Abstract
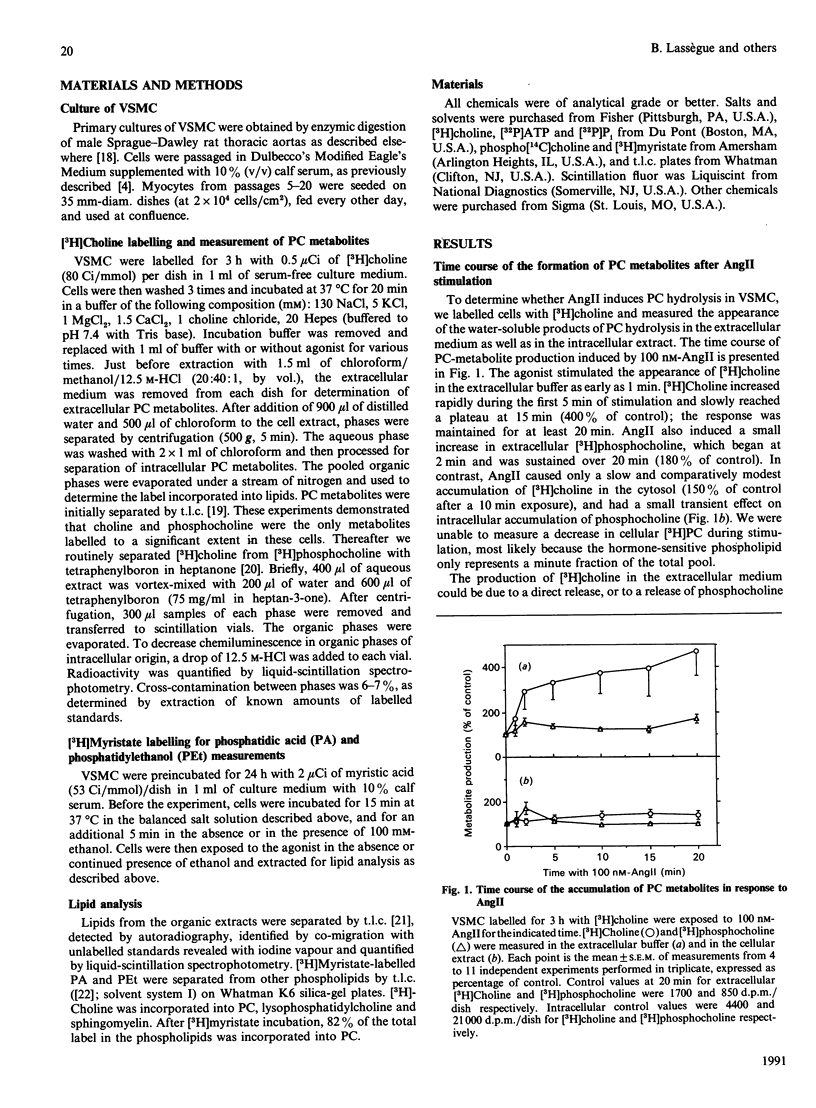
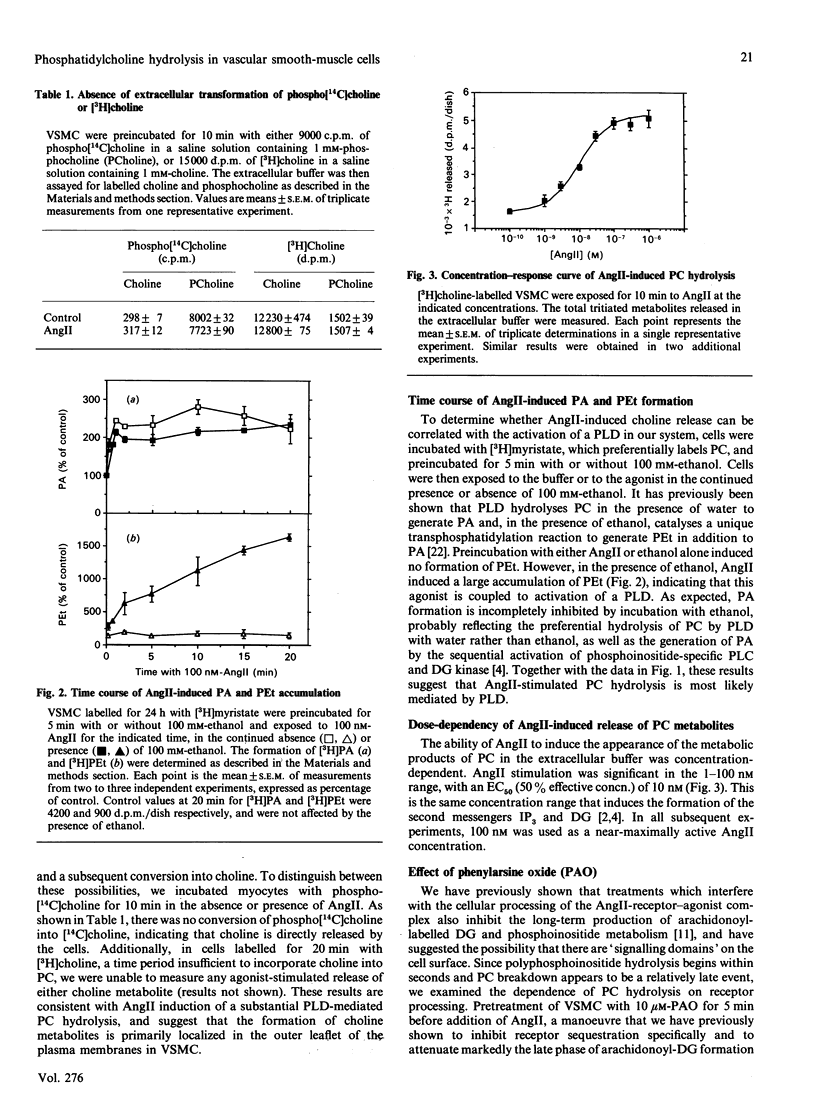
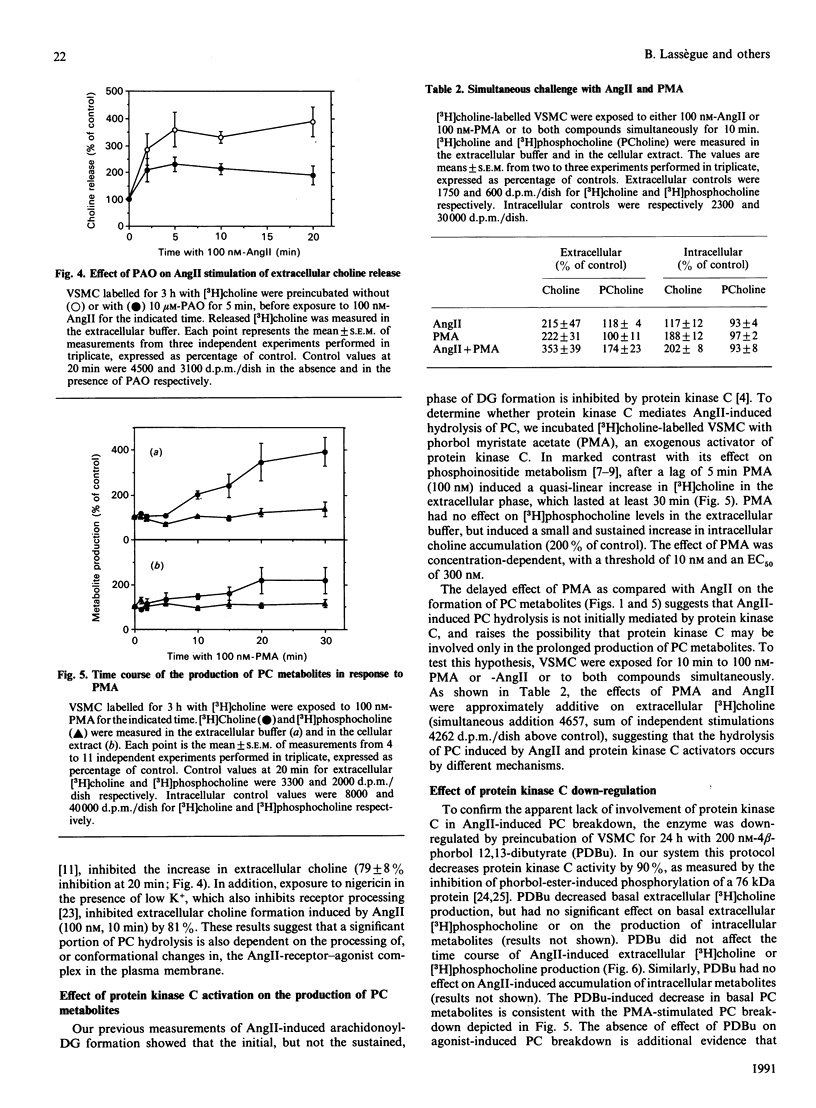
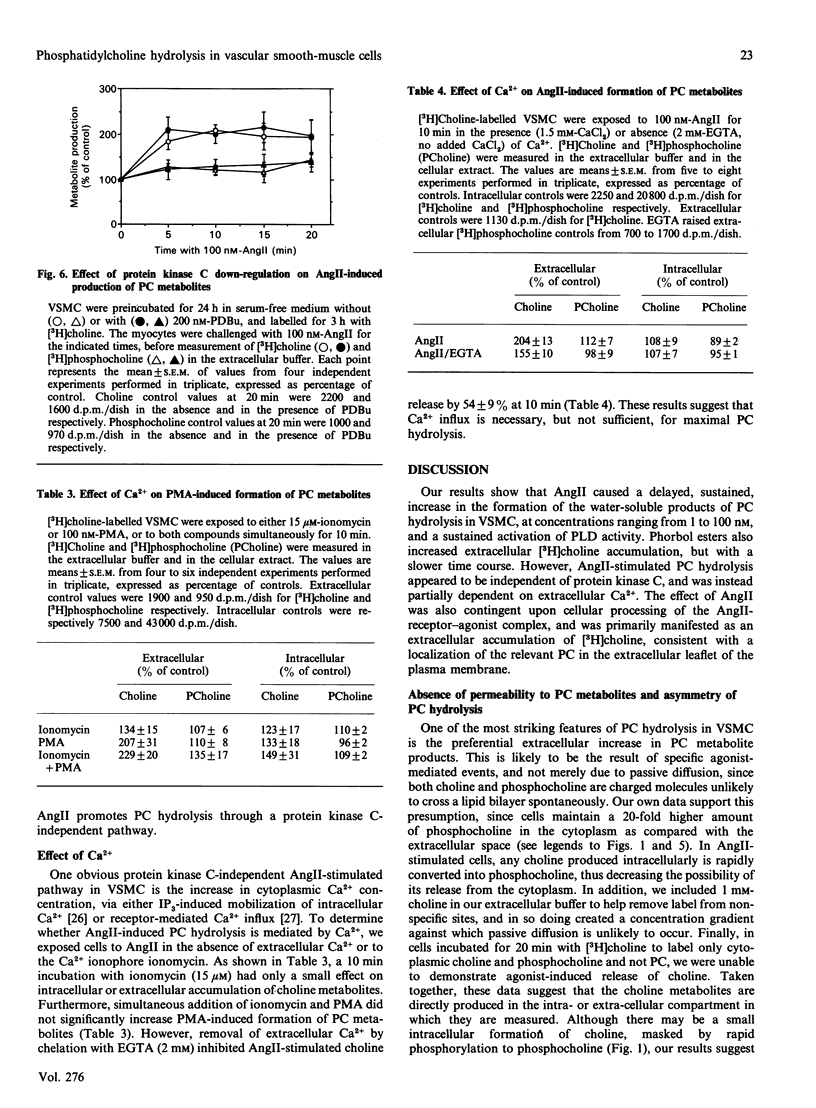
In cultured vascular smooth-muscle cells (VSMC), angiotensin II (AngII) induces a biphasic, sustained increase in diacylglycerol (DG) of unclear origin. To determine whether hydrolysis of phosphatidylcholine (PC) is a possible source of DG, we labelled cellular PC with [3H]choline, and measured the formation of intra- and extra-cellular [3H]choline and [3H]phosphocholine after stimulation with AngII. AngII induced a concentration-dependent release of choline from VSMC that was significant at 2 min and was sustained over 20 min. In contrast, accumulation of choline inside the cells was very slight. AngII also increased the formation of [3H]myristate-labelled phosphatidic acid, and, in the presence of ethanol, of [3H]phosphatidylethanol, characteristic of a phospholipase D (PLD) activity. Extracellular release of choline was partially inhibited by removal of extracellular Ca2+ (54 +/- 9% inhibition at 10 min) or inhibition of receptor processing by phenylarsine oxide (79 +/- 8% inhibition at 20 min). The protein kinase C activator phorbol myristate acetate also stimulated a large release of choline after a 5 min lag, which was unaffected by the Ca2+ ionophore ionomycin, but was additive with AngII stimulation. Down-regulation of protein kinase C by a 24 h incubation with phorbol dibutyrate (200 nM) decreased basal choline release, but had no effect on AngII stimulation. We conclude that AngII induces a major PC hydrolysis, probably mainly via PLD activation. This reaction is partially dependent on Ca2+ and is independent of protein kinase C, and appears to be mediated by cellular processing of the receptor-agonist complex. Our results are consistent with a preferential hydrolysis of PC from the external leaflet of the plasmalemma, and raise the possibility that PC hydrolysis occurs in specialized 'signalling domains' in VSMC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Rittenhouse S. E. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension. 1985 May-Jun;7(3 Pt 1):447–451. [PubMed] [Google Scholar]
