Abstract
N-Acetylneuraminate lyase produced by Escherichia coli was purified and crystallized from a genetically engineered strain (E. coli SF8/pNAL1). The enzyme showed apparent molecular masses of 105,000 Da on gel filtration and 35,000 Da on SDS/PAGE, suggesting that the enzyme is a trimer. The apparent optimum pH and temperature were found to be 6.5-7.0 and 80 degrees C respectively. The Km values for N-acetylneuraminate and N-glycollylneuraminate were 3.3 and 3.3 mM respectively. The enzyme was inhibited by reduction with NaBH4 in the presence of the substrate, indicating that the enzyme belongs to the Schiff-base-forming Class I aldolases. The enzyme was strongly inhibited by Cu2+ ions, p-chloromercuribenzoate and N-bromosuccinimide, and also inhibited competitively by the reaction product, pyruvate, and its structurally related compounds, dihydroxyacetone and DL-glyceraldehyde.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Uwajima T. Cloning and constitutive expression of the N-acetylneuraminate lyase gene of Escherichia coli. Appl Environ Microbiol. 1986 Mar;51(3):562–565. doi: 10.1128/aem.51.3.562-565.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden S. B., Chang W. H., Barksdale L. Distribution of neuraminidase and n-acetylneuraminate lyase activities among corynebacteria, mycobacteria, and nocardias. J Bacteriol. 1972 Dec;112(3):1206–1212. doi: 10.1128/jb.112.3.1206-1212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. The sialic acids. I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960 Sep;235:2529–2537. [PubMed] [Google Scholar]
- Carrillo N., Vallejos R. H. Essential histidyl residues of ferredoxin-NADP+ oxidoreductase revealed by diethyl pyrocarbonate inactivation. Biochemistry. 1983 Dec 6;22(25):5889–5897. doi: 10.1021/bi00294a031. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DeVries G. H., Binkley S. B. N-acetylneuraminic acid aldolase of Clostridium perfringens: purification, properties and mechanism of action. Arch Biochem Biophys. 1972 Jul;151(1):234–242. doi: 10.1016/0003-9861(72)90493-6. [DOI] [PubMed] [Google Scholar]
- Drzeniek R., Scharmann W., Balke E. Neuraminidase and N-acetylneuraminate pyruvate-lyase of Pasteurella multocida. J Gen Microbiol. 1972 Sep;72(2):357–368. doi: 10.1099/00221287-72-2-357. [DOI] [PubMed] [Google Scholar]
