Abstract
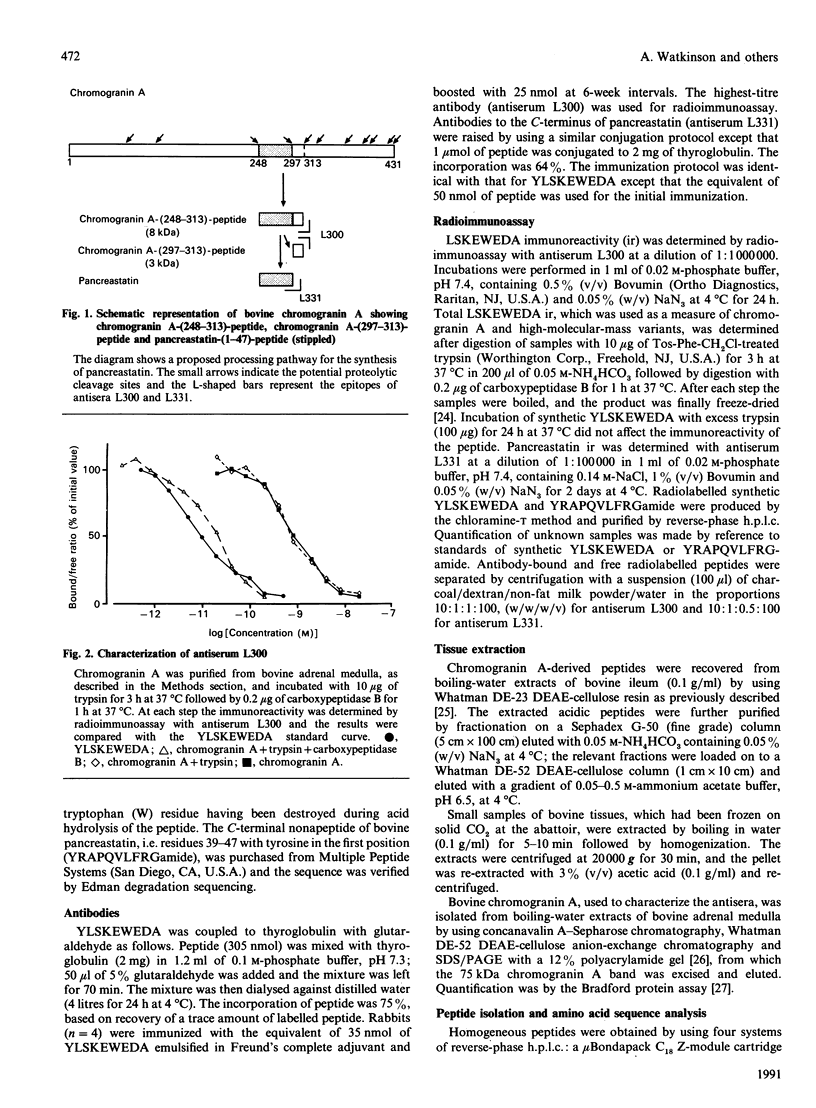
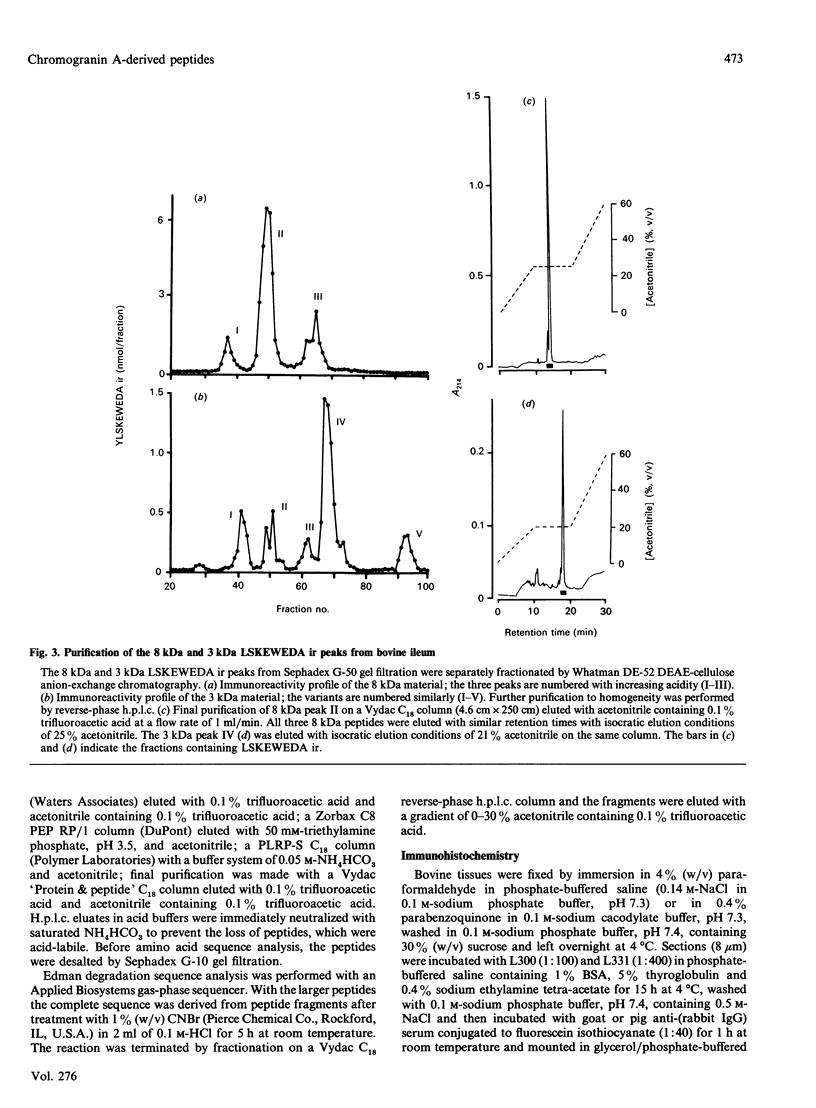
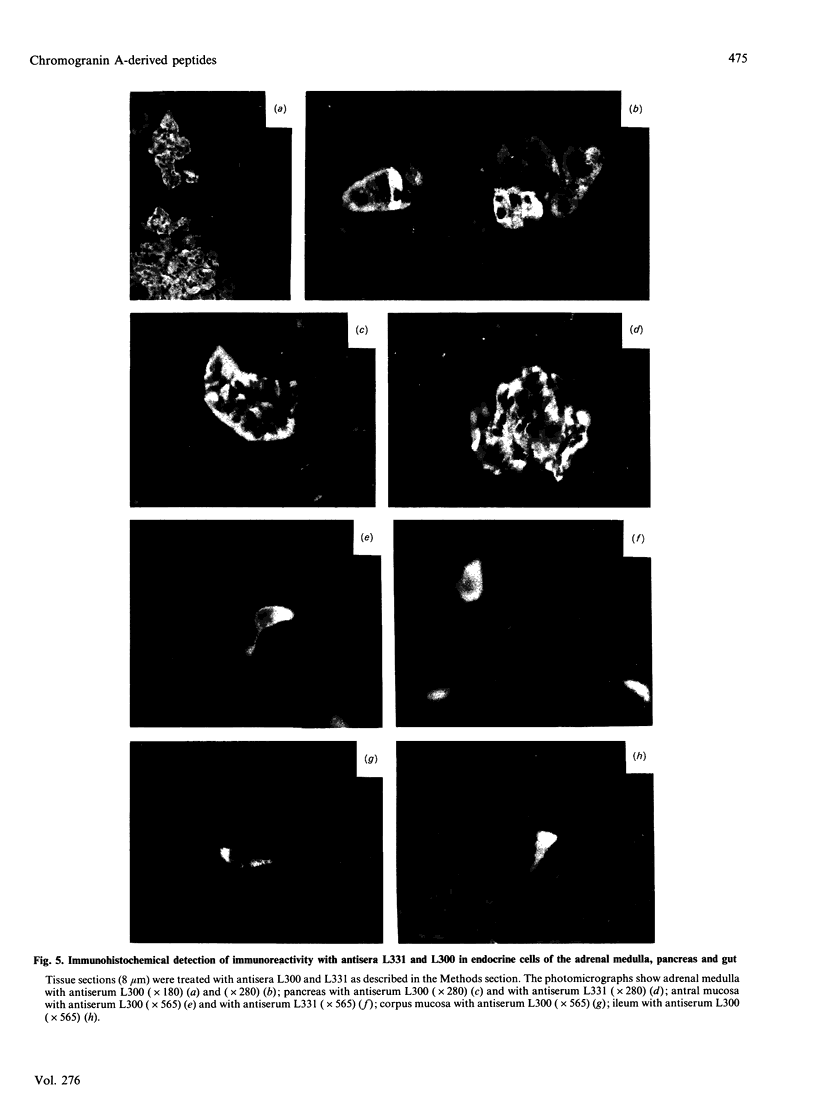
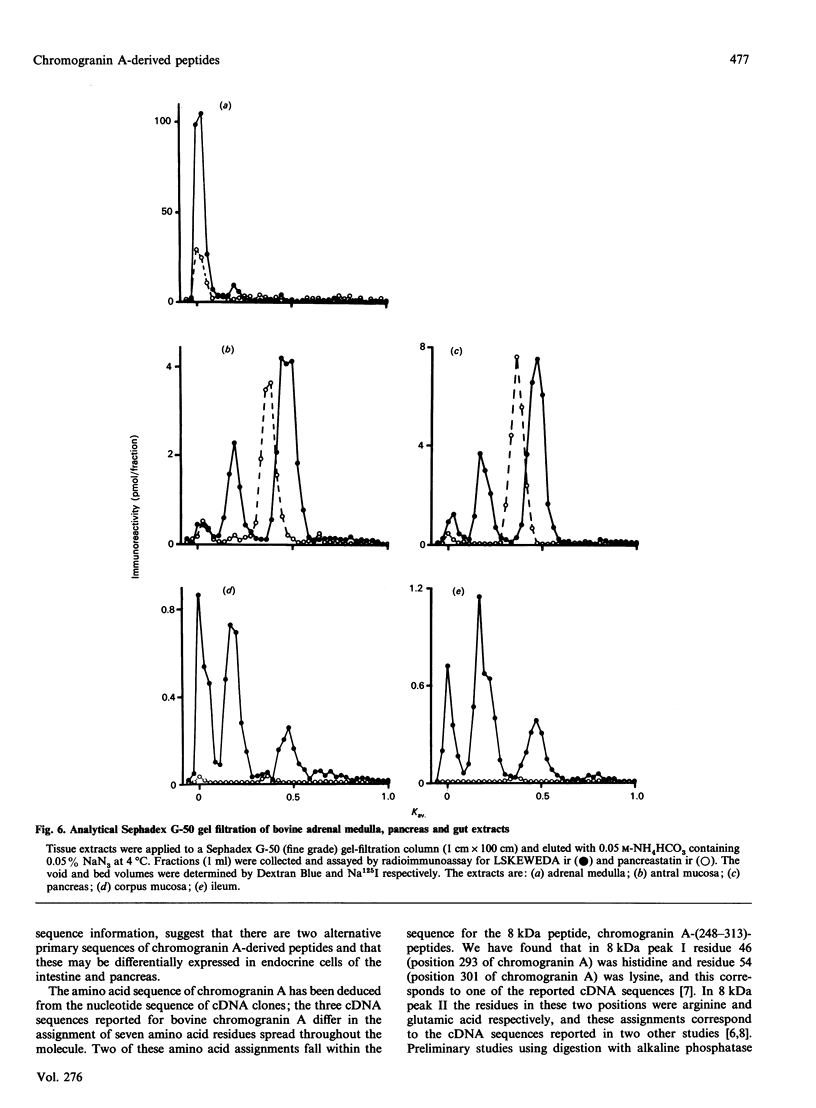
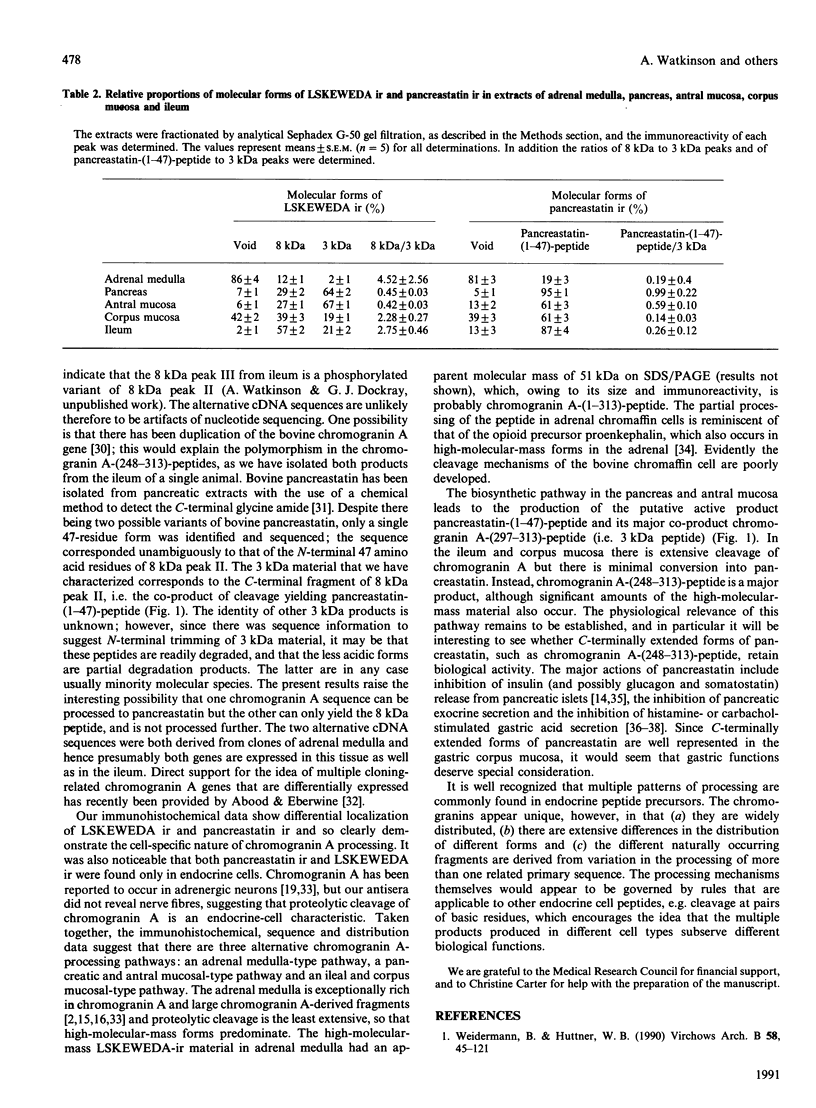
Chromogranin A is produced in many endocrine cell types, and is widely used as a marker in endocrine-cell pathology and secretory-cell biology. There is some evidence that it may be proteolytically processed to yield the putative pancreatic regulatory peptide, pancreastatin, and, in order to characterize the relevant pathways in gastrointestinal and pancreatic endocrine cells, we have used, in radioimmunoassay, site-directed antibodies to pancreastatin itself (L331) and to a sequence of chromogranin A immediately C-terminal to pancreastatin (L300). The latter antibody revealed three major forms of immunoreactivity of 8 kDa and five peptides of approx. 3 kDa in bovine pancreas and gut extracts. The 8 kDa peptides were characterized as chromogranin A-(248-313)-peptides, i.e. C-terminally extended forms of pancreastatin; two of the 8 kDa variants differed in two positions, confirming a polymorphism predicted from cDNA sequencing. One of the 3 kDa peptides was characterized as chromogranin A-(297-313)-peptide, i.e. the C-terminal heptadecapeptide of the 8 kDa peptide that would be liberated after cleavage to yield pancreastatin. On the basis of chromatographic studies, immunohistochemistry and the stoichiometry of different immunoreactive peptides, three different pathways of chromogranin A