- Augert G., Bocckino S. B., Blackmore P. F., Exton J. H. Hormonal stimulation of diacylglycerol formation in hepatocytes. Evidence for phosphatidylcholine breakdown. J Biol Chem. 1989 Dec 25;264(36):21689–21698. [PubMed] [Google Scholar]
- Barritt G. J., Dalton K. A., Whiting J. A. Evidence that phosphatidic acid stimulates the uptake of calcium by liver cells but not calcium release from mitochondria. FEBS Lett. 1981 Mar 23;125(2):137–140. doi: 10.1016/0014-5793(81)80703-x. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Endogenous and exogenous domain markers of the rat hepatocyte plasma membrane. J Cell Biol. 1985 Apr;100(4):1126–1138. doi: 10.1083/jcb.100.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Av P., Liscovitch M. Phospholipase D activation by the mitogens platelet-derived growth factor and 12-O-tetradecanoylphorbol 13-acetate in NIH-3T3 cells. FEBS Lett. 1989 Dec 18;259(1):64–66. doi: 10.1016/0014-5793(89)81495-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Blau L., Weissmann G. Transmembrane calcium movements mediated by ionomycin and phosphatidate in liposomes with Fura 2 entrapped. Biochemistry. 1988 Jul 26;27(15):5661–5666. doi: 10.1021/bi00415a040. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Cao H. T., Chabbott H. The phosphatidylcholine pathway of diacylglycerol formation stimulated by phorbol diesters occurs via phospholipase D activation. FEBS Lett. 1988 Jun 6;233(1):153–157. doi: 10.1016/0014-5793(88)81374-7. [DOI] [PubMed] [Google Scholar]
