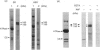Abstract
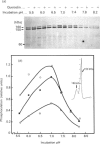
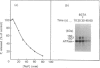
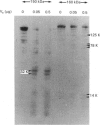
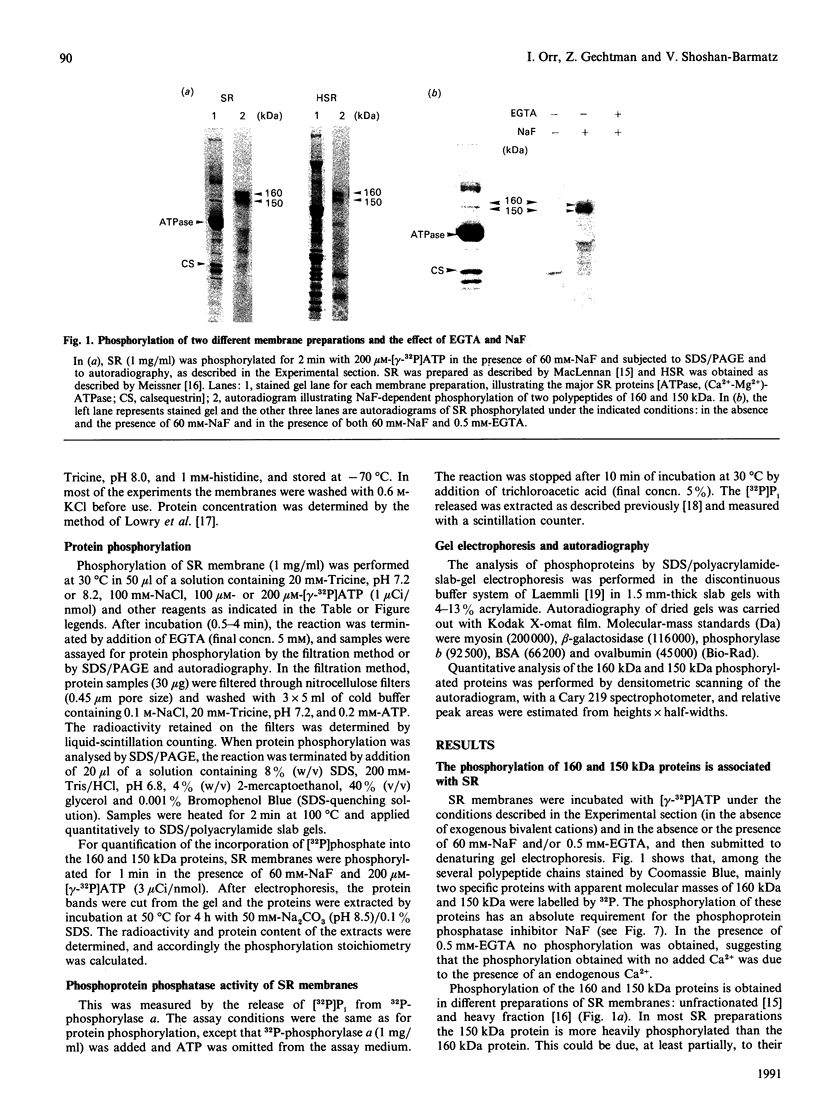
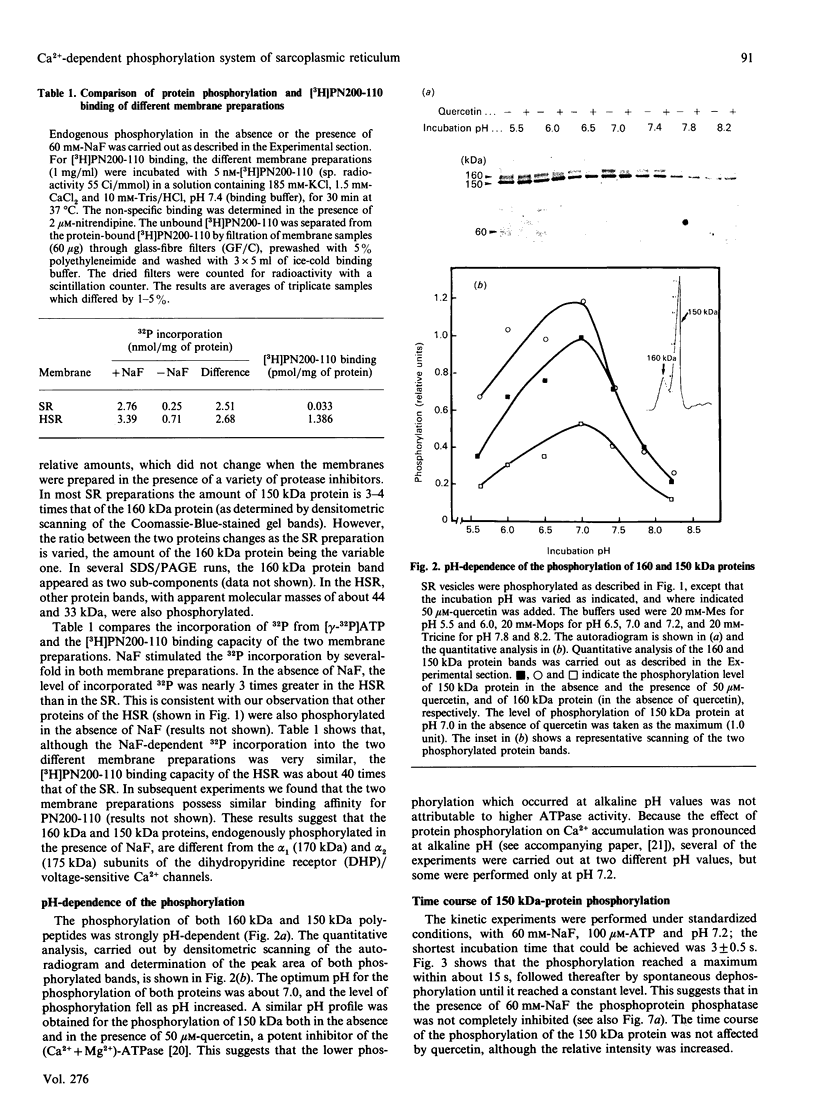
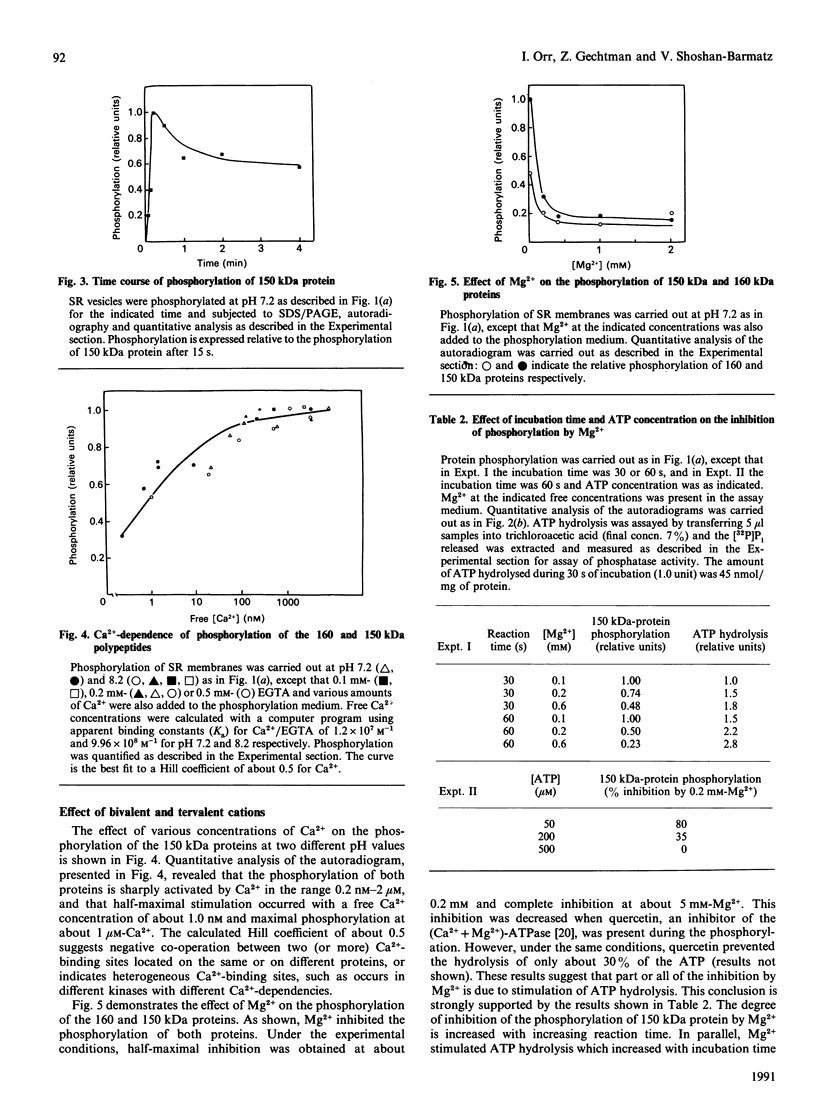
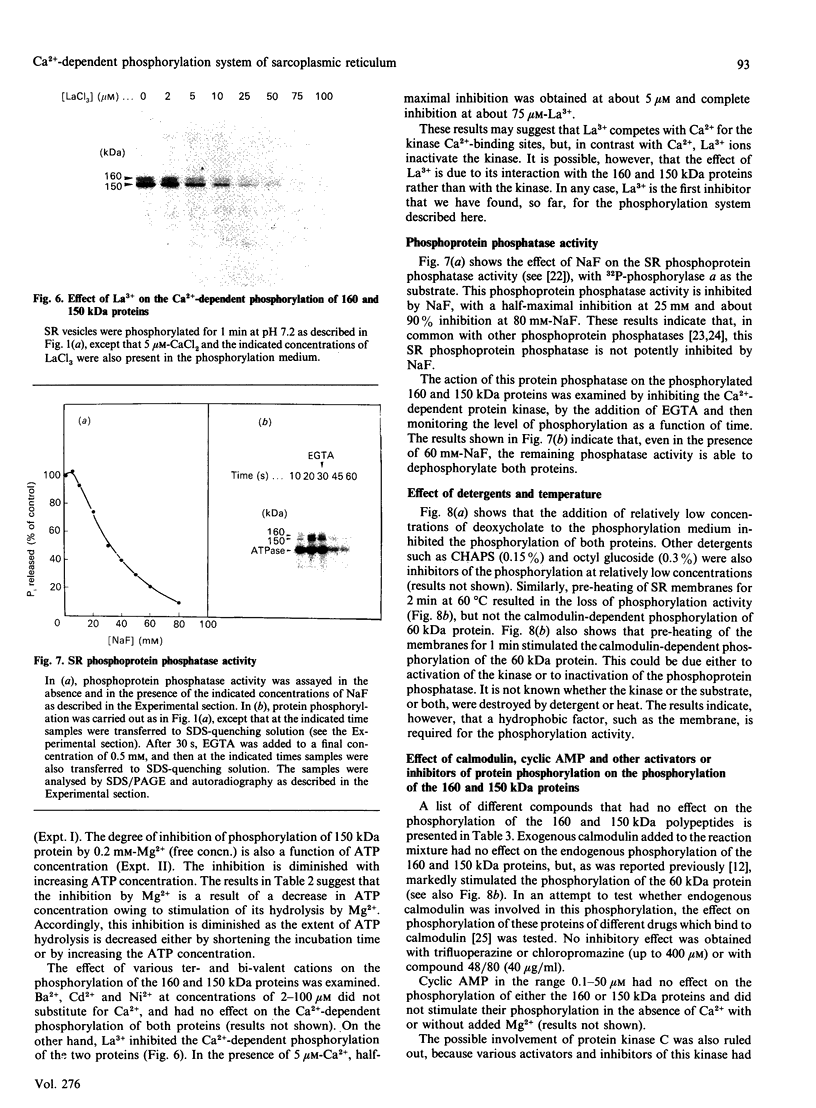
The 160 and 150 kDa proteins of sarcoplasmic reticulum (SR) are phosphorylated endogenously. The phosphorylation of both proteins has a marked requirement for Ca2+. Half-maximal and maximal phosphorylation was obtained at about 1 nM- and 1 microM-Ca2+ respectively, and a Hill coefficient of about 0.5 was calculated. The phosphorylation is also dependent on NaF as an inhibitor of the SR phosphoprotein phosphatase. The phosphorylation of these proteins is very rapid, and maximal phosphorylation is achieved in less than 15 s. The phosphorylation of the 160 kDa and 150 kDa polypeptides is completely inhibited by 5 mM-MgCl2 and by 75 microM-LaCl3, by very low concentrations of different detergents, and by preincubation of the SR for 2 min at 60 degrees C. The inhibition by Mg2+ is due to stimulation of ATP hydrolysis, thereby decreasing ATP concentration. Different phosphorylated peptides were obtained by digestion with protease V8 of the 160 kDa and 150 kDa protein bands, suggesting that the 160 kDa and 150 kDa proteins are distinct. The two phosphorylated proteins are present in different fractions and preparations of SR, with or without [3H]PN200-110 binding capacity. These and other results suggest that the phosphorylated SR proteins are distinct from the alpha 1 