Abstract
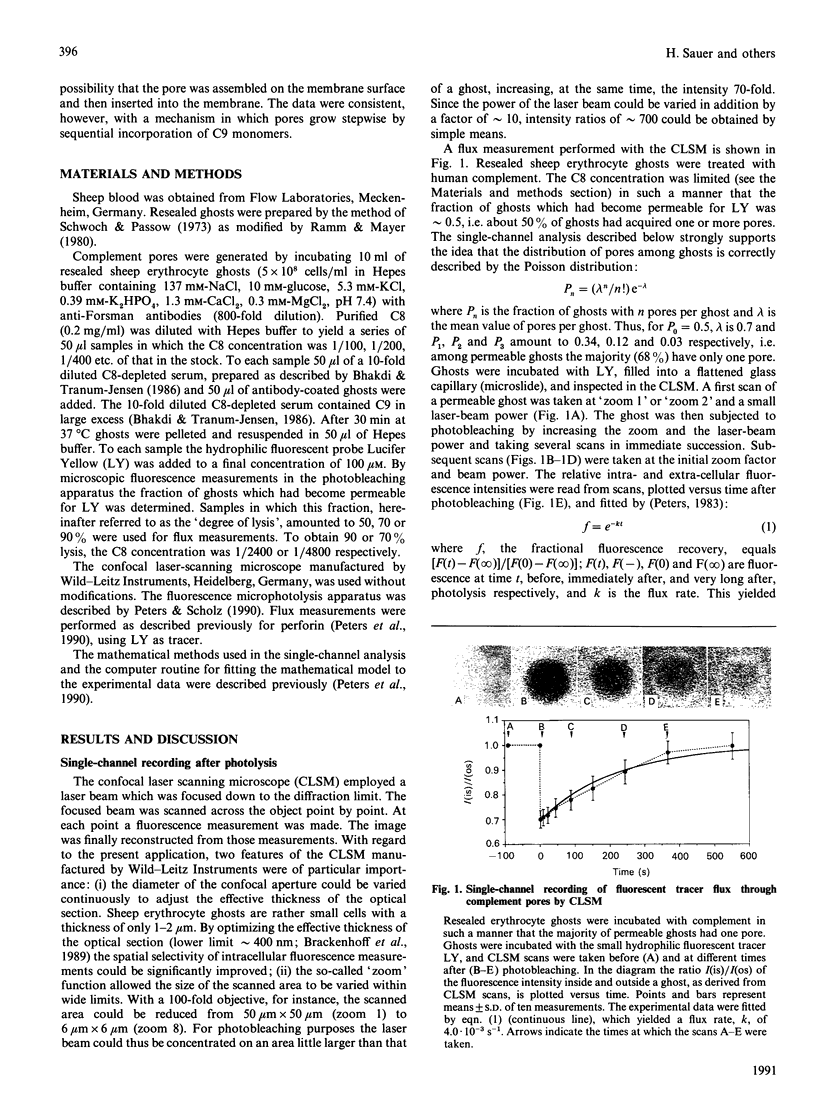
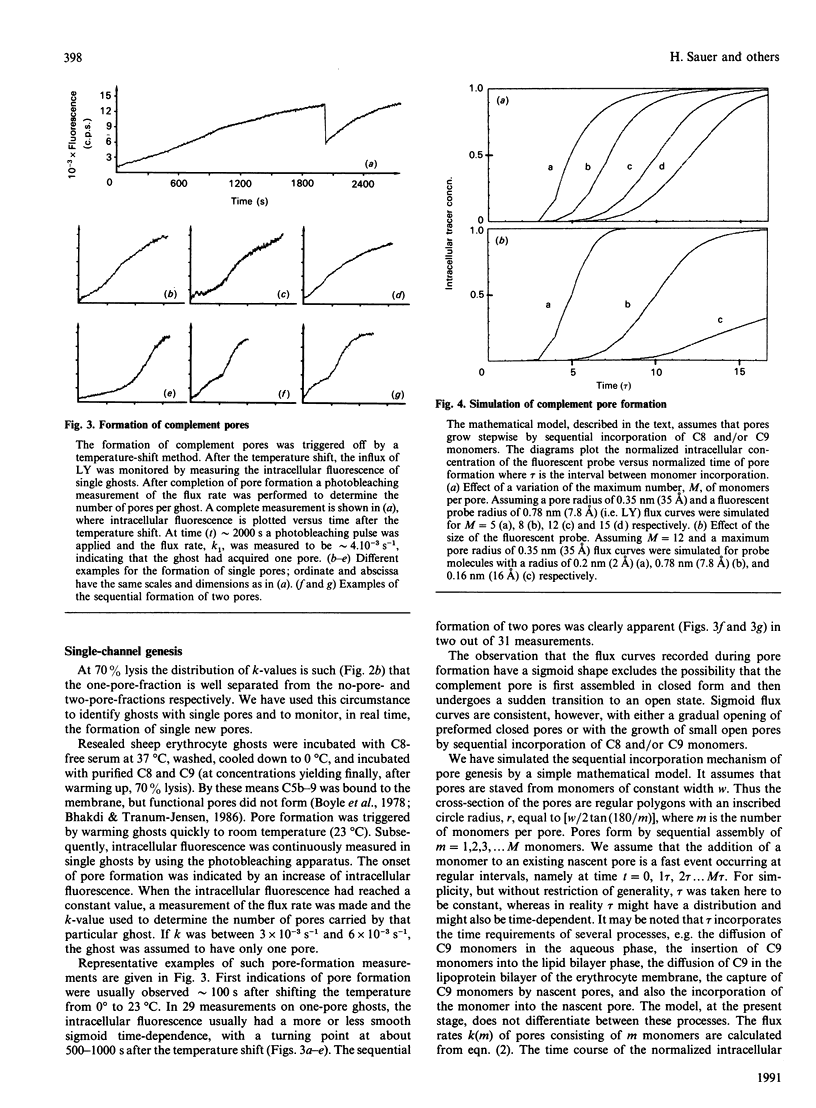
The formation and opening of single complement pores could be directly observed in erythrocyte ghosts by confocal laser-scanning microscopy employing the recently introduced method of fluorescence microscopic single-channel recording. Resealed sheep erythrocyte ghosts were incubated with human complement. By limiting the concentration of C8, the eighth component of complement, the fraction of cells rendered permeable for the small polar fluorescent probe Lucifer Yellow was varied between 0.50 and 0.90. Under each condition the flux rate, k, of Lucifer Yellow was determined for a substantial number of ghosts. By analysing the sample population distribution of k the flux rate k1 of ghosts with a single pore was found to be (4.8 +/- 1.1) x 10(-3) s-1 consistent with a pore radius of about 3.5 nm (35 A). The genesis of single complement pores was studied by continuous influx measurements while triggering pore formation by a temperature shift. Pore genesis was found to be a very slow process, proceeding on a time scale of several minutes. During pore genesis the influx curves had a sigmoid shape, which excluded the possibility that the pore was preformed on the membrane surface and subsequently inserted. However, the influx curves could be well simulated by a model which assumed that pores grow stepwise by sequential incorporation of C9 monomers. The model predicts conditions under which the incorporation of single monomers can be directly revealed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J. C5b-9 assembly: average binding of one C9 molecule to C5b-8 without poly-C9 formation generates a stable transmembrane pore. J Immunol. 1986 Apr 15;136(8):2999–3005. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. III. Distinction between the insertion of C9 and the formation of a transmembrane channel. J Immunol. 1978 May;120(5):1721–1725. [PubMed] [Google Scholar]
- Brakenhoff G. J., van Spronsen E. A., van der Voort H. T., Nanninga N. Three-dimensional confocal fluorescence microscopy. Methods Cell Biol. 1989;30:379–398. doi: 10.1016/s0091-679x(08)60987-5. [DOI] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Jackson M. B., Stephens C. L., Lecar H. Single channel currents induced by complement in antibody-coated cell membranes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6421–6425. doi: 10.1073/pnas.78.10.