processing were identified: in adrenal chromaffin cells chromogranin A existed mainly as the unmodified intact protein, in pancreatic islet and gastric antral endocrine cells pancreastatin and the 3 kDa peptides were major products, but in small intestine and gastric corpus endocrine cells there was little nor no pancreastatin and the 8 kDa cleavage product predominated. There are therefore important differences in the distribution of chromogranin A-derived peptides between quite closely related populations of endocrine cells that are attributable not only to variable post-translational cleavage but also to the expression of different primary sequences. It seems possible that in different cell types chromogranin A-derived peptides might subserve a variety of different functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Eberwine J. H. Characterization and regulation of a cDNA clone for rat pancreastatin. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1079–1085. doi: 10.1016/0006-291x(90)90633-x. [DOI] [PubMed] [Google Scholar]
- Ahn T. G., Cohn D. V., Gorr S. U., Ornstein D. L., Kashdan M. A., Levine M. A. Primary structure of bovine pituitary secretory protein I (chromogranin A) deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5043–5047. doi: 10.1073/pnas.84.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Cetin Y., Müller-Köppel L., Aunis D., Bader M. F., Grube D. Chromogranin A (CgA) in the gastro-entero-pancreatic (GEP) endocrine system. II. CgA in mammalian entero-endocrine cells. Histochemistry. 1989;92(4):265–275. doi: 10.1007/BF00500540. [DOI] [PubMed] [Google Scholar]