and alpha 2 subunits of the voltage-gated Ca2+ channel of the T-system membranes. Different inhibitors and activators of protein kinase C and calmodulin-dependent protein kinase have no effect on the endogenous phosphorylation of both polypeptides, suggesting that the phosphorylation is regulated solely by Ca2+. A possible regulatory function for this phosphorylation system is described in the accompanying paper [Gechtman. Orr & Shoshan-Barmatz (1991) Biochem. J. 276.97-102].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Cala S. E., Jones L. R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem. 1983 Oct 10;258(19):11932–11936. [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Phosphorylation of a 60,000-dalton protein. J Biol Chem. 1982 Feb 10;257(3):1238–1246. [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H. Purification and characterization of the 53,000-dalton glycoprotein from the sarcoplasmic reticulum. J Biol Chem. 1981 May 10;256(9):4626–4632. [PubMed] [Google Scholar]
- Campbell K. P., Shamoo A. E. Phosphorylation of heavy sarcoplasmic reticulum vesicles: identification and characterization of three phosphorylated proteins. J Membr Biol. 1980 Oct 31;56(3):241–248. doi: 10.1007/BF01869479. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Carafoli E. The regulation of Ca2+ transport by fast skeletal muscle sarcoplasmic reticulum. Role of calmodulin and of the 53,000-dalton glycoprotein. J Biol Chem. 1982 Jan 25;257(2):984–991. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Gechtman Z., Orr I., Shoshan-Barmatz V. Involvement of protein phosphorylation in activation of Ca2+ efflux from sarcoplasmic reticulum. Biochem J. 1991 May 15;276(Pt 1):97–102. doi: 10.1042/bj2760097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Guillon G., Mouillac B., Balestre M. N. Activation of polyphosphoinositide phospholipase C by fluoride in WRK1 cell membranes. FEBS Lett. 1986 Aug 18;204(2):183–188. doi: 10.1016/0014-5793(86)80808-0. [DOI] [PubMed] [Google Scholar]
- Gundersen R. E., Nelson D. L. A novel Ca2+-dependent protein kinase from Paramecium tetraurelia. J Biol Chem. 1987 Apr 5;262(10):4602–4609. [PubMed] [Google Scholar]
- Hauschildt S., Hirt W., Bessler W. Modulation of protein kinase C activity by NaF in bone marrow derived macrophages. FEBS Lett. 1988 Mar 28;230(1-2):121–124. doi: 10.1016/0014-5793(88)80654-9. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Brown M. S., Lee E., Pathak R. K., Anderson R. G., Goldstein J. L. Purification of a sarcoplasmic reticulum protein that binds Ca2+ and plasma lipoproteins. J Biol Chem. 1989 May 15;264(14):8260–8270. [PubMed] [Google Scholar]
- Hofmann S. L., Goldstein J. L., Orth K., Moomaw C. R., Slaughter C. A., Brown M. S. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem. 1989 Oct 25;264(30):18083–18090. [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatra B. S., Soderling T. R. Reversible inhibition of skeletal muscle phosphoprotein phosphatase by ATP, phosphate and fluoride. Biochem Biophys Res Commun. 1978 Nov 29;85(2):647–654. doi: 10.1016/0006-291x(78)91211-1. [DOI] [PubMed] [Google Scholar]
