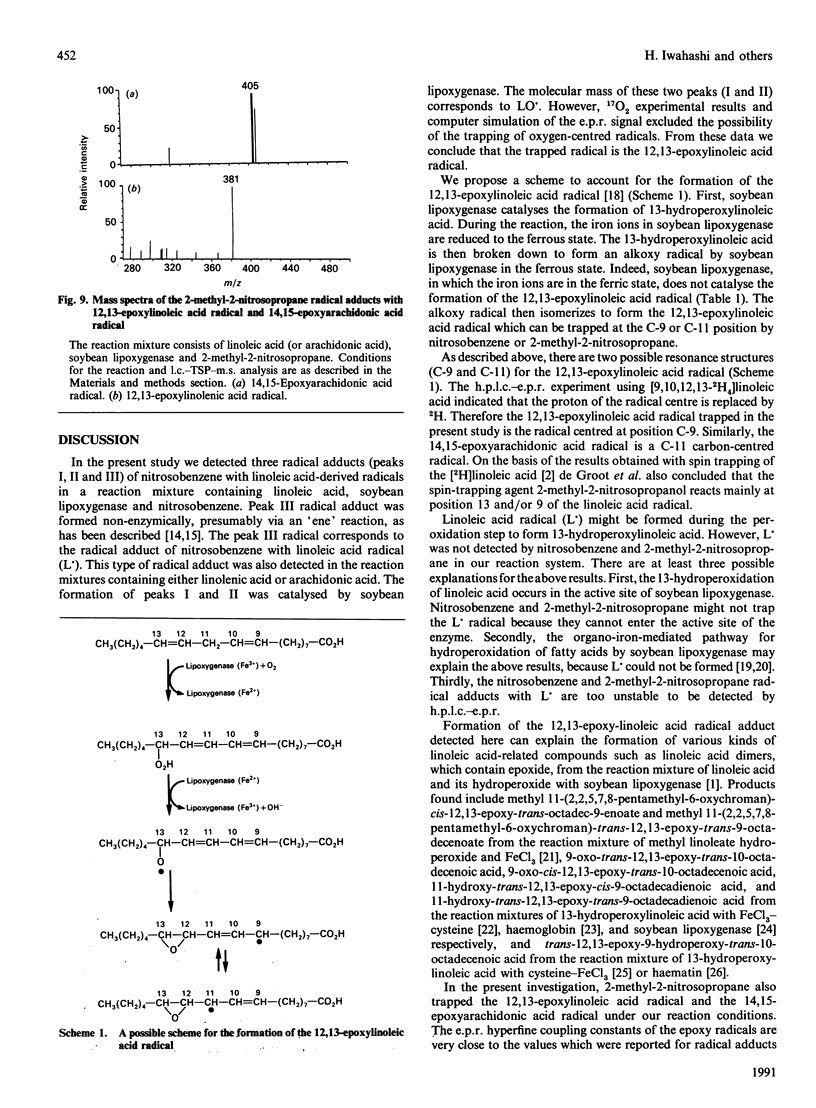
Abstract
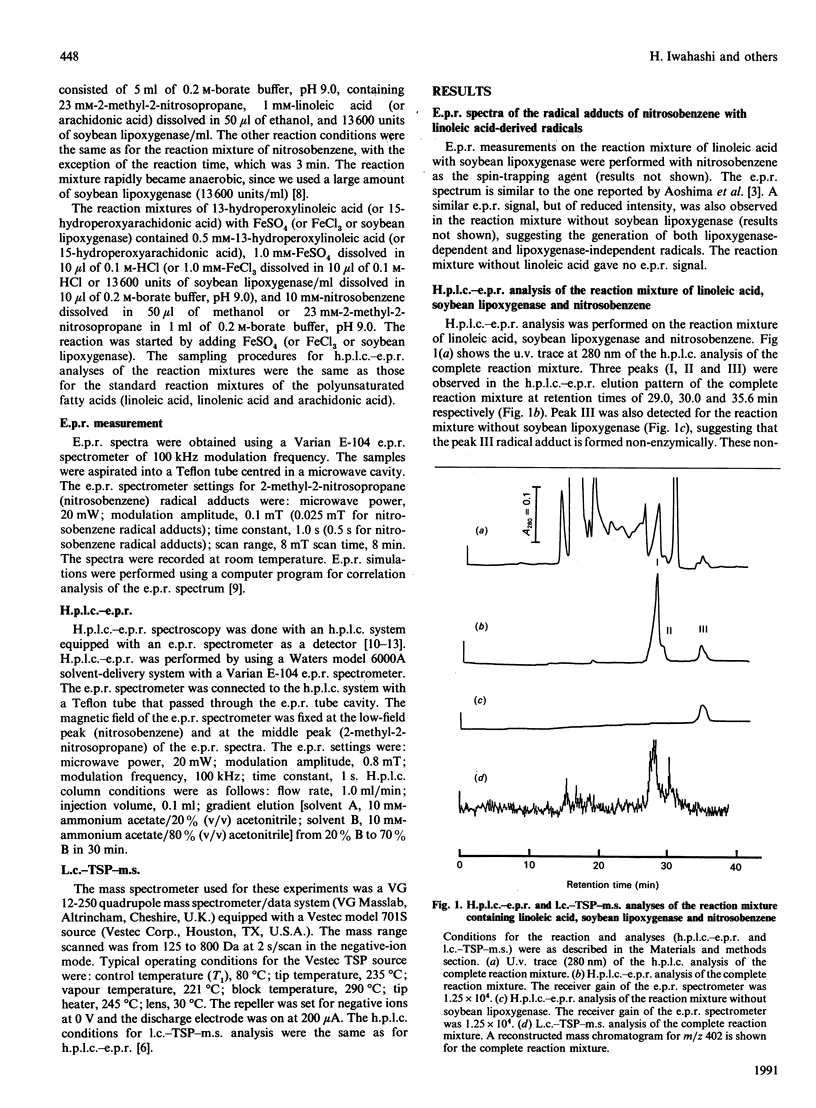
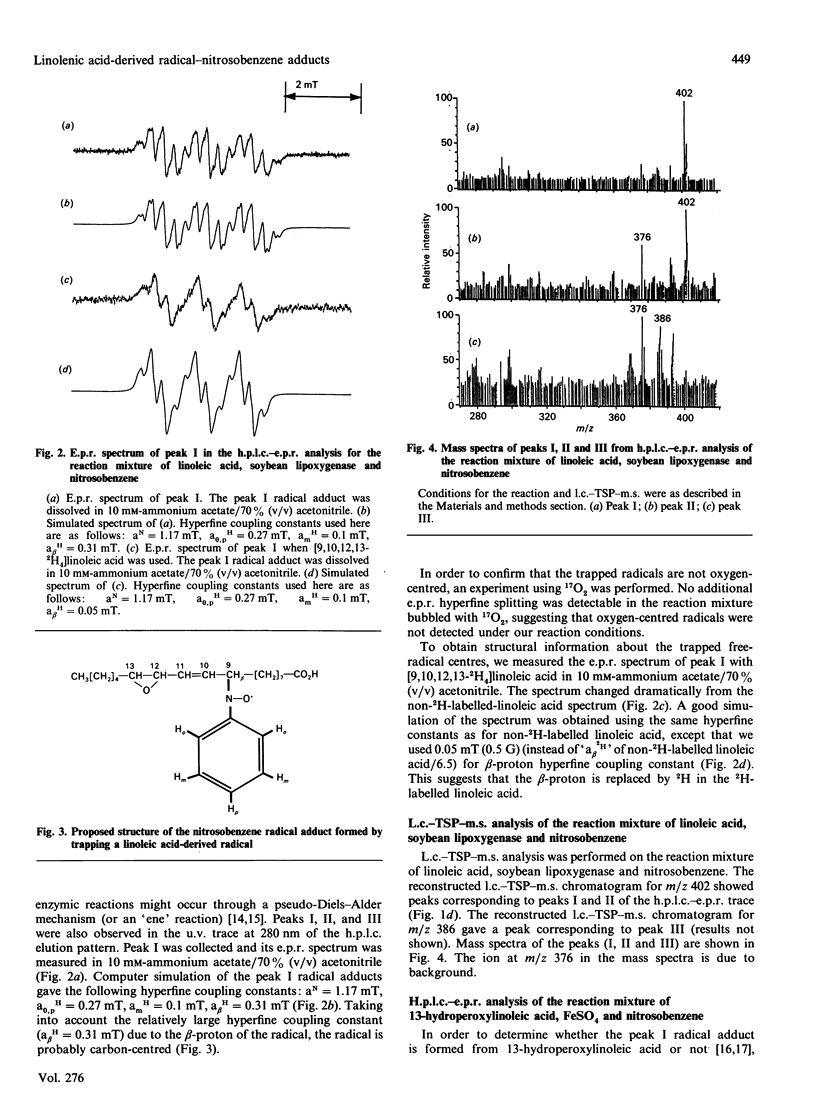
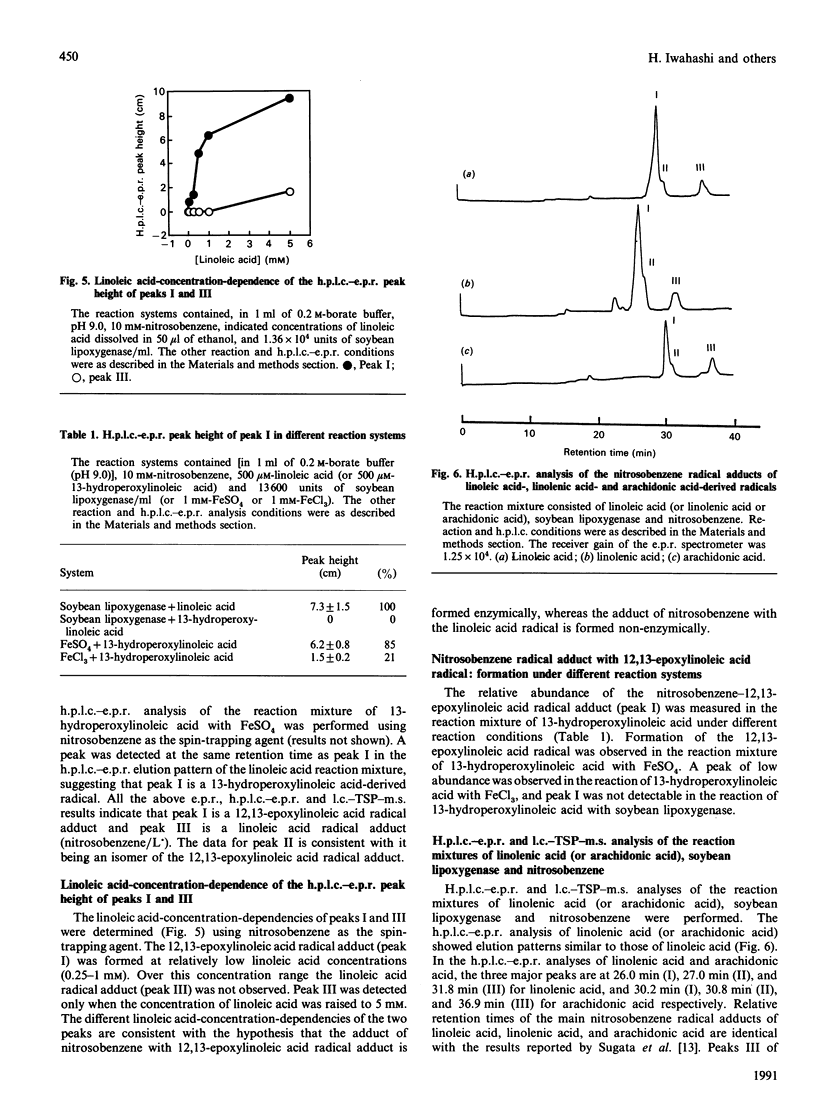
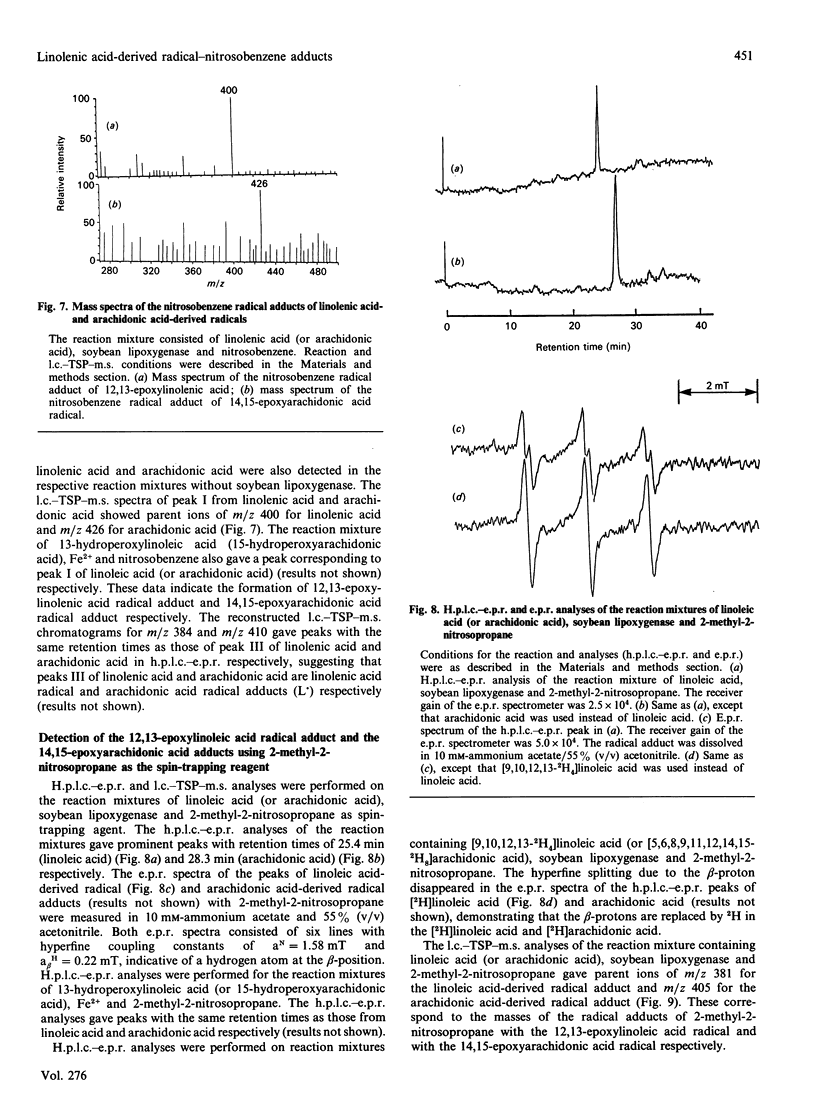
Linoleic acid-derived radicals, which are formed in the reaction of linoleic acid with soybean lipoxygenase, were trapped with nitrosobenzene and the resulting radical adducts were analysed by h.p.l.c.-e.p.r. and liquid chromatography-thermospray-m.s. Three nitrosobenzene radical adducts (peaks I, II and III) were detected; these gave the following parent ion masses: 402 for peak I, 402 for peak II, and 386 for peak III. The masses of peaks I and II correspond to the linoleic acid radicals with one more oxygen atom [L(O).]. The radicals are probably carbon-centred, because the use of 17O2 did not result in an additional hyperfine splitting. Computer simulation of the peak I radical adduct e.p.r. spectrum also suggested that the radical is carbon-centred. The peak I radical was also detected in the reaction of 13-hydroperoxylinoleic acid with FeSO4. From the above results, peak I is probably the 12,13-epoxylinoleic acid radical. An h.p.l.c.-e.p.r. experiment using [9,10,12,13-2H4]linoleic acid suggested that the 12,13-epoxylinoleic acid radical is a C-9-centred radical. Peak II is possibly an isomer of peak I. Peak III, which was observed in the reaction mixture without