Abstract
The contribution of immune reconstitution following antiretroviral treatment to the prevention or treatment of human immunodeficiency virus-related primary or reactivation tuberculosis remains unknown. Macaque models of simian immunodeficiency virus-Mycobacterium bovis BCG (SIV/BCG) coinfection were employed to determine the extent to which anti-Mycobacterium tuberculosis immunity can be restored by antiretroviral therapy. Both SIV-infected macaques with active BCG reinfection and naive animals with simultaneous SIV/BCG coinfection were evaluated. The suppression of SIV replication by antiretroviral treatment resulted in control of the active BCG infection and blocked development of the fatal SIV-related tuberculosis-like disease. The resolution of this disease coincided with the restoration of BCG purified protein derivative (PPD)-specific T-cell immune responses. In contrast, macaques similarly coinfected with SIV/BCG but not receiving antiretroviral therapy had depressed PPD-specific primary and memory T-cell immune responses and died from tuberculosis-like disease. These results provide in vivo evidence that the restoration of anti-mycobacterial immunity by antiretroviral agents can improve the clinical outcome of an AIDS virus-related tuberculosis-like disease.
Mycobacterium tuberculosis-induced tuberculosis remains one of the world's leading killers. Human immunodeficiency virus (HIV)-infected patients appear particularly susceptible to both primary and reactivation tuberculosis even at moderate levels of immune suppression. Currently accepted tuberculosis therapy requires prolonged treatment with multiple anti-mycobacterial drugs. The absence of complete compliance for the prolonged treatment can certainly lead to the occurrence of drug resistance. In fact, the emergence of multidrug-resistant tuberculosis (MDRTB) has made tuberculosis extremely difficult to treat in HIV type 1 (HIV-1)-infected humans (10, 12, 19). Although recent studies have demonstrated that rates of tuberculosis decline coincident with the introduction of highly active antiretroviral treatment (HAART) (9, 14), many important questions regarding the pathogenesis of HIV-M. tuberculosis coinfection and the effect of HAART on the clinical improvement of HIV-related tuberculosis have not been addressed. It is therefore important to elucidate the precise defects in anti-mycobacterial immunity caused by HIV-1 infection and to determine the degree to which anti-tuberculosis immunity can be restored in HIV-1-infected individuals by HARRT.
We have recently demonstrated that simian immunodeficiency virus (SIV)-infected macaques inoculated intravenously with Mycobacterium bovis BCG (SIV/BCG) develop an SIV-related tuberculosis-like disease characterized clinically by a syndrome of fever, anorexia, diarrhea, and weight loss and pathologically by the formation of disseminated granulomas (4, 26; Y. Shen et al., submitted for publication). The coinfected macaques likely to develop this fatal SIV-related tuberculosis-like disease have features of enhanced decline of CD4+ peripheral blood lymphocyle (PBL) counts and an associated high level of SIV replication. It is therefore possible that the control of SIV infections might reverse the SIV-mediated suppression of anti-mycobacterial T-cell responses and reduce the susceptibility of SIV/BCG-coinfected macaques to this fatal SIV-related BCG-induced disease. To test this hypothesis, we sought to determine whether antiretroviral drug therapy could restore anti-mycobacterial immunity and block clinical progression to this fatal tuberculosis-like disease in SIV/BCG-coinfected monkeys.
MATERIALS AND METHODS
Animals and virus.
Rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) macaques, 2 to 5 years of age, were used in these studies. These animals were maintained in accordance with the guidelines of the Committee on Animals for Harvard Medical School and the Guide for the Care and Use of Laboratory Animals. For SIV infection, macaques were inoculated intravenously with 106 50% tissue culture infective doses of strain SIV mac 251, as described previously (3).
M. bovis BCG infection.
M. bovis BCG (Pasteur strain) was stored in liquid nitrogen and thawed immediately before inoculation. To examine antiretroviral therapy-induced restoration of memory anti-mycobacterial immunity, six macaques were infected sequentially with BCG, then with SIV, and finally with BCG again, at 2-month intervals. To test the effect of antiretroviral therapy on the development of primary T-cell responses to BCG, four macaques naive to SIV and BCG were simultaneously inoculated intravenously with SIVmac251 and BCG. For BCG infections, macaques were inoculated intravenously with 108 CFU of BCG. After BCG inoculation, the monkeys were assessed prospectively for the status of their SIV and BCG infections, as well as for the development of clinical illness.