- Caramelo C., Tsai P., Schrier R. W. Mechanism of cellular effect of phorbol esters on action of arginine vasopressin and angiotensin II on rat vascular smooth muscle cells in culture. Biochem J. 1988 Sep 15;254(3):625–629. doi: 10.1042/bj2540625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Watson J. E., Acevedo-Duncan M., Pollet R. J., Standaert M. L., Farese R. V. Retention of specific protein kinase C isozymes following chronic phorbol ester treatment in BC3H-1 myocytes. Biochem Biophys Res Commun. 1989 May 30;161(1):327–334. doi: 10.1016/0006-291x(89)91600-8. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Leeb-Lundberg L. M., Hagen P. O., Lefkowitz R. J., Caron M. G. Phorbol ester effects on alpha 1-adrenoceptor binding and phosphatidylinositol metabolism in cultured vascular smooth muscle cells. Life Sci. 1985 Dec 23;37(25):2389–2398. doi: 10.1016/0024-3205(85)90106-7. [DOI] [PubMed] [Google Scholar]
- Delafontaine P., Griendling K. K., Gimbrone M. A., Jr, Alexander R. W. Potassium depletion selectively inhibits sustained diacylglycerol formation from phosphatidylinositol in angiotensin II-stimulated, cultured vascular smooth muscle cells. J Biol Chem. 1987 Oct 25;262(30):14549–14554. [PubMed] [Google Scholar]
- Diaz-Meco M. T., Larrodera P., Lopez-Barahona M., Cornet M. E., Barreno P. G., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is activated by muscarinic agonists. Biochem J. 1989 Oct 1;263(1):115–120. doi: 10.1042/bj2630115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Godson C., Weiss B. A., Insel P. A. Differential activation of protein kinase C alpha is associated with arachidonate release in Madin-Darby canine kidney cells. J Biol Chem. 1990 May 25;265(15):8369–8372. [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Alexander R. W. Evidence that Na+/H+ exchange regulates angiotensin II-stimulated diacylglycerol accumulation in vascular smooth muscle cells. J Biol Chem. 1988 Aug 5;263(22):10620–10624. [PubMed] [Google Scholar]