soybean lipoxygenase, corresponds to a linoleic acid radical (L.). The 12,13-epoxylinoleic acid radical, 12,13-epoxylinolenic acid radical and 14,15-epoxyarachidonic acid radical were also detected in the reactions of linoleic acid, linolenic acid and arachidonic acid respectively, with soybean lipoxygenase using nitrosobenzene and 2-methyl-2-nitrosopropane as spin-trapping agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Lott K. A., Slater T. F., Stier A., Symons M. C., Tomasi A. Spin-trapping studies on the free-radical products formed by metabolic activation of carbon tetrachloride in rat liver microsomal fractions isolated hepatocytes and in vivo in the rat. Biochem J. 1982 May 15;204(2):593–603. doi: 10.1042/bj2040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshima H., Kajiwara T., Hatanaka A., Hatano H. Electron spin resonance studies on the lipoxygenase reaction by spin trapping and spin labelling methods. J Biochem. 1977 Dec;82(6):1559–1565. doi: 10.1093/oxfordjournals.jbchem.a131850. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Leiboff R. H., Mergner G. W., Dickens B. F., Weglicki W. B. Spin trapping of oxygen and carbon-centered free radicals in ischemic canine myocardium. Free Radic Biol Med. 1987;3(5):313–316. doi: 10.1016/s0891-5849(87)80037-0. [DOI] [PubMed] [Google Scholar]