Antiretroviral treatment of SIV/BCG-coinfected macaques.
Two groups of SIV/BCG-coinfected macaques were used in studies to assess the ability of antiretroviral therapy to restore effective anti-mycobacterial immunity. To test the effect of antiretroviral therapy on memory T-cell responses to BCG, six macaques were sequentially infected with BCG, SIV, and again with BCG at 2-month intervals, as described above. Three of these macaques were treated daily with antiretroviral drugs 3 to 5 days after they developed the clinical syndrome of anorexia, diarrhea, and weight loss (up to 20% loss of body weight). The other three macaques were used as controls and never received antiretroviral drugs. The antiretroviral drug regimen was comprised of [(R)-9-(2-phosphonomethoxy)propyl] adenine (PMPA) and two protease inhibitors, Crixivan (Merck, Inc.) and Viracept (Agouron, Inc.). Each antiretroviral drug was in the form of an injectable powder and was dissolved in saline according to the manufacturer's instruction (for Viracept, dimethyl sulfoxide was added as a solvent). The in vivo use of PMPA and its efficacy in anti-SIV treatment (16, 24) is well described. Our in vitro studies showed that Crixivan and Viracept can suppress SIV replication in macaque PBL to undectectable levels, although 3- to 10-fold higher concentrations of drug are needed to achieve a potency comparable to that used to inhibit HIV-1 replication. In fact, the daily injection of SIV-infected macaques with these two drugs together for 1 week resulted in up to a 2-log reduction of SIV RNA levels in plasma (data not shown). The in vivo dosages of Crixivan and Viracept were chosen based on the results of in vitro testing and the half-lives of the drugs in animals. PMPA was given by subcutaneous injection once daily at a dosage of 30 mg/kg; Crixivan and Viracept were administered subcutaneously twice daily at dosages of 20 and 5 mg/kg, respectively. Viracept was discontinued 3 weeks after the initiation of treatment because of the occurrence of skin rash in some monkeys. The antiretroviral drugs were given for up to 10 weeks. The SIV/BCG-coinfected macaques were followed up clinically and pathologically for up to 12 months after the administration of antiretroviral agents.
A second group of animals was used to assess the effects of antiretroviral therapy on the development of primary T-cell immunity to mycobacterial infection. For this purpose, four naive macaques were inoculated simultaneously with SIV and BCG. Antiretroviral treatment was initiated when the animals developed the clinical syndrome of anorexia, diarrhea, and weight loss. Two of the SIV/BCG-coinfected macaques were injected subcutaneously with 30 mg of PMPA daily for 8 weeks. The use PMPA alone for the treatment protocol in the second cohort was based on its success in the drug-induced control of the marked increase in levels of SIV RNA following staphylococcus enterotoxin B superantigen challenge in SIV-infected animals (data not shown). As controls, the other two coinfected macaques were inoculated with saline.
Immune flow cytometry analyses of CD4+ T cells.
CD4+ PBL counts were calculated based on the results of complete blood counts and immune flow cytometry data showing the percentage of CD4+ PBL. Three-color analyses of whole blood were performed with an XL flow cytometer (Coulter, Hialeah, Fla.). The following anti-human monoclonal antibodies that cross-react with the corresponding macaque antigens were used: phycoerythrin (PE)-conjugated anti-rhesus monkey CD3 (FN18; Biosource, Camarillo, Calif.), PE-conjugated anti-human CD4 (Ortho Diagnostic Systems, Raritan, N.J.), and PE-conjugated anti-human CD8 (Dako Corporation, Carpinteria, Calif.).
Proliferation assay.