- Efendić S., Tatemoto K., Mutt V., Quan C., Chang D., Ostenson C. G. Pancreastatin and islet hormone release. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7257–7260. doi: 10.1073/pnas.84.20.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Schober M. Isolation and characterization of chromogranins A, B, and C from bovine chromaffin granules and a rat pheochromocytoma. J Neurochem. 1987 Jan;48(1):262–270. doi: 10.1111/j.1471-4159.1987.tb13157.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi A., Miyasaka K., Nakamura R., Kitani K., Tatemoto K. Inhibitory effect of pancreastatin on pancreatic exocrine secretion in the conscious rat. Regul Pept. 1989 May;25(2):157–166. doi: 10.1016/0167-0115(89)90257-7. [DOI] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Gorr S. U., Shioi J., Cohn D. V. Interaction of calcium with porcine adrenal chromogranin A (secretory protein-I) and chromogranin B (secretogranin I). Am J Physiol. 1989 Aug;257(2 Pt 1):E247–E254. doi: 10.1152/ajpendo.1989.257.2.E247. [DOI] [PubMed] [Google Scholar]
- Grimes M., Iacangelo A., Eiden L. E., Godfrey B., Herbert E. Chromogranin A: the primary structure deduced from cDNA clones reveals the presence of pairs of basic amino acids. Ann N Y Acad Sci. 1987;493:351–378. doi: 10.1111/j.1749-6632.1987.tb27218.x. [DOI] [PubMed] [Google Scholar]
