun 10;16(11):5197–5197. doi: 10.1093/nar/16.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Coddeville B., Montreuil J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie. 1988 Nov;70(11):1459–1469. doi: 10.1016/0300-9084(88)90283-0. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Tsuda E., Goto M., Murakami A., Akai K., Ueda M., Kawanishi G., Takahashi N., Sasaki R., Chiba H., Ishihara H. Comparative structural study of N-linked oligosaccharides of urinary and recombinant erythropoietins. Biochemistry. 1988 Jul 26;27(15):5646–5654. doi: 10.1021/bi00415a038. [DOI] [PubMed] [Google Scholar]