Abstract
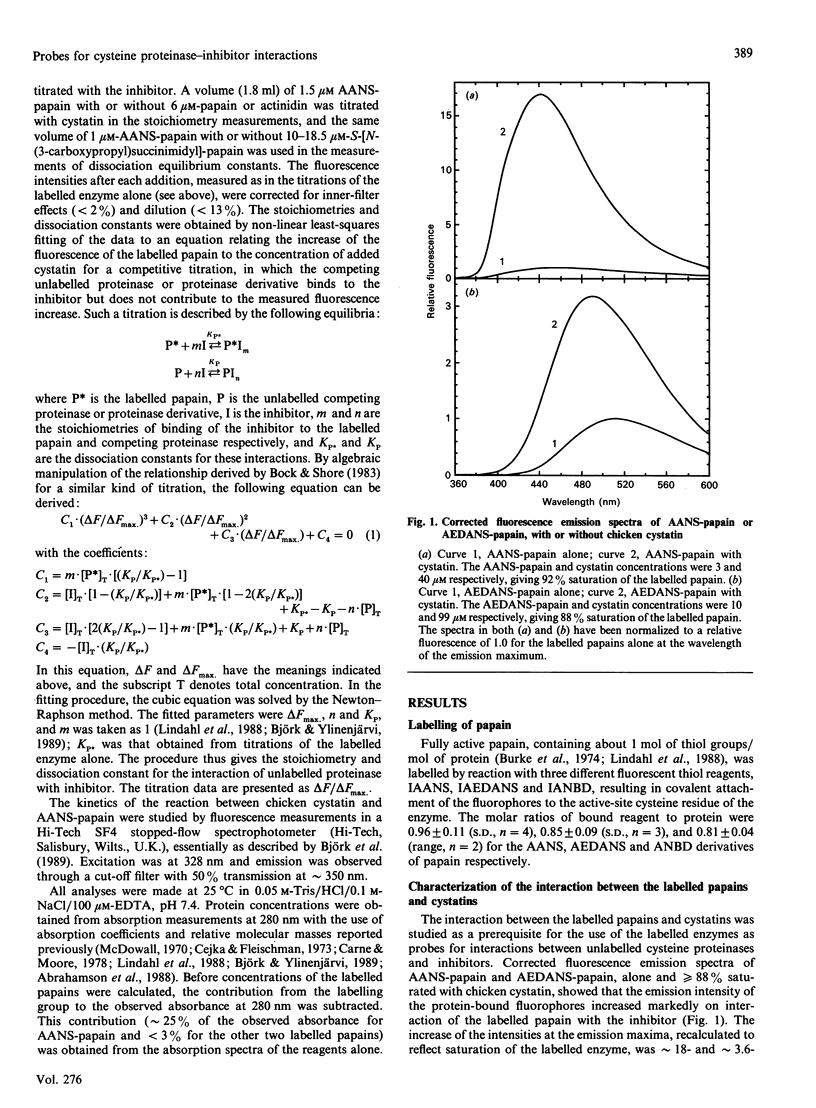
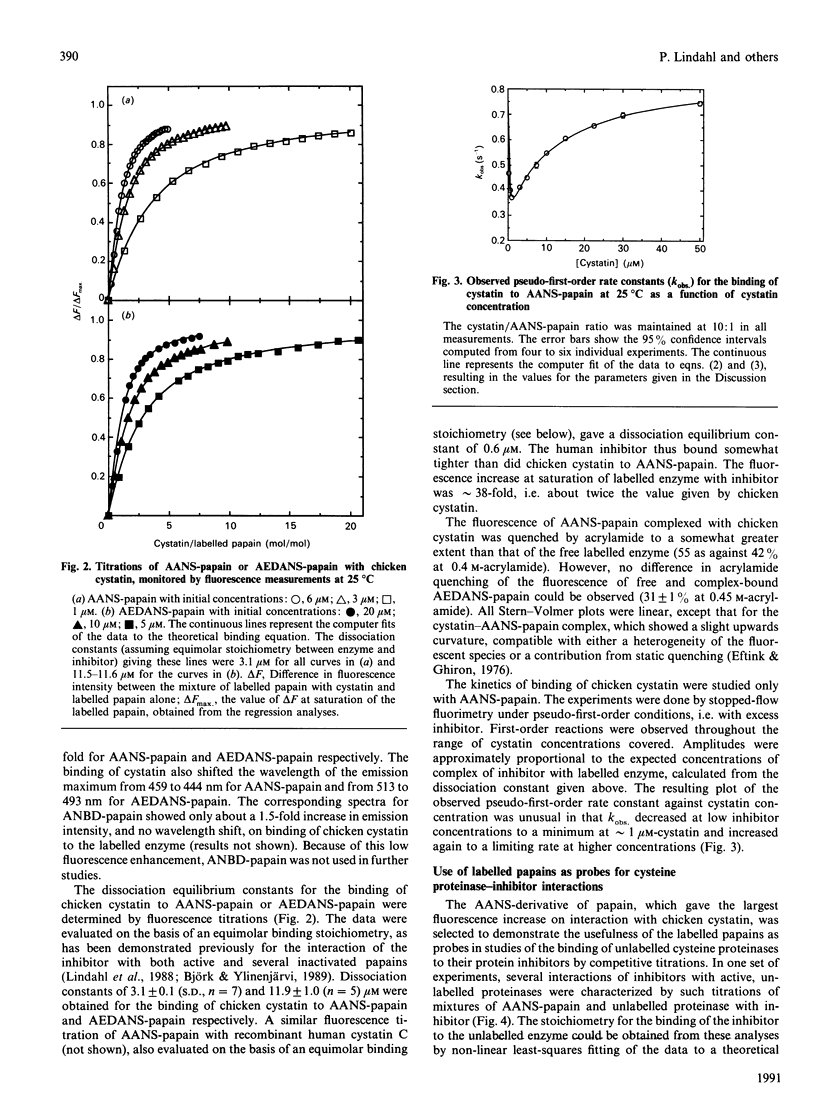
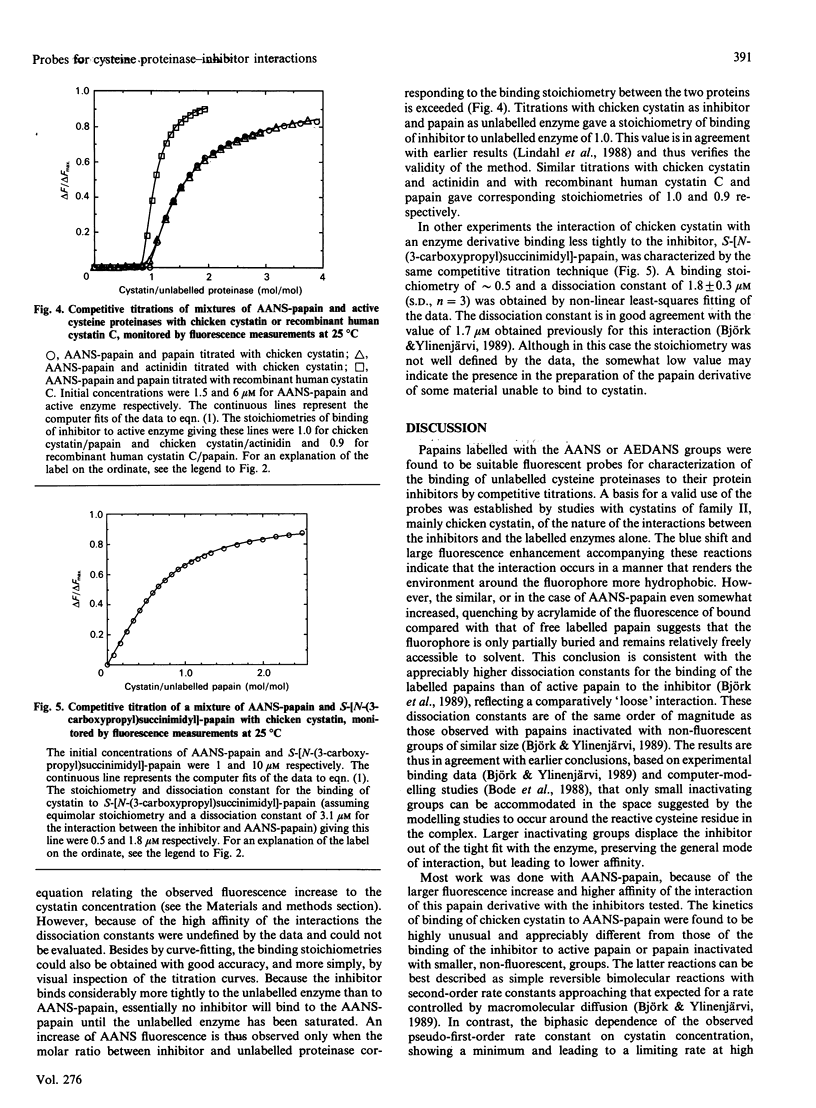
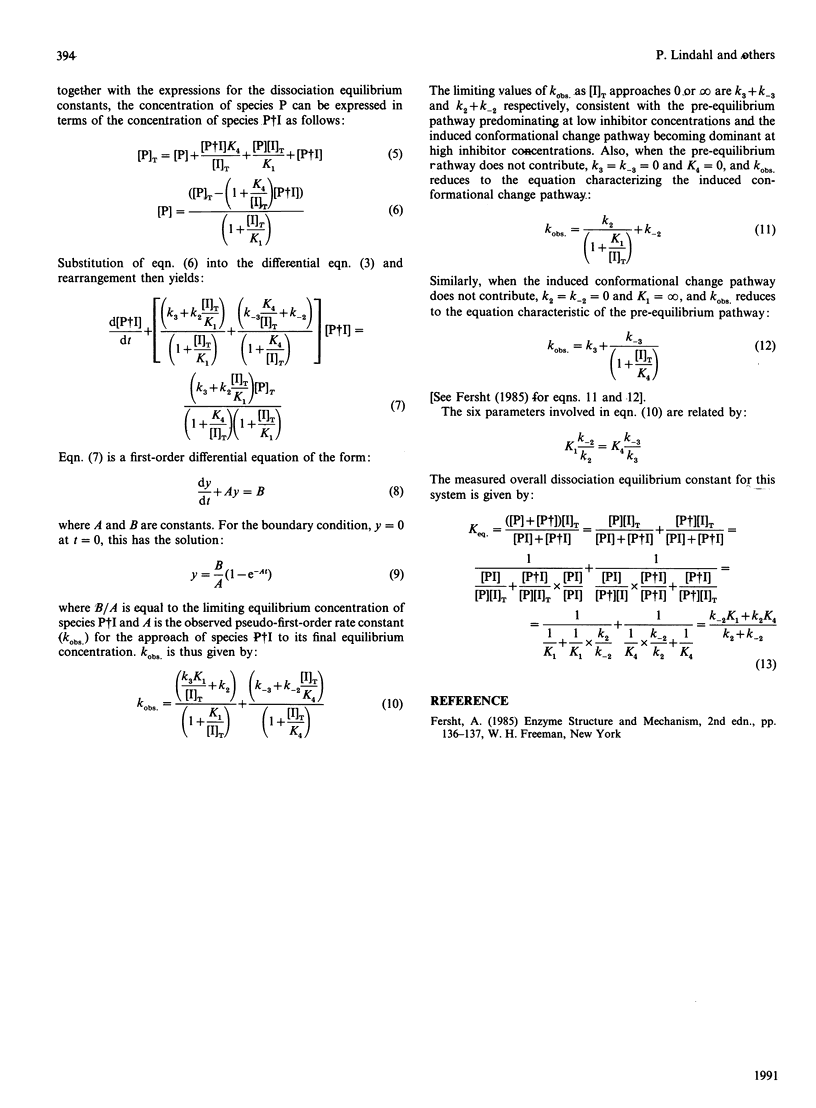
Papain was labelled by attachment of the fluorescent groups 2-(4'-acetamidoanilino)naphthalene-6-sulphonic acid (AANS) or N-(acetylaminoethyl)-8-naphthylamine-1-sulphonic acid (AEDANS) to the active-site cysteine residue, with the aim of using the labelled papains as probes in competitive titrations of unlabelled cysteine proteinases with their inhibitors. The interaction between the labelled papains and cystatins was accompanied by an increase in fluorescence emission of up to 38-fold for AANS-papain and approximately 3.5-fold for AEDANS-papain. Fluorescence titrations gave dissociation equilibrium constants of 3.1 and 0.6 microM for the binding of chicken cystatin and recombinant human cystatin C respectively to AANS-papain and of 11.9 microM for the binding of chicken cystatin to AEDANS-papain. The kinetics of interaction of chicken cystatin with AANS-papain showed an unusual biphasic dependence of the observed pseudo-first-order rate constant on inhibitor concentration, consistent with the reaction occurring via both pathways of a general two-step binding mechanism. AANS-papain was selected as the most suitable probe for competitive titrations of unlabelled active or inactivated cysteine proteinases with inhibitors. This technique, which provides stoichiometries and dissociation constants for the interaction between unlabelled enzyme and inhibitor, allows monitoring of the interactions by a large fluorescent signal in a wavelength region where the interacting proteins