- Chamulitrat W., Mason R. P. Lipid peroxyl radical intermediates in the peroxidation of polyunsaturated fatty acids by lipoxygenase. Direct electron spin resonance investigations. J Biol Chem. 1989 Dec 15;264(35):20968–20973. [PubMed] [Google Scholar]
- Connor H. D., Fischer V., Mason R. P. A search for oxygen-centered free radicals in the lipoxygenase/linoleic acid system. Biochem Biophys Res Commun. 1986 Dec 15;141(2):614–621. doi: 10.1016/s0006-291x(86)80217-0. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Conversion of linoleic acid hydroperoxide to hydroxy, keto, epoxyhydroxy, and trihydroxy fatty acids by hematin. J Biol Chem. 1985 May 10;260(9):5351–5357. [PubMed] [Google Scholar]
- Feix J. B., Kalyanaraman B. Spin trapping of lipid-derived radicals in liposomes. Biochim Biophys Acta. 1989 Aug 18;992(2):230–235. doi: 10.1016/0304-4165(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Soong L. M., Stuart M. A., Reigh D. L. Free radicals and carcinogenesis. Some properties of the nitroxyl free radicals produced by covalent binding of 2-nitrosofluorene to unsaturated lipids of membranes. Arch Biochem Biophys. 1978 Jan 30;185(2):450–457. doi: 10.1016/0003-9861(78)90188-1. [DOI] [PubMed] [Google Scholar]
- Funk M. O., Isacc R., Porter N. A. Preparation and purification of lipid hydroperoxides from arachidonic and gamma-linolenic acids. Lipids. 1976 Feb;11(2):113–117. doi: 10.1007/BF02532660. [DOI] [PubMed] [Google Scholar]