- Kim D. H., Ikemoto N. Involvement of 60-kilodalton phosphoprotein in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1986 Sep 5;261(25):11674–11679. [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberer E., Charuk J. H., Green N. M., MacLennan D. H. Molecular cloning and expression of cDNA encoding a lumenal calcium binding glycoprotein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6047–6051. doi: 10.1073/pnas.86.16.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A. T., Imagawa T., Campbell K. P. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent Ca2+ channel from rabbit skeletal muscle. Evidence for two distinct high molecular weight subunits. J Biol Chem. 1987 Jun 15;262(17):7943–7946. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Mackenzie C. W., 3rd, Bulbulian G. J., Bishop J. S. Use of fluoride to inactivate phosphorylase a phosphatases from rat liver cytosol. Presence of fluoride-insensitive glycogen synthase-specific phosphatase. Biochim Biophys Acta. 1980 Aug 7;614(2):413–424. doi: 10.1016/0005-2744(80)90231-4. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Plank B., Wyskovsky W., Hohenegger M., Hellmann G., Suko J. Inhibition of calcium release from skeletal muscle sarcoplasmic reticulum by calmodulin. Biochim Biophys Acta. 1988 Feb 8;938(1):79–88. doi: 10.1016/0005-2736(88)90124-1. [DOI] [PubMed] [Google Scholar]
- Rubtsov A. M., Murphy A. J. Caffeine interaction with the Ca-release channels of heavy sarcoplasmic reticulum. Evidence that 170 kD Ca-binding protein is a caffeine receptor of the Ca-channels. Biochem Biophys Res Commun. 1988 Jul 15;154(1):462–468. doi: 10.1016/0006-291x(88)90709-7. [DOI] [PubMed] [Google Scholar]