Conventional proliferation assays were carried out as described previously. Briefly, unfractionated PBL (105 cells per well) or CD4+ lymphocyte-enriched PBL were cultured in triplicate in 96-well plates in the presence of BCG purified protein derivative (PPD) (1, 5, or 25 μg/ml), concanavalin A (5 μg/ml), bovine serum albumin (3 μg/ml), or medium alone. Five days later, cells were pulsed with [3H]thymidine at 1.0 μCi per well, and uptake was measured 8 h later by using a 1450 Microbeta scintillation counter (Wallac, Gaithersburg, Md.). Stimulation index was defined as the ratio of the mean counts per minute of PPD- or concanavalin A-stimulated wells relative to the mean counts per minute of control wells (medium alone). The CD4+ lymphocyte-enriched PBL were obtained through the depletion of CD8+ lymphocytes by using anti-CD8 antibody-conjugated Dynabeads (Dynal, Inc.; Great Neck, N.Y.), as described previously (25). PBL were incubated with these immunomagnetic beads for 30 min at room temperature and then selected in two cycles with a magnetic particle concentrator. The CD4+ lymphocyte-enriched cells obtained by this selection contained less than 5% CD8+ T cells. Similarly, the CD8+ lymphocyte-enriched T cells were purified by using anti-CD4 antibody-conjugated Dynabeads. In the control experiments using CD4+ or CD8+ lymphocyte-enriched PBL from BCG-infected macaques, PPD was shown to stimulate a significant proliferation of CD4+ lymphocyte-enriched, but not CD8+ lymphocyte-enriched, PBL.
Quantitative measurement of SIV RNA in plasma.
This was done using quantitative competitive-PCR (QC-PCR) as we described previously (25). Briefly, viral RNA was extracted following the instructions for the RNA Extraction Kit from Qiagen (Valencia, Calif.). The extracted RNA was divided into six different tubes, each of which contained defined copies of SIV gag competitor RNA. The RNA mixtures were reverse transcribed to cDNA and competitively amplified by a 35-cycle PCR, using a pair of SIV gag-specific primers (25). The amplified PCR products containing the wild type and competitor were separated on 2% agarose gels and measured for their densities in a GS 700 Imaging Densitometer (Bio-Rad). Quantitation was achieved by data analysis using the Molecular Analyst system software (Bio-Rad). The intra- and interassay coefficient variation using this protocol was less than 20%. The sensitivity of the QC-PCR was 4 × 102 RNA copies in 1 ml of plasma. As a complementary study, the level of SIV RNA in plasma was also quantitated by the branched DNA assay (Chiron, Emeryville, Calif.). This assay allows for the detection of a minimum of 1,500 RNA copies/ml.
BCG colony counts.
Viable BCG in the lymph nodes was determined by the quantitation of BCG CFU in cell lysates from 106 lymph node cells from SIV/BCG-coinfected macaques. Cell pellets prepared from 106 lymph node cells were lysed with 10% saponin to release intracellular BCG. Fivefold dilutions of the lysate were plated in duplicate on Middlebrook 7H10 agar plates (Difco) (22). The CFU were counted after a 3-week incubation at 37°C.
Statistical analysis.
The Student's t test or nonparametric methods were employed as described previously (6) to examine whether any differences in viral loads, CD4+ PBL counts, or BCG loads identified before and after antiretroviral treatment or between treated and untreated groups were statistically significant. In addition, the correlation coefficient was calculated by Prism software to determine the correlation between changes in BCG loads and numbers or function of CD4+ T cells.
RESULTS
Antiretroviral therapy controlled SIV-induced disease in SIV/BCG-coinfected macaques.
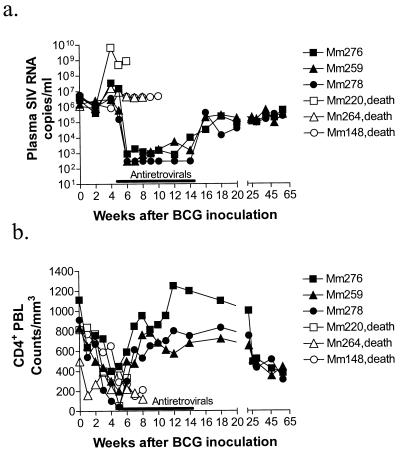
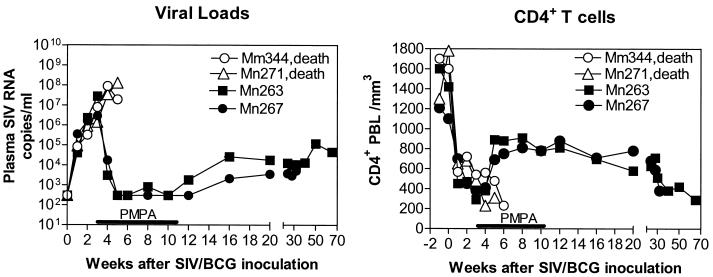
