Abstract
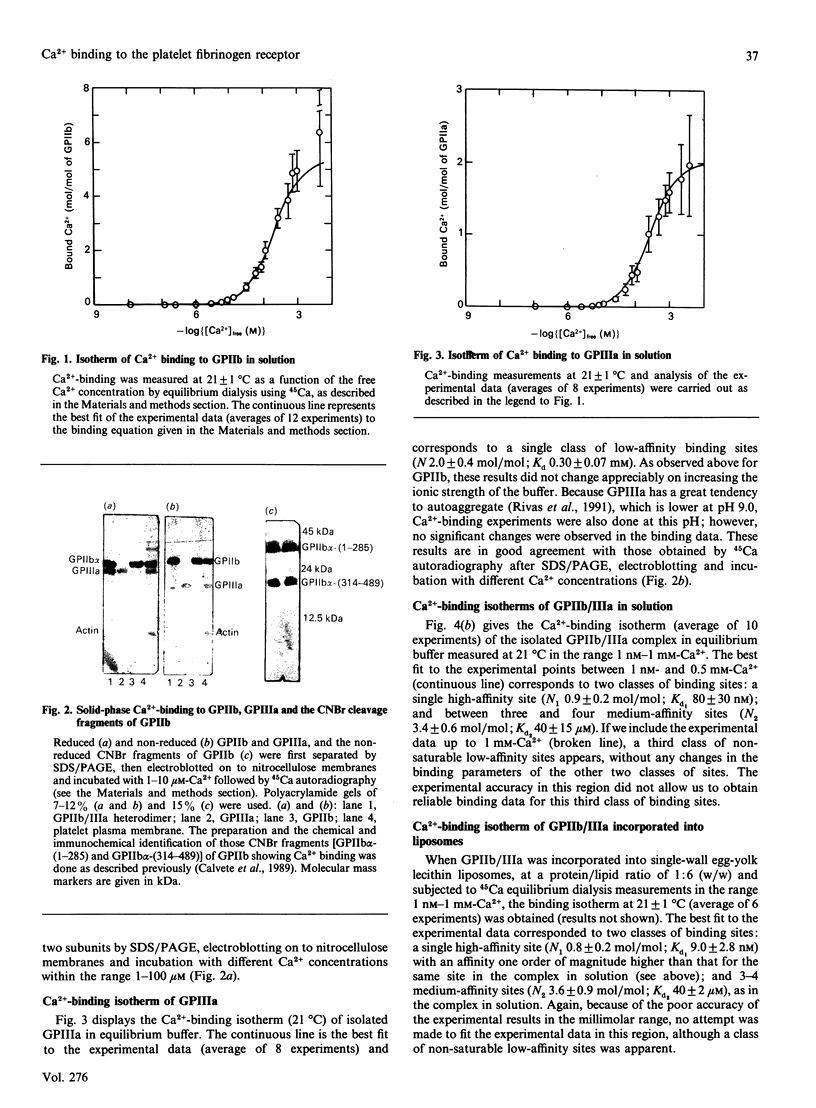
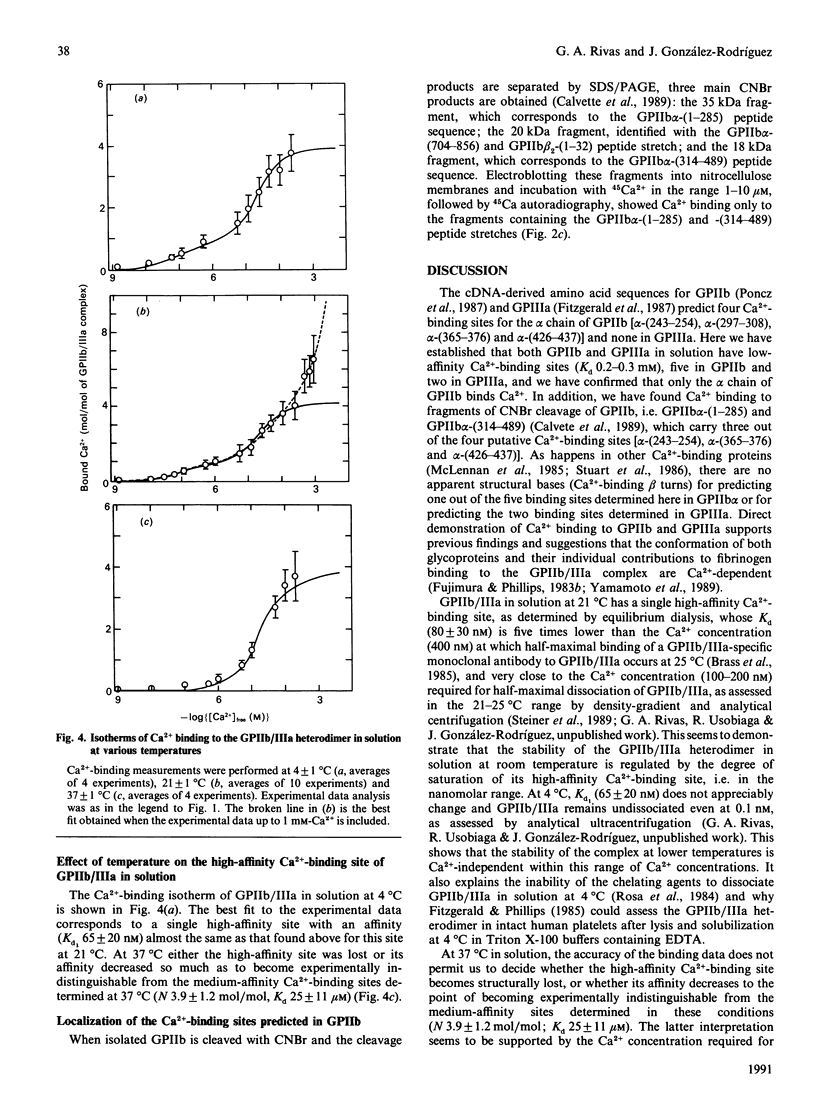
Platelet plasma membrane glycoproteins IIb (GPIIb) and IIIa (GPIIIa) form a Ca(2+)-dependent heterodimer. GPIIb/IIIa, which serves as the receptor for fibrinogen and other adhesive proteins at the surface of activated platelets. Using equilibrium dialysis measurements, it was established that both GPIIb and GPIIIa in solution have low-affinity Ca(2-)-binding sites (Kd0.2-0.3 mM), five in GPIIb and two in GPIIIa, and it was confirmed that only the alpha-chain of GPIIb (GPIIb alpha) binds Ca2+. Furthermore, Ca2+ binding was found with two CNBr fragments of GPIIb, GPIIb alpha-(1-285) and GPIIb alpha-(314-489), which carry three out of the four putative Ca(2+)-binding sites. GPIIb/IIIa in solution has a single high-affinity Ca(2+)-binding site (Kd1 80 +/- 30 nM at 21 degrees C), whose degree of saturation regulates the state of association of GPIIb and GPIIIa in the GPIIb/IIIa heterodimer at room temperature, and 3-4 medium-affinity Ca(2+)-binding sites (Kd2 40 +/- 15 microM at 21 degrees C). When GPIIb/IIIa was incorporated into liposomes, Kd1 decreased by an order of magnitude (9 +/- 3 nM at 21 degrees C) and reached the dissociation constant estimated for the high-affinity Ca(2+)-binding sites at the platelet surface [Brass & Shattil (1982) J. Biol. Chem. 257, 1400-1405], whereas Kd2 remained unchanged. The high-affinity Ca(2+)-binding site of GPIIb/IIIa in solution at 4 degrees C has almost the same affinity (Kd1 65 +/- 20 nM) as at 21 degrees C; however, at 37 degrees C, either its affinity decreases enough so as to become experimentally indistinguishable from the medium-affinity Ca(2+)-binding sites determined at this temperature (number of binding sites 3.9 +/- 1.2 mol of Ca2+/mol of GP, Kd 25 +/- 11 microM), or vanishes altogether. Studies on Ca(2+)-dependent dissociation of GPIIIb/IIIa at 37 degrees C in solution seem to support the former interpretation. Further work will be necessary to decide whether the dissociation of GPIIb/IIIa in the platelet membrane at 37 degrees C is regulated by the degree of saturation of the high-affinity Ca(2+)-binding site, as occurs in solution. It is suggested that the high-affinity Ca(2+)-binding site could be related to the putative GPIIIa-binding region in GPIIb (residues 558-747 of the alpha chain).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. EFFECTS OF INORGANIC IONS AND OF PLASMA PROTEINS ON THE AGGREGATION OF BLOOD PLATELETS BY ADENOSINE DIPHOSPHATE. J Physiol. 1964 Mar;170:397–414. doi: 10.1113/jphysiol.1964.sp007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Protein kinase C interaction with calcium: a phospholipid-dependent process. Biochemistry. 1990 Aug 21;29(33):7624–7630. doi: 10.1021/bi00485a012. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F. Ca2+ transport across the platelet plasma membrane. A role for membrane glycoproteins IIB and IIIA. J Biol Chem. 1985 Feb 25;260(4):2231–2236. [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J. Identification and function of the high affinity binding sites for Ca2+ on the surface of platelets. J Clin Invest. 1984 Mar;73(3):626–632. doi: 10.1172/JCI111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Calvete J. J., Alvarez M. V., Rivas G., Hew C. L., Henschen A., González-Rodríguez J. Interchain and intrachain disulphide bonds in human platelet glycoprotein IIb. Localization of the epitopes for several monoclonal antibodies. Biochem J. 1989 Jul 15;261(2):551–560. doi: 10.1042/bj2610551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Arias J., Alvarez M. V., Lopez M. M., Henschen A., Gonzalez-Rodriguez J. Further studies on the topography of human platelet glycoprotein IIb. Localization of monoclonal antibody epitopes and the putative glycoprotein IIa- and fibrinogen-binding regions. Biochem J. 1991 Feb 1;273(Pt 3):767–775. doi: 10.1042/bj2730767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., González-Rodríguez J. Isolation and biochemical characterization of the alpha- and beta-subunits of glycoprotein IIb of human platelet plasma membrane. Biochem J. 1986 Nov 15;240(1):155–161. doi: 10.1042/bj2400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirín M. T., Calvete J. J., González-Rodríguez J. New isolation procedure and further biochemical characterization of glycoproteins IIb and IIIa from human platelet plasma membrane. Biochem J. 1986 Nov 15;240(1):147–153. doi: 10.1042/bj2400147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Phillips D. R. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 15;260(20):11366–11374. [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Forstner J., Manery J. F. Calcium binding by human erythrocyte membranes. Biochem J. 1971 Sep;124(3):563–571. doi: 10.1042/bj1240563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Glenney J. Phospholipid-dependent Ca2+ binding by the 36-kDa tyrosine kinase substrate (calpactin) and its 33-kDa core. J Biol Chem. 1986 Jun 5;261(16):7247–7252. [PubMed] [Google Scholar]
- Gogstad G. O., Krutnes M. B., Solum N. O. Calcium-binding proteins from human platelets. A study using crossed immunoelectrophoresis and 45Ca2+. Eur J Biochem. 1983 Jun 1;133(1):193–199. doi: 10.1111/j.1432-1033.1983.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Johnston G. I., Heptinstall S. Identity of saturable calcium-binding sites on blood platelets and their involvement in platelet aggregation. Thromb Haemost. 1988 Feb 25;59(1):54–61. [PubMed] [Google Scholar]