- Griendling K. K., Delafontaine P., Rittenhouse S. E., Gimbrone M. A., Jr, Alexander R. W. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987 Oct 25;262(30):14555–14562. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Grillone L. R., Clark M. A., Godfrey R. W., Stassen F., Crooke S. T. Vasopressin induces V1 receptors to activate phosphatidylinositol- and phosphatidylcholine-specific phospholipase C and stimulates the release of arachidonic acid by at least two pathways in the smooth muscle cell line, A-10. J Biol Chem. 1988 Feb 25;263(6):2658–2663. [PubMed] [Google Scholar]
- He N. B., Hui S. W. Electron microscopic observation of domain movement in reconstituted erythrocyte membranes. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7304–7308. doi: 10.1073/pnas.82.21.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling M., Vaartjes W. J., van Golde L. M. Isozymic forms of protein kinase C in regenerating rat liver. FEBS Lett. 1989 Apr 24;247(2):487–491. doi: 10.1016/0014-5793(89)81397-3. [DOI] [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Isakov N., McMahon P., Altman A. Selective post-transcriptional down-regulation of protein kinase C isoenzymes in leukemic T cells chronically treated with phorbol ester. J Biol Chem. 1990 Feb 5;265(4):2091–2097. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Stimulation of phosphatidylinositol 4,5-bisphosphate phospholipase C activity by phosphatidic acid. Arch Biochem Biophys. 1989 Feb 1;268(2):516–524. doi: 10.1016/0003-9861(89)90318-4. [DOI] [PubMed] [Google Scholar]
- Kariya K., Takai Y. Distinct functions of down-regulation-sensitive and -resistant types of protein kinase C in rabbit aortic smooth muscle cells. FEBS Lett. 1987 Jul 13;219(1):119–124. doi: 10.1016/0014-5793(87)81202-4. [DOI] [PubMed] [Google Scholar]