To explore the utility of antiretroviral therapy for restoring effective anti-mycobacterial immunity in SIV/BCG-coinfected rhesus monkeys, animals were inoculated sequentially with BCG, then with SIV, and finally with BCG again, at 2-month intervals. We have recently demonstrated that SIV/BCG coinfection in macaques causes rapid destruction of CD4+ T cells and subsequently induces an SIV-related tuberculosis-like disease (4, 25; Shen et al., submitted). As expected, the BCG reinfection of these SIV-infected macaques resulted in an increase in the level of SIV RNA in plasma and a marked decline of CD4+ PBL counts (Fig. 1). Moreover, these SIV/BCG-coinfected macaques developed a clinical syndrome characterized by diarrhea, anorexia, and up to a 20% weight loss 4 weeks after the second BCG inoculation. Five days after the monkeys developed this clinical syndrome, the experimental group of SIV/BCG-coinfected animals received an antiretroviral regimen comprised of PMPA and two protease inhibitors, Crixivan and Viracept. This antiretroviral regimen effectively controlled the SIV-induced disease in SIV/BCG-coinfected monkeys. During treatment, levels of SIV RNA in plasma fell to low or undetectable levels in the macaques (Fig. 1a). Consistently, containment of SIV replication was associated with a marked increase in CD4+ PBL counts in the treated animals (Fig. 1b). In contrast, the group of control monkeys similarly coinfected with SIV/BCG maintained a persistent high level of SIV RNA in plasma and developed progressive declines of their CD4+ PBL counts (Fig. 1). These results therefore indicate that antiretroviral treatment can effectively contain SIV replication and control the BCG infection-accelerated decline of CD4+ PBL counts in SIV/BCG-coinfected monkeys.
FIG. 1.
Antiretroviral therapy controlled SIV-induced disease in SIV/BCG-coinfected macaques. Shown are the changes in the levels of SIV RNA in plasma (a) and CD4+ PBL counts (b) in SIV-infected macaques after BCG reinfection (second inoculation). The data for SIV RNA in plasma were generated by QC-PRC. Antiretroviral drugs were initiated within 5 days after SIV/BCG-coinfected macaques developed the clinical syndrome of anorexia, diarrhea, and 20% loss of body weight. Antiretroviral treatment with PMPA, Crixivan, and Viracept was given for up to 10 weeks. Macaque 276 (Mm276) received treatment 5 days later than macaques 259 and 278 due to 1-week delays in their developing this clinical syndrome after BCG coinfection. Macaques 220, 264, and 148 constituted the control group of SIV/BCG-coinfected animals (death).
The control of BCG-enhanced SIV-induced disease by antiretroviral treatment resulted in clinical improvement of SIV-related tuberculosis-like disease in SIV/BCG-coinfected macaques.
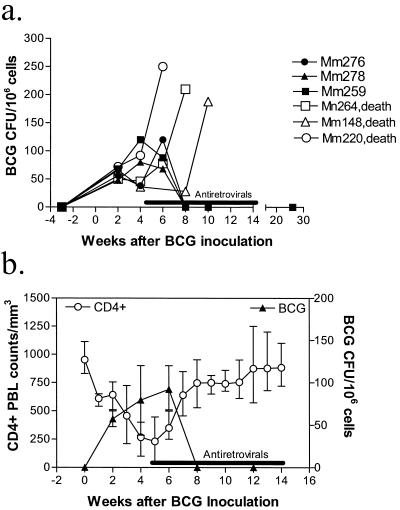
If a BCG-induced exacerbation of SIV-induced disease plays a critical role in triggering the development of this SIV-related tuberculosis-like disease (4, 25; Shen et al., submitted), the control of SIV replication by antiretroviral drugs may block development of SIV-related tuberculosis-like disease. As expected, the control of SIV replication during antiretroviral treatment coincided with an improvement of the clinical syndrome of anorexia, diarrhea, and weight loss in the SIV/BCG-coinfected macaques. This syndrome completely resolved in the monkeys by 3 weeks after the initiation of antiretroviral treatment. Resolution of the clinical syndrome was associated with a decrease in BCG loads in the lymph nodes of the monkeys (Fig. 2). Furthermore, these treated animals did not show any clinical evidence of a tuberculosis-like disease during the 12-month follow-up period after treatment. In contrast, the control group of monkeys not treated with antiretroviral agents manifested a clinical deterioration and died from the tuberculosis-like disease 1.5 to 3 months after BCG reinfection. Necropsy studies showed disseminated granulomas in multiple organs (data not shown). These results suggest that the control of SIV replication by antiretroviral treatment can improve the clinical syndrome induced by SIV/BCG coinfection and block the development of fatal SIV-related tuberculosis-like disease in monkeys.
