Abstract
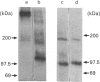
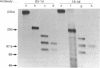
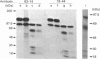
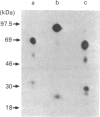
The tyrosine kinase of the insulin receptor can be activated by trypsin treatment. The concomitant abolition of insulin binding has been postulated to result from proteolytic destruction of the receptor. A discrepancy between the decrease in insulin binding and receptor immunoreactivity after trypsin treatment led us to investigate more closely the structure of the trypsin-treated receptor. After trypsin treatment of the CHOT cell line, which over-expresses transfected human insulin receptors, insulin binding was significantly decreased, but reactivity with five alpha-subunit monoclonal antibodies was either unaffected or only moderately decreased, indicating that the alpha-subunit was substantially intact. Examination of receptor structure after trypsin treatment, receptor autophosphorylation and gel electrophoresis revealed a single band at 110 kDa in non-reduced gels, comprising a small fragment (21 kDa) of the alpha-subunit linked to the beta-subunit by class II disulphides. When the receptor was radio-labelled with 125I, two additional alpha-subunit bands of 142 kDa and 81 kDa (composed of identical reduced bands) were observed on non-reduced gels, which contained disulphide-linked (class I) fragments. All fragments could be precipitated by antibodies to both alpha- and beta-subunits. However, only antibodies directed towards the N-terminus of the receptor could immunoblot trypsin-treated fragments. Thus activation of the receptor tyrosine kinase by trypsin occurs after cleavage, but not loss of the alpha-subunit. This finding has implications for the mechanism of transmembrane activation of the receptor kinase by insulin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Clark S., Cheng D. J., Hsuan J. J., Haley J. D., Waterfield M. D. Loss of three major auto phosphorylation sites in the EGF receptor does not block the mitogenic action of EGF. J Cell Physiol. 1988 Mar;134(3):421–428. doi: 10.1002/jcp.1041340313. [DOI] [PubMed] [Google Scholar]
- Clark S., DeLuise M., Larkins R. G., Melick R. A., Harrison L. C. The effects of digestive enzymes on characteristics of placental insulin receptor. Comparison of particulate and soluble receptor preparations. Biochem J. 1978 Jul 15;174(1):37–43. doi: 10.1042/bj1740037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Ellis L., Sissom J., Levitan A. Truncation of the ectodomain of the human insulin receptor results in secretion of a soluble insulin binding protein from transfected CHO cells. J Mol Recognit. 