do not contribute to the observed fluorescence. Such competitive titrations of active papain or actinidin with chicken cystatin or recombinant human cystatin C all gave inhibitor/enzyme stoichiometries of close to 1.0. A dissociation constant of 1.8 microM for the reaction of chicken cystatin with a papain derivative, S-[N-(3-carboxypropyl)succinimidyl]-papain, was also determined by the same technique. These results show the usefulness of the fluorescent papains for the characterization of interactions between cysteine-proteinase inhibitors and their target enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Alriksson E., Ylinenjärvi K. Kinetics of binding of chicken cystatin to papain. Biochemistry. 1989 Feb 21;28(4):1568–1573. doi: 10.1021/bi00430a022. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction between chicken cystatin and the cysteine proteinases actinidin, chymopapain A, and ficin. Biochemistry. 1990 Feb 20;29(7):1770–1776. doi: 10.1021/bi00459a016. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction of chicken cystatin with inactivated papains. Biochem J. 1989 May 15;260(1):61–68. doi: 10.1042/bj2600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock P. E., Shore J. D. Protein-protein interactions in contact activation of blood coagulation. Characterization of fluorescein-labeled human high molecular weight kininogen-light chain as a probe. J Biol Chem. 1983 Dec 25;258(24):15079–15086. [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. E., Lewis S. D., Shafer J. A. A two-step procedure for purification of papain from extract of papaya latex. Arch Biochem Biophys. 