- Garssen G. J., Veldink G. A., Vliegenthart J. F., Boldingh J. The formation of threo-11-hydroxy-trans-12: 13-epoxy-9-cis-octadecenoic acid by enzymic isomerisation of 13-L-hydroperoxy-9-cis, 11-transoctadecadienoic acid by soybean lipoxygenase-1. Eur J Biochem. 1976 Feb 2;62(1):33–36. doi: 10.1111/j.1432-1033.1976.tb10094.x. [DOI] [PubMed] [Google Scholar]
- Garssen G. J., Vliegenthart J. F., Boldingh J. The origin and structures of dimeric fatty acids from the anaerobic reaction between soya-bean lipoxygenase, linoleic acid and its hydroperoxide. Biochem J. 1972 Nov;130(2):435–442. doi: 10.1042/bj1300435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. Decomposition of unsaturated fatty acid hydroperoxides by hemoglobin: Structures of major products of 13L-hydroperoxy-9,11-octadecadienoic acid. Lipids. 1975 Feb;10(2):87–92. doi: 10.1007/BF02532161. [DOI] [PubMed] [Google Scholar]
- Hughes M. F., Chamulitrat W., Mason R. P., Eling T. E. Epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene via a hydroperoxide-dependent mechanism catalyzed by lipoxygenases. Carcinogenesis. 1989 Nov;10(11):2075–2080. doi: 10.1093/carcin/10.11.2075. [DOI] [PubMed] [Google Scholar]
- Iwahashi H., Ikeda A., Negoro Y., Kido R. Detection of radical species in haematin-catalysed retinoic acid 5,6-epoxidation by using h.p.l.c.