- Grube D. Immunoreactivities of gastrin (G-) cells. II. Non-specific binding of immunoglobulins to G-cells by ionic interactions. Histochemistry. 1980;66(2):149–167. doi: 10.1007/BF00494642. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Hansen F., Peshavaria M. beta-Granins: 21 kDa co-secreted peptides of the insulin granule closely related to adrenal medullary chromogranin A. FEBS Lett. 1985 Sep 2;188(2):336–340. doi: 10.1016/0014-5793(85)80398-7. [DOI] [PubMed] [Google Scholar]
- Iacangelo A. L., Fischer-Colbrie R., Koller K. J., Brownstein M. J., Eiden L. E. The sequence of porcine chromogranin A messenger RNA demonstrates chromogranin A can serve as the precursor for the biologically active hormone, pancreastatin. Endocrinology. 1988 May;122(5):2339–2341. doi: 10.1210/endo-122-5-2339. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Ishizuka J., Asada I., Poston G. J., Lluis F., Tatemoto K., Greeley G. H., Jr, Thompson J. C. Effect of pancreastatin on pancreatic endocrine and exocrine secretion. Pancreas. 1989;4(3):277–281. doi: 10.1097/00006676-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Benedum U. M., Gerdes H. H., Huttner W. B. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987 Dec 15;262(35):17026–17030. [PubMed] [Google Scholar]
- Lewis J. J., Zdon M. J., Adrian T. E., Modlin I. M. Pancreastatin: a novel peptide inhibitor of parietal cell secretion. Surgery. 1988 Dec;104(6):1031–1036. [PubMed] [Google Scholar]
- Nakano I., Funakoshi A., Miyasaka K., Ishida K., Makk G., Angwin P., Chang D., Tatemoto K. Isolation and characterization of bovine pancreastatin. Regul Pept. 1989 May;25(2):207–213. doi: 10.1016/0167-0115(89)90262-0. [DOI] [PubMed] [Google Scholar]