FIG. 2.
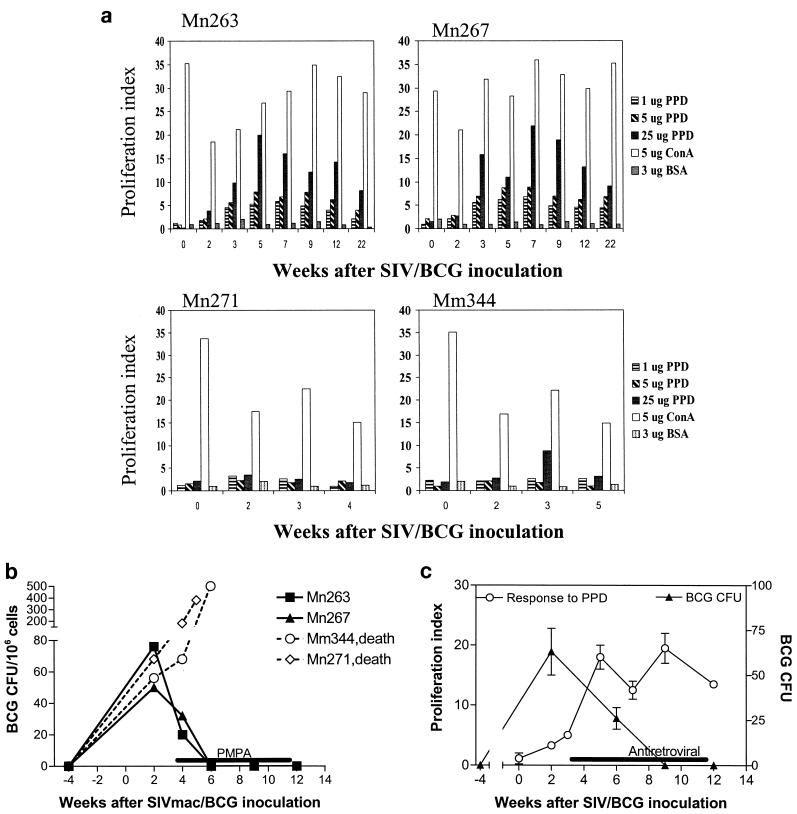
Control of accelerated SIV-induced disease after antiretroviral treatment coincided with resolution of BCG infection and fatal SIV-related tuberculosis-like disease. (a) Change in CFU of BCG in the lysates of 106 lymph node cells obtained from monkeys after BCG reinfection. (b) Temporal correlation (r = 0.88) between the recovery of CD4+ PBL counts and the decline in BCG colony counts. Shown are means, with error bars, from three monkeys.
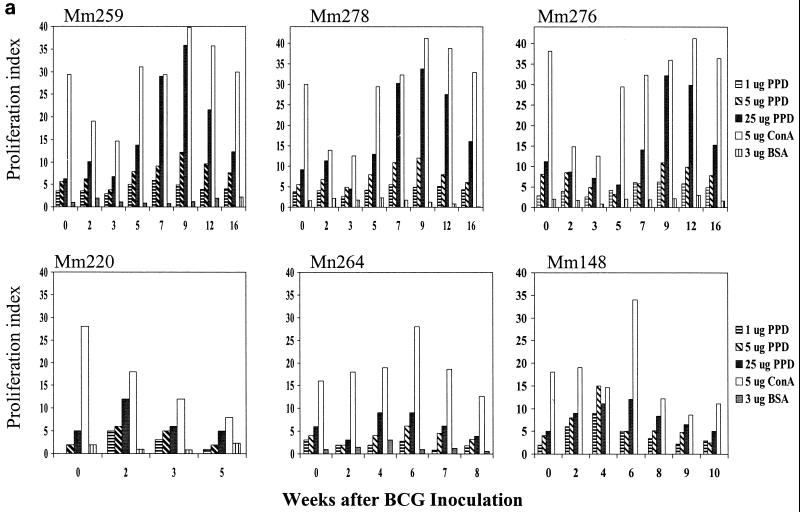
Control of SIV-related tuberculosis-like disease correlated with the restoration of BCG PPD-specific T-cell responses during antiretroviral treatment.