- Karpatkin S., Ferziger R., Dorfman D. Crossed immunoelectrophoresis of human platelet membranes. Effect of charge on association and dissociation of the glycoprotein GPIIb-GPIIIa membrane complex. J Biol Chem. 1986 Oct 25;261(30):14266–14272. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., Ginsberg M. H. Platelet membrane glycoprotein IIb heavy chain forms a complex with glycoprotein IIIa that binds Arg-Gly-Asp peptides. Blood. 1989 May 1;73(6):1513–1518. [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G., Chagniel G., Suscillon M. The binding of calcium to bovine fibrinogen. Biochim Biophys Acta. 1977 Jan 25;490(1):94–103. doi: 10.1016/0005-2795(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987 Aug;70(2):475–483. [PubMed] [Google Scholar]
- Nozaki Y., Lasic D. D., Tanford J. A. Size analysis of phospholipid vesicle preparations. Science. 1982 Jul 23;217(4557):366–367. doi: 10.1126/science.7089571. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. pH and magnesium alter 45calcium binding to platelets at sites other than glycoproteins I or IIb/IIIa. Proc Soc Exp Biol Med. 1985 Jun;179(2):232–239. doi: 10.3181/00379727-179-42092. [DOI] [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Potter J. D., Strang-Brown P., Walker P. L., Iida S. Ca2+ binding to calmodulin. Methods Enzymol. 1983;102:135–143. doi: 10.1016/s0076-6879(83)02014-5. [DOI] [PubMed] [Google Scholar]
- Rosa J. P., Kieffer N., Didry D., Pidard D., Kunicki T. J., Nurden A. T. The human platelet membrane glycoprotein complex GP IIb-IIIa expresses antigenic sites not exposed on the dissociated glycoproteins. Blood. 1984 Dec;64(6):1246–1253. [PubMed] [Google Scholar]
- Rybak M. E., Renzulli L. A., Bruns M. J., Cahaly D. P. Platelet glycoproteins IIb and IIIa as a calcium channel in liposomes. Blood. 1988 Aug;72(2):714–720. [PubMed] [Google Scholar]
- Steiner B., Cousot D., Trzeciak A., Gillessen D., Hadváry P. Ca2+-dependent binding of a synthetic Arg-Gly-Asp (RGD) peptide to a single site on the purified platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989 Aug 5;264(22):13102–13108. [PubMed] [Google Scholar]
- Stuart D. I., Acharya K. R., Walker N. P., Smith S. G., Lewis M., Phillips D. C. Alpha-lactalbumin possesses a novel calcium binding loop. Nature. 1986 Nov 6;324(6092):84–87. doi: 10.1038/324084a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]
- Yamaguchi A., Yamamoto N., Kitagawa H., Tanoue K., Yamazaki H. Ca2+ influx mediated through the GPIIb/IIIa complex during platelet activation. FEBS Lett. 1987 Dec 10;225(1-2):228–232. doi: 10.1016/0014-5793(87)81163-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Kitagawa H., Yamamoto K., Tanoue K., Yamazaki H. Calcium ions and the conformation of glycoprotein IIIa that is essential fibrinogen binding to platelets: analysis by a new monoclonal anti-GP IIIa antibody, TM83. Blood. 1989 May 1;73(6):1552–1560. [PubMed] [Google Scholar]