1974 Sep;164(1):30–36. doi: 10.1016/0003-9861(74)90004-6. [DOI] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka J., Fleischmann L. E. Post- -globulin: isolation and physicochemical characterization. Arch Biochem Biophys. 1973 Jul;157(1):168–176. doi: 10.1016/0003-9861(73)90402-5. [DOI] [PubMed] [Google Scholar]
- Dunn S. M., Conti-Tronconi B. M., Raftery M. A. Separate sites of low and high affinity for agonists on Torpedo californica acetylcholine receptor. Biochemistry. 1983 May 10;22(10):2512–2518. doi: 10.1021/bi00279a031. [DOI] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Ohkubo I., Ishiguro H., Kunimatsu M., Sawaki K., Sasaki M. Human high molecular weight kininogen as a thiol proteinase inhibitor: presence of the entire inhibition capacity in the native form of heavy chain. Biochemistry. 1986 Apr 8;25(7):1669–1675. doi: 10.1021/bi00355a034. [DOI] [PubMed] [Google Scholar]
- Hudson E. N., Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973 Oct 9;12(21):4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Collins J. H., Robertson S. P., Potter J. D. A fluorescent probe study of Ca2+ binding to the Ca2+-specific sites of cardiac troponin and troponin C. J Biol Chem. 1980 Oct 25;255(20):9635–9640. [PubMed] [Google Scholar]
- Laber B., Krieglstein K., Henschen A., Kos J., Turk V., Huber R., Bode W. The cysteine proteinase inhibitor chicken cystatin is a phosphoprotein. FEBS Lett. 1989 May 8;248(1-2):162–168. doi: 10.1016/0014-5793(89)80453-3. [DOI] [PubMed] [Google Scholar]