- Nolan J. A., Trojanowski J. Q., Hogue-Angeletti R. Neurons and neuroendocrine cells contain chromogranin: detection of the molecule in normal bovine tissues by immunochemical and immunohistochemical methods. J Histochem Cytochem. 1985 Aug;33(8):791–798. doi: 10.1177/33.8.3894497. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Chromogranin A: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci. 1983 Oct 24;33(17):1657–1663. doi: 10.1016/0024-3205(83)90721-x. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984 Mar 10;259(5):3237–3247. [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry. 1986 Jul 29;25(15):4402–4406. doi: 10.1021/bi00363a034. [DOI] [PubMed] [Google Scholar]
- Rindi G., Buffa R., Sessa F., Tortora O., Solcia E. Chromogranin A, B and C immunoreactivities of mammalian endocrine cells. Distribution, distinction from costored hormones/prohormones and relationship with the argyrophil component of secretory granules. Histochemistry. 1986;85(1):19–28. doi: 10.1007/BF00508649. [DOI] [PubMed] [Google Scholar]
- Settleman J., Fonseca R., Nolan J., Angeletti R. H. Relationship of multiple forms of chromogranin. J Biol Chem. 1985 Feb 10;260(3):1645–1651. [PubMed] [Google Scholar]
- Simon J. P., Bader M. F., Aunis D. Proteolytic processing of chromogranin A in cultured chromaffin cells. Biochim Biophys Acta. 1990 Feb 19;1051(2):123–130. doi: 10.1016/0167-4889(90)90183-e. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Kilpatrick D. L. Biochemistry of the enkephalins and enkephalin-containing peptides. Arch Biochem Biophys. 1983 Mar;221(2):309–323. doi: 10.1016/0003-9861(83)90149-2. [DOI] [PubMed] [Google Scholar]
- Watkinson A., Dockray G. J., Young J. N-linked glycosylation of a proenkephalin A-derived peptide. Evidence for the glycosylation of an NH2-terminally extended Met-enkephalin Arg6-Gly7-Leu8 variant. J Biol Chem. 1988 May 25;263(15):7147–7152. [PubMed] [Google Scholar]
- Watkinson A., O'Sullivan A. J., Burgoyne R. D., Dockray G. J. Differential accumulation of catecholamines, proenkephalin- and chromogranin A-derived peptides in the medium after chronic nicotine stimulation of cultured bovine adrenal chromaffin cells. Peptides. 1990 May-Jun;11(3):435–441. doi: 10.1016/0196-9781(90)90039-8. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Waldherr R., Buhr H., Hille A., Rosa P., Huttner W. B. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology. 1988 Nov;95(5):1364–1374. doi: 10.1016/0016-5085(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Apps D. K., Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986 Jun;18(2):261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Wohlfarter T., Fischer-Colbrie R., Hogue-Angeletti R., Eiden L. E., Winkler H. Processing of chromogranin A within chromaffin granules starts at C- and N-terminal cleavage sites. FEBS Lett. 1988 Apr 11;231(1):67–70. doi: 10.1016/0014-5793(88)80704-x. [DOI] [PubMed] [Google Scholar]