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenheld M. G., Olsen K. J., Lu P., Lowrey D. M., Hameed A., Hengartner H., Podack E. R. Structure and function of human perforin. Nature. 1988 Sep 29;335(6189):448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- Malinski J. A., Nelsestuen G. L. Membrane permeability to macromolecules mediated by the membrane attack complex. Biochemistry. 1989 Jan 10;28(1):61–70. doi: 10.1021/bi00427a010. [DOI] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Peters R. Nuclear envelope permeability measured by fluorescence microphotolysis of single liver cell nuclei. J Biol Chem. 1983 Oct 10;258(19):11427–11429. [PubMed] [Google Scholar]
- Peters R. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 1984 Aug;3(8):1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Sauer H., Tschopp J., Fritzsch G. Transients of perforin pore formation observed by fluorescence microscopic single channel recording. EMBO J. 1990 Aug;9(8):2447–2451. doi: 10.1002/j.1460-2075.1990.tb07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Mayer M. M. Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol. 1980 May;124(5):2281–2287. [PubMed] [Google Scholar]
- SEARS D. A., WEED R. I., SWISHER S. N. DIFFERENCES IN THE MECHANISM OF IN VITRO IMMUNE HEMOLYSIS RELATED TO ANTIBODY SPECIFICITY. J Clin Invest. 1964 May;43:975–985. doi: 10.1172/JCI104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Takio K., Okumura K. Homology of perforin to the ninth component of complement (C9). Nature. 1988 Aug 11;334(6182):525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K. The molecular mechanism of complement C9 insertion and polymerisation in biological membranes. Curr Top Microbiol Immunol. 1989;140:49–65. doi: 10.1007/978-3-642-73911-8_5. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S. Freeze-fracture analysis of the membrane lesion of human complement. J Cell Biol. 1983 Sep;97(3):618–626. doi: 10.1083/jcb.97.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A., Podack E. R. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: structural, immunological, and functional similarities. Science. 1986 Jul 11;233(4760):184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]