1988 Feb;1(1):25–31. doi: 10.1002/jmr.300010106. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Flier J., Itin A., Kahn C. R., Roth J. Radioimmunoassay of the insulin receptor: a new probe of receptor structure and function. Science. 1979 Feb 9;203(4380):544–547. doi: 10.1126/science.83675. [DOI] [PubMed] [Google Scholar]
- Hsuan J. J., Downward J., Clark S., Waterfield M. D. Proteolytic generation of constitutive tyrosine kinase activity of the human insulin receptor. Biochem J. 1989 Apr 15;259(2):519–527. doi: 10.1042/bj2590519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Disulfide reduction converts the insulin receptor of human placenta to a low affinity form. J Clin Invest. 1980 Dec;66(6):1424–1427. doi: 10.1172/JCI109996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Schwartz C., Creacy S., Larner J. Independent control of selected insulin-sensitive cell membrane and intracellular functions-the linkage of cell membrane and intracellular events controlled by insulin. III. The influence of trypsin on cell membrane hexose transport and on glycogen synthase and mitochondrial pyruvate dehydrogenase activation. Mol Cell Biochem. 1981 Jul 7;37(2):125–130. doi: 10.1007/BF02354936. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Insulin-like effects of trypsin on fat cells. Localization of the metabolic steps and the cellular site affected by the enzyme. J Biol Chem. 1971 Oct 25;246(20):6204–6209. [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leef J. W., Larner J. Insulin-mimetic effect of trypsin on the insulin receptor tyrosine kinase in intact adipocytes. J Biol Chem. 1987 Oct 25;262(30):14837–14842. [PubMed] [Google Scholar]
- Loughnan M. S., Sanderson C. J., Nossal G. J. Soluble interleukin 2 receptors are released from the cell surface of normal murine B lymphocytes stimulated with interleukin 5. Proc Natl Acad Sci U S A. 1988 May;85(9):3115–3119. doi: 10.1073/pnas.85.9.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. Role of disulfides in the subunit structure of the insulin receptor. Reduction of class I disulfides does not impair transmembrane signalling. J Biol Chem. 1982 Jun 25;257(12):6729–6738. [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Plate binding assay for monoclonal anti-receptor antibodies. Endocrinology. 1985 Mar;116(3):1224–1226. doi: 10.1210/endo-116-3-1224. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Axelrod J. D., Colello J., Czech M. P. Unimpaired signal transduction by the adipocyte insulin receptor following its partial proteolytic fragmentation. J Biol Chem. 1981 Feb 25;256(4):1570–1575. [PubMed] [Google Scholar]