- GRAZI E., MELOCHE H., MARTINEZ G., WOOD W. A., HORECKER B. L. Evidence for Schiff base formation in enzymatic aldol condensations. Biochem Biophys Res Commun. 1963 Jan 18;10:4–10. doi: 10.1016/0006-291x(63)90257-2. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H., Möhler H., Decker K. A., Wood W. A. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase. I. Physical evidence for a three-subunit molecule. J Biol Chem. 1971 Apr 10;246(7):2069–2074. [PubMed] [Google Scholar]
- Heimer R., Meyer K. STUDIES ON SIALIC ACID OF SUBMAXILLARY MUCOID. Proc Natl Acad Sci U S A. 1956 Oct;42(10):728–734. doi: 10.1073/pnas.42.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kolisis F. N., Sotiroudis T. G., Evangelopoulos A. E. The protection role of pyruvate against heat inactivation of N-acetylneuraminate lyase. FEBS Lett. 1980 Dec 1;121(2):280–282. doi: 10.1016/0014-5793(80)80362-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nees S., Schauer R., Mayer F. Purification and characterization of N-acetylneuraminate lyase from Clostridium perfringens. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):839–853. doi: 10.1515/bchm2.1976.357.1.839. [DOI] [PubMed] [Google Scholar]
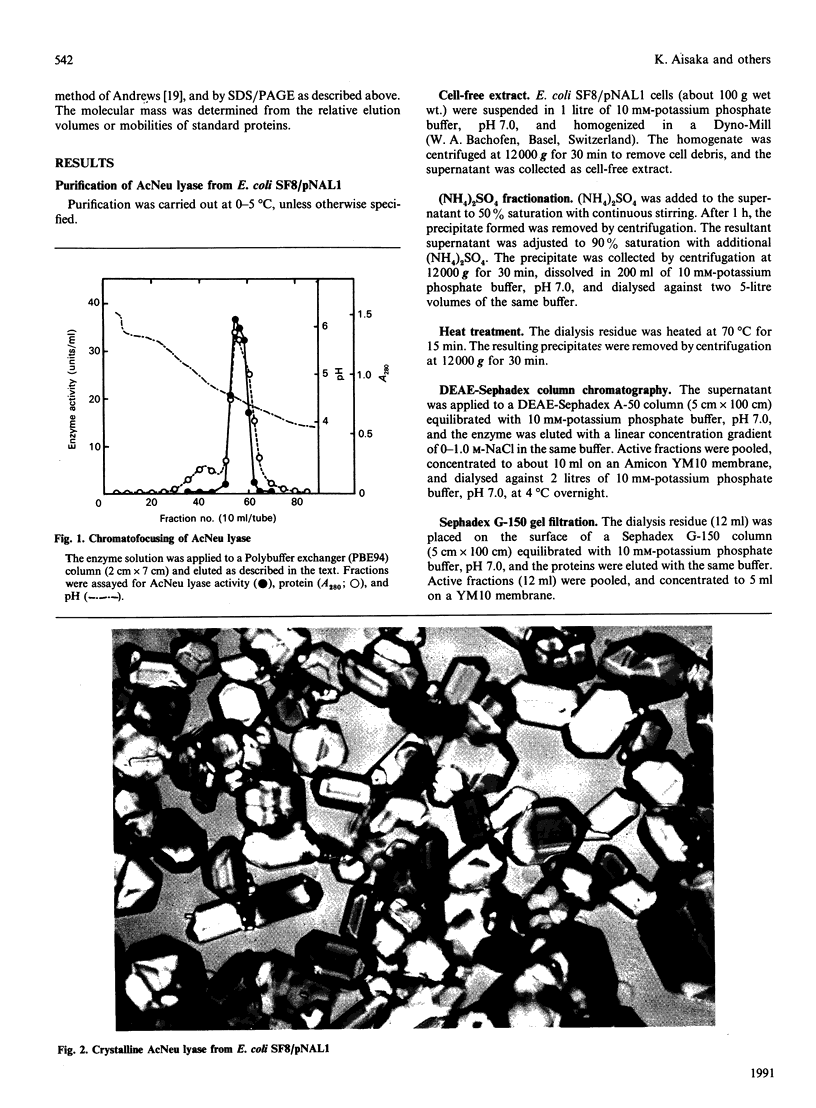
- Ohta Y., Watanabe K., Kimura A. Complete nucleotide sequence of the E. coli N-acetylneuraminate lyase. Nucleic Acids Res. 1985 Dec 20;13(24):8843–8852. doi: 10.1093/nar/13.24.8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Robertson D. C., Hammerstedt R. H., Wood W. A. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase. II. Chemical evidence for a three-subunit molecule. J Biol Chem. 1971 Apr 10;246(7):2075–2083. [PubMed] [Google Scholar]
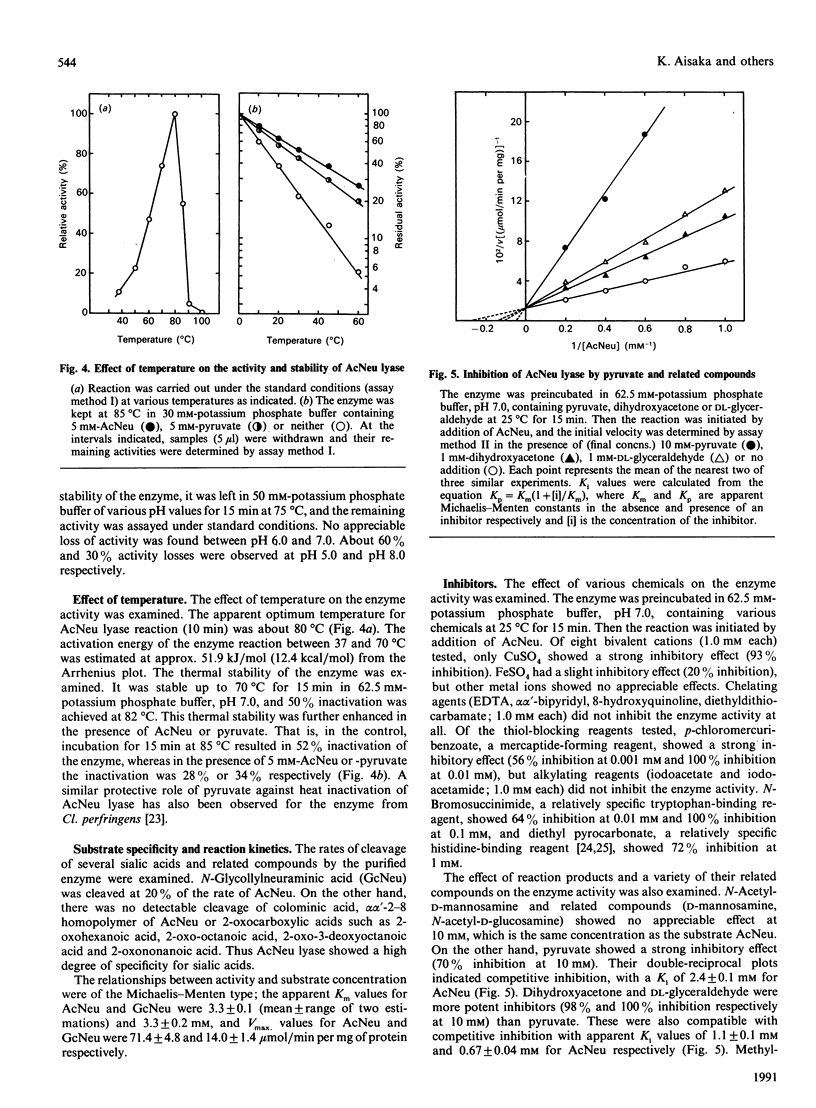
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Schauer R. Inhibition of acylneuraminate pyruvate-lyase: evidence of intermediary Schiff's base formation and of a possible role of histidine residues. Hoppe Seylers Z Physiol Chem. 1971 Nov;352(11):1517–1523. doi: 10.1515/bchm2.1971.352.2.1517. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Purification and properties of N-acetylneuraminate lyase from Escherichia coli. J Biochem. 1984 Aug;96(2):507–522. doi: 10.1093/oxfordjournals.jbchem.a134863. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985 Nov;164(2):845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 1985 Nov;164(2):854–860. doi: 10.1128/jb.164.2.854-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Dekker E. E., Lewinski N. D., Winter H. C. Physical and chemical evidence for the trimeric subunit structure of 2-keto-4-hydroxyglutarate aldolase from Escherichia coli K-12. J Biol Chem. 1981 Feb 25;256(4):1793–1800. [PubMed] [Google Scholar]