We then sought to determine whether containment of the SIV-related tuberculosis-like disease correlated with the restoration of BCG-specific T-cell responses after antiretroviral treatment. To address this issue, PPD-driven proliferative responses of PBL from the coinfected macaques were assessed. The control group of SIV/BCG-coinfected macaques showed persistent suppression of memory BCG-specific T-cell responses, associated with development of the clinical syndrome and disseminated granulomas (Shen et al., submitted, and data not shown). In contrast, the SIV/BCG-coinfected macaques treated with antiretroviral drugs had a recovery of memory PPD-specific T-cell responses (Fig. 3a). Importantly, restoration of PPD-specific T-cell proliferative responses was observed coincident with a resolution of the clinical syndrome. Moreover, restoration of the T-cell proliferative response to PPD correlated with the clearance of detectable BCG in the lymph nodes of SIV/BCG-coinfected monkeys (Fig. 3b). These results suggest that restoration of anti-mycobacterial T-cell responses contributes to the control of active BCG replication and SIV-related tuberculosis-like disease in SIV-infected macaques.
FIG. 3.
Control of fatal SIV-related tuberculosis-like disease in SIV/BCG-coinfected macaques correlated temporally with the restoration of BCG PPD-specific T-cell responses during the period of antiretroviral treatment. The data shown in panel a were generated in proliferation assays, using PBL from the coinfected macaques depleted of CD8+ lymphocytes. (b) Correlation between the resolution of BCG infection and the restored T-cell responses to BCG after antiretroviral treatment. Follow-up studies showed that the ability of T cells to proliferate was suppressed again due to the rebound SIV infection after the discontinuation of antiretroviral treatment (data not shown).
Antiretroviral treatment sustained the development of primary BCG-specific T-cell responses and blocked progression of fatal SIV-related tuberculosis-like disease in naive macaques simultaneously infected with SIV and BCG.
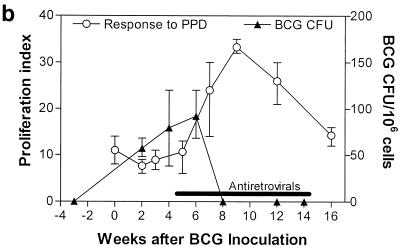
We have recently shown that naive macaques are susceptible to a rapidly fatal SIV-related tuberculosis-like disease following simultaneous inoculation with SIV and BCG (Shen et al., submitted). We reasoned that SIV/BCG coinfection of macaques might suppress the development of primary anti-mycobacterial immunity, which may, in turn, be responsible for the development of the fatal SIV-related tuberculosis-like disease. We therefore sought to determine whether, in a setting of the control of SIV replication by antiretroviral treatment, coinfected monkeys could sustain the development of primary anti-mycobacterial immunity and control the acutely lethal disease. Twenty-five days after simultaneous inoculation with SIV and BCG, the macaques developed the same clinical syndrome observed in the chronically SIV-infected macaques that were subsequently infected with BCG. The experimental group of these coinfected macaques received antiretroviral treatment with PMPA 2 days after they developed this clinical syndrome; the control group of macaques was injected daily with saline. PMPA treatment of the macaques simultaneously coinfected with SIV/BCG was associated with a clearance of SIV viremia and control of the decline of CD4+ PBL counts (Fig. 4). These PMPA-treated monkeys also developed primary CD4+ T-cell responses to PPD (Fig. 5a). The development of this primary CD4+ T-cell response was associated with a control of BCG replication and the tuberculosis-like disease (Fig. 5b and c). Furthermore, no evidence of clinical disease was seen in these treated macaques during an 8-month follow-up period. In contrast, PBL of the control macaques had a weak or undectectable T-cell proliferative response to PPD and the monkeys died as a result of SIV and BCG dissemination (Fig. 4 and 5). These results in naive macaques therefore complement those seen in the macaques sequentially infected with SIV and BCG and support the observation that antiretroviral therapy can restore BCG-specific CD4+ T-cell responses and facilitate the control of SIV-related BCG-induced disease in macaques.