- Prigent S. A., Stanley K. K., Siddle K. Identification of epitopes on the human insulin receptor reacting with rabbit polyclonal antisera and mouse monoclonal antibodies. J Biol Chem. 1990 Jun 15;265(17):9970–9977. [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Honegger A. M., Schlessinger J., Ullrich A. Cytoplasmic domains determine signal specificity, cellular routing characteristics and influence ligand binding of epidermal growth factor and insulin receptors. EMBO J. 1989 Oct;8(10):2943–2954. doi: 10.1002/j.1460-2075.1989.tb08444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Schaefer E. M., Siddle K., Ellis L. Deletion analysis of the human insulin receptor ectodomain reveals independently folded soluble subdomains and insulin binding by a monomeric alpha-subunit. J Biol Chem. 1990 Aug 5;265(22):13248–13253. [PubMed] [Google Scholar]
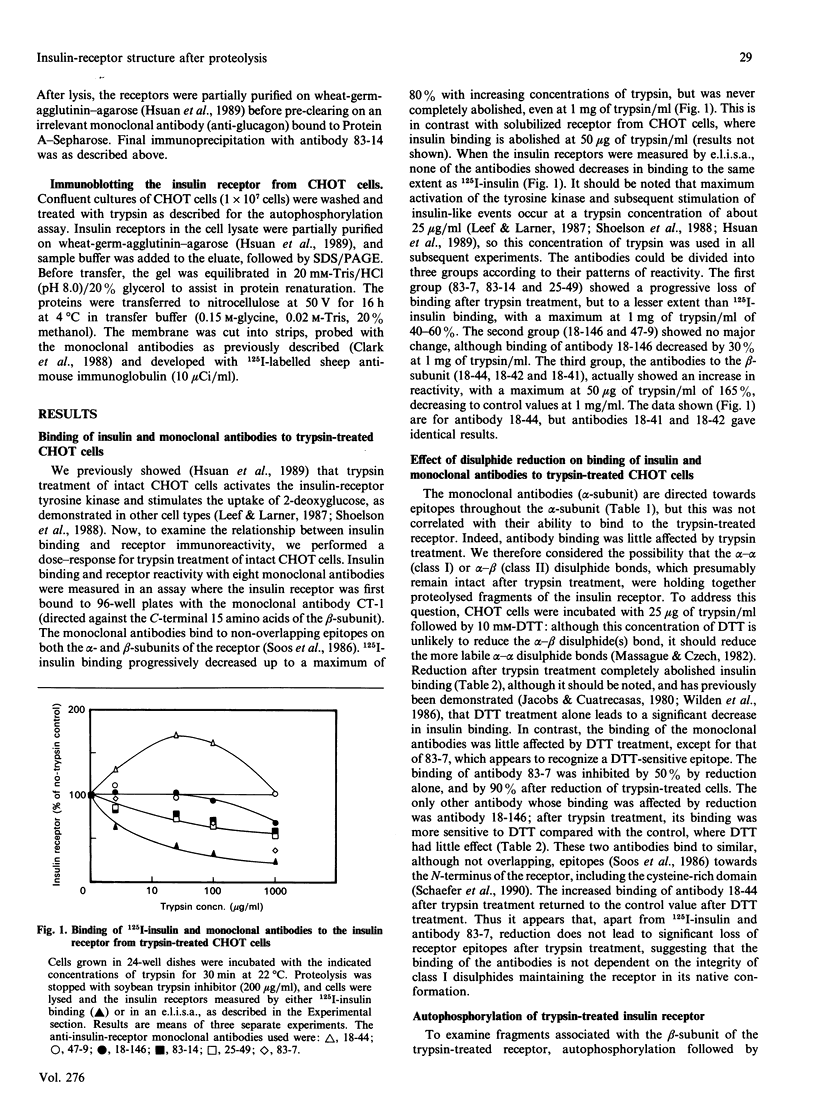
- Shoelson S. E., White M. F., Kahn C. R. Tryptic activation of the insulin receptor. Proteolytic truncation of the alpha-subunit releases the beta-subunit from inhibitory control. J Biol Chem. 1988 Apr 5;263(10):4852–4860. [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Fujita-Yamaguchi Y., Larner J. Insulin-like effect of trypsin on the phosphorylation of rat adipocyte insulin receptor. J Biol Chem. 1983 Dec 25;258(24):14749–14752. [PubMed] [Google Scholar]
- Taylor R., Soos M. A., Wells A., Argyraki M., Siddle K. Insulin-like and insulin-inhibitory effects of monoclonal antibodies for different epitopes on the human insulin receptor. Biochem J. 1987 Feb 15;242(1):123–129. doi: 10.1042/bj2420123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Livingston J. N., Backer J. M., Lauris V., Dull T. J., Ullrich A., Kahn C. R. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell. 1988 Aug 26;54(5):641–649. doi: 10.1016/s0092-8674(88)80008-4. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Boyle T. R., Swanson M. L., Sweet L. J., Pessin J. E. Alteration of intramolecular disulfides in insulin receptor/kinase by insulin and dithiothreitol: insulin potentiates the apparent dithiothreitol-dependent subunit reduction of insulin receptor. Biochemistry. 1986 Jul 29;25(15):4381–4388. doi: 10.1021/bi00363a031. [DOI] [PubMed] [Google Scholar]
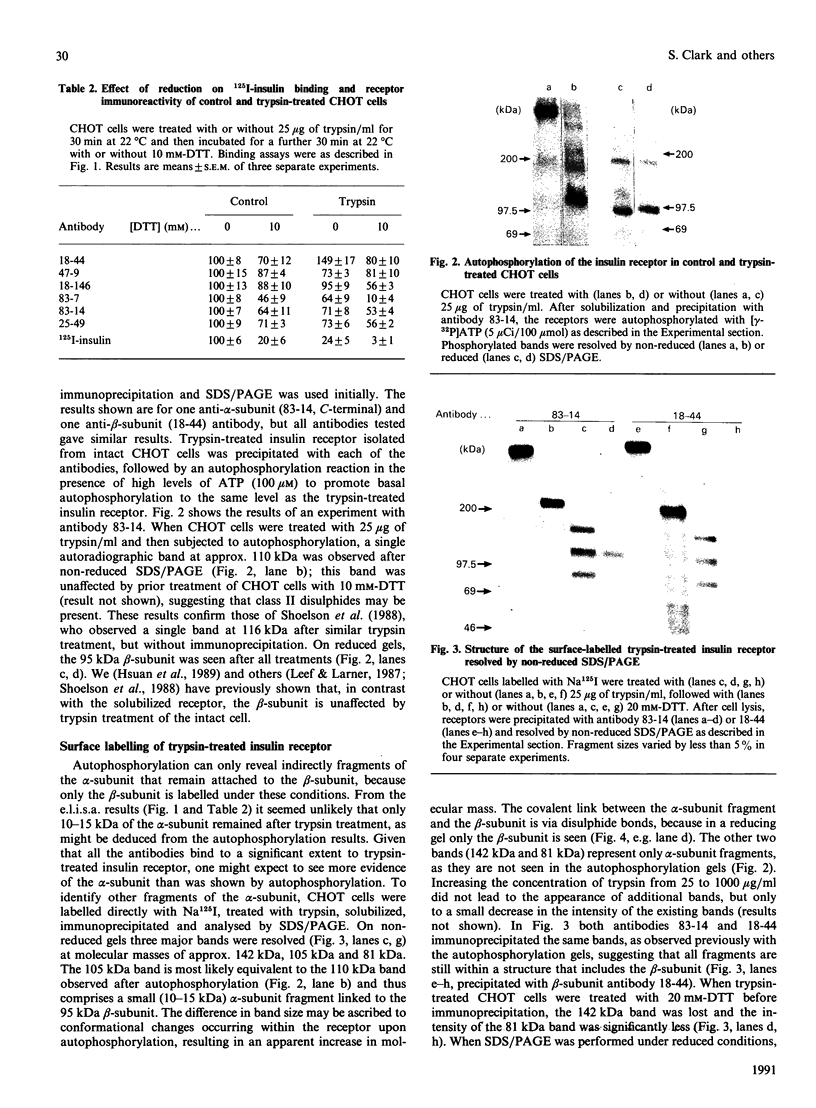
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]