The authors would like to make the following corrections to this published paper [1].
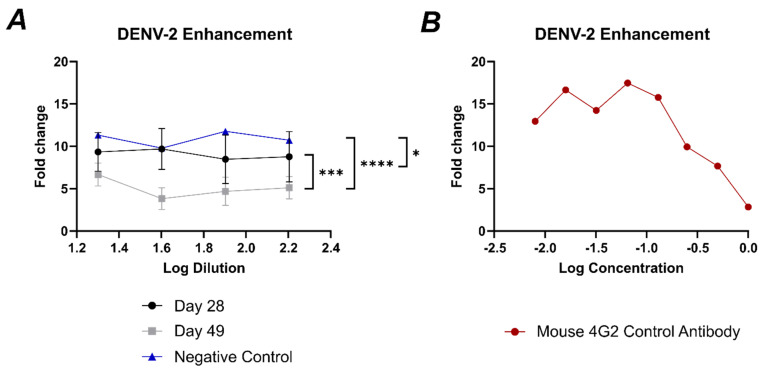
In the original manuscript, Figure 10A contained an error in the labeling of the X-axis (Log Dilution). The correct dilutions used for the serum were from 1/20 to 1/160, which correspond to log dilutions approximately from log 1.3 to log 2.2. Therefore, the X-axis should have been labeled as 1.2 to 2.4, rather than 2.2 to 3.4. We apologize for this oversight and have corrected the figure accordingly to reflect the accurate dilution range. The corrected Figure 10 is listed below:
Figure 10.
Antibodies induced by vaccination with DV1-AP205/AP205-DV4 do not enhance DENV−2 infection. (A) Fold change of DENV-2 infection of the serum-induced by vaccination with DV1-AP205/AP205-DV4. Day 28 is shown in black, and day 49 is shown in grey. Negative Control (naïve serum) is shown in blue. The serum dilution is shown in log values. (B) Fold change of DENV-2 infection of mouse 4G2 antibody used as a positive control. Antibody concentration is shown in log values. Statistical analysis (mean ± SEM) using one-way ANOVA. Vaccine group n = 6. One representative of two similar experiments is shown. The value of p < 0.05 was considered statistically significant (* p < 0.05, *** p < 0.001, **** p < 0.0001).
The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Reference
- 1.Rothen D.A., Dutta S.K., Krenger P.S., Vogt A.-C.S., Lieknina I., Sobczak J.M., Osterhaus A.D.M.E., Mohsen M.O., Vogel M., Martina B., et al. Preclinical Evaluation of Novel Sterically Optimized VLP-Based Vaccines against All Four DENV Serotypes. Vaccines. 2024;12:874. doi: 10.3390/vaccines12080874. [DOI] [PMC free article] [PubMed] [Google Scholar]