FIG. 4.
Antiretroviral treatment contained SIV replication (left) and restored CD4+ PBL counts (right) in naive macaques simultaneously inoculated with SIV and BCG. The levels of SIV RNA in plasma were greater than 1,500 copies/ml and were generated by branched DNA assay, whereas those below 1,500 copies/ml were generated by QC-PCR. PMPA treatment was initiated at the time the animals began to display clinical abnormalities.
FIG. 5.
The control of SIV-induced disease during antiretroviral treatment was associated with the generation of BCG-specific T-cell responses and control of the progression of SIV-related tuberculosis-like disease in the naive macaques simultaneously infected with SIV and BCG. PMPA treatment facilitated the development of proliferative T-cell responses to PPD (a) and blocked the evolution of fatal BCG-induced disease (b). The proliferation data were generated by using CD4+ lymphocyte-enriched PBL from the coinfected macaques. BCG CFU were assessed using the lysates of 106 lymph node cells obtained from the macaques after simultaneous inoculation with SIV and BCG. (c) Correlation between the restored T-cell proliferative responses to PPD and the decrease in BCG CFU during antiretroviral treatment of these monkeys. Shown are means, with error bars, from two monkeys.
DISCUSSION
The present studies employed the macaque model of SIV/BCG coinfection to explore the utility of antiretroviral treatment in controlling an AIDS virus-related tuberculosis-like disease. These studies demonstrate that a short course of antiretroviral treatment can result in the control of this fatal SIV-related tuberculosis-like disease. Most strikingly, no evidence of BCG-induced disease or reactivation of the active BCG infection was seen during a 12-month follow-up period after antiretroviral treatment. These results are consistent with accumulating recent findings in this model (25; Shen et al., submitted). Previous studies have shown that BCG-stimulated activation of CD4+ T cells results in an increase in viral loads and an accelerated decline of CD4+ PBL counts in SIV/BCG-coinfected macaques. Moreover, BCG infection of monkeys with progressive SIV-induced disease leads to the development of an SIV-related tuberculosis-like disease (4, 25; Shen et al., submitted). The present studies show that through containing SIV replication and blocking BCG-accelerated depletion of CD4+ T cells, the development of a fatal SIV-related tuberculosis-like disease can be interrupted. No reactivation of SIV-related tuberculosis-like disease after the discontinuation of antiretroviral therapy may be attributed to the low SIV and/or BCG loads in these monkeys. The high SIV and/or BCG loads seen in the early days following infection may be required for the initiation of BCG-enhanced SIV disease and the subsequent development of SIV-related tuberculosis-like disease (Shen et al., submitted).
Results of the studies in macaques suggest that CD4+ T cells play an important role in acquired immunity to mycobacteria. While the contribution of CD4+ T cells to protective immunity against tuberculosis has been well described in small animal models of M. tuberculosis infections (17, 18, 20), a role for CD4+ T cells in acquired immunity against tuberculosis has not formally been demonstrated in humans (8, 23). The self-limiting course of tuberculosis pleurisy in humans is associated with a local accumulation of CD4+ T cells (2). Studies in HIV clinics have also shown that HIV-infected individuals are susceptible to developing tuberculosis (1, 5, 21). Now, in the macaque models of SIV/BCG coinfection, we have shown that the restoration of numbers and function of CD4+ T cells after antiretroviral treatment coincides with the clinical improvement and control of SIV-related tuberculosis-like disease. The contribution of CD4+ T cells to anti-mycobacterial immune function is also supported by the finding in the control, untreated macaques that the BCG-accelerated decline of CD4+ PBL counts and the associated suppression of functional PPD-specific CD4+ T-cell responses correlate with the development of SIV-related tuberculosis-like disease. While these results implicate CD4+ T cells as important in controlling mycobacterial infection, other T-cell populations may cooperate with CD4+ T cells in the development of anti-mycobacterial immunity. It is also possible that changes in the cytokine environment induced by antiretroviral drugs may also play a role in the modulation of immune responses to BCG coinfection
The findings in this study may have clinical implications for the immune treatment of MDRTB and M. tuberculosis relapse and/or reinfection. The results in SIV/BCG-coinfected macaques suggest that HAART alone may be able to restore anti-mycobacterial immunity and facilitate treating an M. tuberculosis coinfection, including MDRTB in HIV-infected individuals. In fact, HAART has been shown to improve T-cell responses or clinical conditions in AIDS patients with opportunistic infections or Kaposi's sarcoma (7, 11, 13, 15). An optimal antiretroviral regimen may improve M. tuberculosis disease in that subset of HIV-M. tuberculosis-coinfected humans whose anti-M. tuberculosis immunity is profoundly suppressed but restorable. Thus, our findings in the macaque model strongly support the principle that the restoration of anti-mycobacterial immunity can be an important component of therapy for treating AIDS virus-related tuberculosis. The results in macaques and the data from human cohort studies suggest that a prolonged restoration of T-cell function by HAART can reduce the incidence of primary infection and the relapse and/or reinfection of M. tuberculosis in HIV-infected individuals.