- Kroll M. H., Zavoico G. B., Schafer A. I. Second messenger function of phosphatidic acid in platelet activation. J Cell Physiol. 1989 Jun;139(3):558–564. doi: 10.1002/jcp.1041390315. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Martinson E. A., Goldstein D., Brown J. H. Muscarinic receptor activation of phosphatidylcholine hydrolysis. Relationship to phosphoinositide hydrolysis and diacylglycerol metabolism. J Biol Chem. 1989 Sep 5;264(25):14748–14754. [PubMed] [Google Scholar]
- Matozaki T., Williams J. A. Multiple sources of 1,2-diacylglycerol in isolated rat pancreatic acini stimulated by cholecystokinin. Involvement of phosphatidylinositol bisphosphate and phosphatidylcholine hydrolysis. J Biol Chem. 1989 Sep 5;264(25):14729–14734. [PubMed] [Google Scholar]
- McGhee J. G., Shoback D. M. Effects of phosphatidic acid on parathyroid hormone release, intracellular free Ca2+, and inositol phosphates in dispersed bovine parathyroid cells. Endocrinology. 1990 Feb;126(2):899–907. doi: 10.1210/endo-126-2-899. [DOI] [PubMed] [Google Scholar]
- Meyer H. W. Lipid domain structures in biological membranes. Exp Pathol. 1983;23(1):3–26. doi: 10.1016/s0232-1513(83)80037-1. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Ochsner M., Whitebread S., De Gasparo M. Down-regulation of protein kinase C potentiates angiotensin II-stimulated polyphosphoinositide hydrolysis in vascular smooth-muscle cells. Biochem J. 1989 Aug 15;262(1):285–291. doi: 10.1042/bj2620285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotton S., Robaye B., Lagneau C., Boeynaems J. M. Adenine nucleotides modulate phosphatidylcholine metabolism in aortic endothelial cells. J Cell Physiol. 1990 Mar;142(3):449–457. doi: 10.1002/jcp.1041420303. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Marshall C. J., Hall A. Stimulation of phosphatidylcholine hydrolysis, diacylglycerol release, and arachidonic acid production by oncogenic ras is a consequence of protein kinase C activation. J Biol Chem. 1989 Oct 5;264(28):16638–16643. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Reusch R. N. A mechanism for phosphoglyceride and Ca2+ transbilayer movement. Chem Phys Lipids. 1985 Apr;37(1):53–67. doi: 10.1016/0009-3084(85)90074-x. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E. Human platelets contain phospholipase C that hydrolyzes polyphosphoinositides. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5417–5420. doi: 10.1073/pnas.80.17.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Slivka S. R., Meier K. E., Insel P. A. Alpha 1-adrenergic receptors promote phosphatidylcholine hydrolysis in MDCK-D1 cells. A mechanism for rapid activation of protein kinase C. J Biol Chem. 1988 Sep 5;263(25):12242–12246. [PubMed] [Google Scholar]
- Smith J. B., Smith L., Brown E. R., Barnes D., Sabir M. A., Davis J. S., Farese R. V. Angiotensin II rapidly increases phosphatidate-phosphoinositide synthesis and phosphoinositide hydrolysis and mobilizes intracellular calcium in cultured arterial muscle cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7812–7816. doi: 10.1073/pnas.81.24.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Rasmussen H. A tumour promoter, 12-O-tetradecanoylphorbol 13-acetate, increases cellular 1,2-diacylglycerol content through a mechanism other than phosphoinositide hydrolysis in Swiss-mouse 3T3 fibroblasts. Biochem J. 1987 May 1;243(3):647–653. doi: 10.1042/bj2430647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Barrett G., Burnstock G. Differences in proliferation of primary cultures of vascular smooth muscle cells taken from male and female rats. Blood Vessels. 1980;17(2):110–116. doi: 10.1159/000158240. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Griendling K. K., Alexander R. W. Angiotensin II stimulates vimentin phosphorylation via a Ca2+-dependent, protein kinase C-independent mechanism in cultured vascular smooth muscle cells. J Biol Chem. 1988 Dec 25;263(36):19758–19763. [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]