- Segal R. A., Luck D. J. Phosphorylation in isolated Chlamydomonas axonemes: a phosphoprotein may mediate the Ca2+-dependent photophobic response. J Cell Biol. 1985 Nov;101(5 Pt 1):1702–1712. doi: 10.1083/jcb.101.5.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S., Wegener A. D., Whang D. D., Hathaway D. R., Jones L. R. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease. J Biol Chem. 1984 Jul 10;259(13):8550–8557. [PubMed] [Google Scholar]
- Shoshan-Barmatz V. Stimulation of Ca2+ efflux from sarcoplasmic reticulum by preincubation with ATP and inorganic phosphate. Biochem J. 1987 Nov 1;247(3):497–504. doi: 10.1042/bj2470497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan V., MacLennan D. H. Quercetin interaction with the (Ca2+ + Mg2+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1981 Jan 25;256(2):887–892. [PubMed] [Google Scholar]
- Smilowitz H., Hadjian R. A., Dwyer J., Feinstein M. B. Regulation of acetylcholine receptor phosphorylation by calcium and calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4708–4712. doi: 10.1073/pnas.78.8.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuana B. S., MacLennan D. H. Calmidazolium and compound 48/80 inhibit calmodulin-dependent protein phosphorylation and ATP-dependent Ca2+ uptake but not Ca2+-ATPase activity in skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1984 Jun 10;259(11):6979–6983. [PubMed] [Google Scholar]
- Varsányi M., Heilmeyer L. M., Jr Ca2+ regulation of sarcoplasmic reticular protein phosphatase activity. Biochemistry. 1979 Oct 30;18(22):4869–4875. doi: 10.1021/bi00589a015. [DOI] [PubMed] [Google Scholar]