-e.p.r. spectrometry. Biochem J. 1986 Jun 1;236(2):509–514. doi: 10.1042/bj2360509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi H., Parker C. E., Mason R. P., Tomer K. B. Radical identification by liquid chromatography/thermospray mass spectrometry. Rapid Commun Mass Spectrom. 1990 Sep;4(9):352–354. doi: 10.1002/rcm.1290040912. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Mason R. P., Perez-Reyes E., Chignell C. F., Wolf C. R., Philpot R. M. Characterization of the free radical formed in aerobic microsomal incubations containing carbon tetrachloride and NADPH. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1065–1072. doi: 10.1016/0006-291x(79)92116-8. [DOI] [PubMed] [Google Scholar]
- Li A. S., Chignell C. F. Spectroscopic studies of cutaneous photosensitizing agents--IX. A spin trapping study of the photolysis of amiodarone and desethylamiodarone. Photochem Photobiol. 1987 Feb;45(2):191–197. doi: 10.1111/j.1751-1097.1987.tb05363.x. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Kalyanaraman B., Tainer B. E., Eling T. E. A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. Spin trapping and oxygen uptake studies. J Biol Chem. 1980 Jun 10;255(11):5019–5022. [PubMed] [Google Scholar]
- Pryor W. A., Prier D. G., Church D. F. Radical production from the interaction of ozone and PUFA as demonstrated by electron spin resonance spin-trapping techniques. Environ Res. 1981 Feb;24(1):42–52. doi: 10.1016/0013-9351(81)90130-4. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Rauckman E. J. Spin trapping of free radicals during hepatic microsomal lipid peroxidation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7346–7349. doi: 10.1073/pnas.78.12.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J., Mason R. P., Eling T. E. Carbon-centered free radical intermediates in the hematin- and ram seminal vesicle-catalyzed decomposition of fatty acid hydroperoxides. Arch Biochem Biophys. 1986 Nov 15;251(1):17–24. doi: 10.1016/0003-9861(86)90046-9. [DOI] [PubMed] [Google Scholar]
- Sugata R., Iwahashi H., Ishii T., Kido R. Separation of polyunsaturated fatty acid radicals by high-performance liquid chromatography with electron spin resonance and ultraviolet detection. J Chromatogr. 1989 Jan 27;487(1):9–16. doi: 10.1016/s0378-4347(00)83002-9. [DOI] [PubMed] [Google Scholar]
- de Groot J. J., Garssen G. J., Vliegenthart J. F., Boldingh J. The detection of linoleic acid radicals in the anaerobic reaction of lipoxygenase. Biochim Biophys Acta. 1973 Nov 29;326(2):279–284. doi: 10.1016/0005-2760(73)90254-3. [DOI] [PubMed] [Google Scholar]
- de Groot J. J., Veldink G. A., Vliegenthart J. F., Boldingh J., Wever R., van Gelder B. F. Demonstration by EPR spectroscopy of the functional role of iron in soybean lipoxygenase-1. Biochim Biophys Acta. 1975 Jan 23;377(1):71–79. doi: 10.1016/0005-2744(75)90287-9. [DOI] [PubMed] [Google Scholar]