ACKNOWLEDGMENTS
This work was supported by NIH RO1 grants RR13601 (to Z.W.C.) and HL64560 (to Z.W.C.) and by the fund from the Pittsfield Anti-Tuberculosis Association (to Z.W.C.).
REFERENCES
- 1.Barnes P F, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 3.Chen Z W, Kou Z C, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor V beta repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian- human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z W, Zhou D, Chalifoux L, Lee-Parritz D, Mansfield K, Lord C I, Letvin N L. Disseminated granulomatous disease in a simian immunodeficiency virus-and bacille Calmette-Guerin-infected rhesus monkey. AIDS. 1997;11:266–267. [PubMed] [Google Scholar]
- 5.Daley C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R, Jr, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 6.Dunn C. Applied statistics. New York, N.Y.: Wiley Inter-Science; 1987. [Google Scholar]
- 7.Dupont C, Vasseur E, Beauchet A, Aegerter P, Berthe H, de Truchis P, Zucman D, Rouveix E, Saiag P. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodeficience humaine. AIDS. 2000;14:987–993. doi: 10.1097/00002030-200005260-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ellner J J, Hirsch C S, Whalen C C. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin Infect Dis. 2000;30(Suppl. 3):S279–S282. doi: 10.1086/313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteelli A, Ippolito G. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 10.Havlir D V, Barnes P F. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340:367–373. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 11.Havlir D V, Schrier R D, Torriani F J, Chervenak K, Hwang J Y, Boom W H. Effect of potent antiretroviral therapy on immune responses to Mycobacterium avium in human immunodeficiency virus-infected subjects. J Infect Dis. 2000;182:1658–1663. doi: 10.1086/317620. [DOI] [PubMed] [Google Scholar]
- 12.Horsburgh C R., Jr The global problem of multidrug-resistant tuberculosis: the genie is out of the bottle. JAMA. 2000;283:2575–2576. doi: 10.1001/jama.283.19.2575. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann G R, Zaunders J, Cooper D A. Immune reconstitution in HIV-1 infected subjects treated with potent antiretroviral therapy. Sex Transm Infect. 1999;75:218–224. doi: 10.1136/sti.75.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk O, Gatell J M, Mocroft A, Pedersen C, Proenca R, Brettle R P, Barton S E, Sudre P, Phillips A N. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–872. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 15.Komanduri K V, Viswanathan M N, Wieder E D, Schmidt D K, Bredt B M, Jacobson M A, McCune J M. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 16.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D K, Kiser R F, Coalter V J, Walsh G, Imming R J, Fisher B, Flynn B M, Bischofberger N, Piatak M, Jr, Hirsch V M, Nowak M A, Wodarz D. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller I, Cobbold S P, Waldmann H, Kaufmann S H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 19.Orme I M. Virulence of recent notorious Mycobacterium tuberculosis isolates. Tuber Lung Dis. 1999;79:379–381. doi: 10.1054/tuld.1999.0221. [DOI] [PubMed] [Google Scholar]
- 20.Scanga C A, Mohan V P, Yu K, Joseph H, Tanaka K, Chan J, Flynn J L. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selwyn P A, Hartel D, Lewis V A, Schoenbaum E E, Vermund S H, Klein R S, Walker A T, Friedland G H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 22.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 23.Silver R F, Li Q, Boom W H, Ellner J J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–2417. [PubMed] [Google Scholar]
- 24.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 25.Zhou D, Shen Y, Chalifoux L, Lee-Parritz D, Simon M, Sehgal P K, Zheng L, Halloran M, Chen Z W. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]