- Lancet D., Pecht I. Spectroscopic and immunochemical studies with nitrobenzoxadiazolealanine, a fluorescent dinitrophenyl analogue. Biochemistry. 1977 Nov 15;16(23):5150–5157. doi: 10.1021/bi00642a031. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Alriksson E., Jörnvall H., Björk I. Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry. 1988 Jul 12;27(14):5074–5082. doi: 10.1021/bi00414a019. [DOI] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- McDowall M. A. Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme. Eur J Biochem. 1970 Jun;14(2):214–221. doi: 10.1111/j.1432-1033.1970.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa T., Towatari T., Ike Y., Katunuma N. Studies on the reactive site of the cystatin superfamily using recombinant cystatin A mutants. Evidence that the QVVAG region is not essential for cysteine proteinase inhibitory activities. FEBS Lett. 1989 Sep 25;255(2):309–314. doi: 10.1016/0014-5793(89)81112-3. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Binding of low-affinity and high-affinity heparin to antithrombin. Ultraviolet difference spectroscopy and circular dichroism studies. Biochemistry. 1978 Aug 8;17(16):3339–3344. doi: 10.1021/bi00609a026. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Lewis S. D., Ballou D. P., Olson S. T., Shafer J. A. Reactivity of small thiolate anions and cysteine-25 in papain toward methyl methanethiosulfonate. Biochemistry. 1986 Sep 23;25(19):5595–5601. doi: 10.1021/bi00367a038. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Parkes C., Abrahamson M., Grubb A., Barrett A. J. Human low-Mr kininogen contains three copies of a cystatin sequence that are divergent in structure and in inhibitory activity for cysteine proteinases. Biochem J. 1986 Mar 1;234(2):429–434. doi: 10.1042/bj2340429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


