Abstract
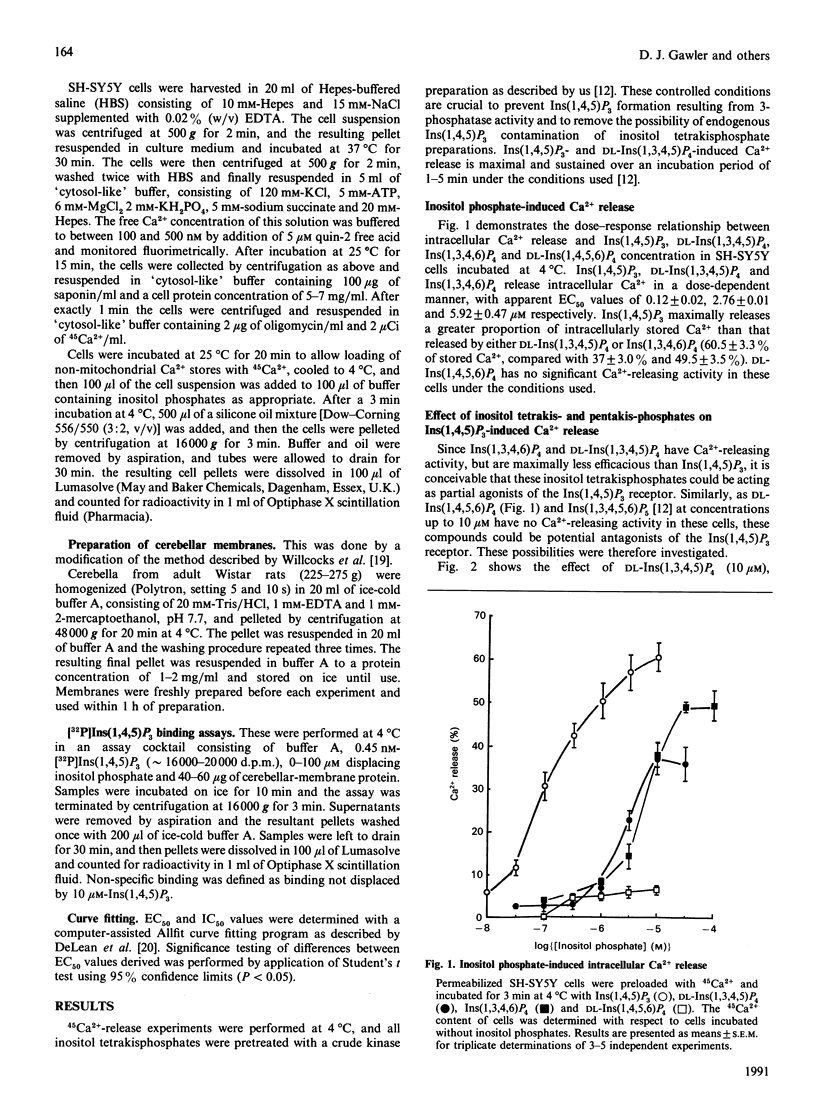
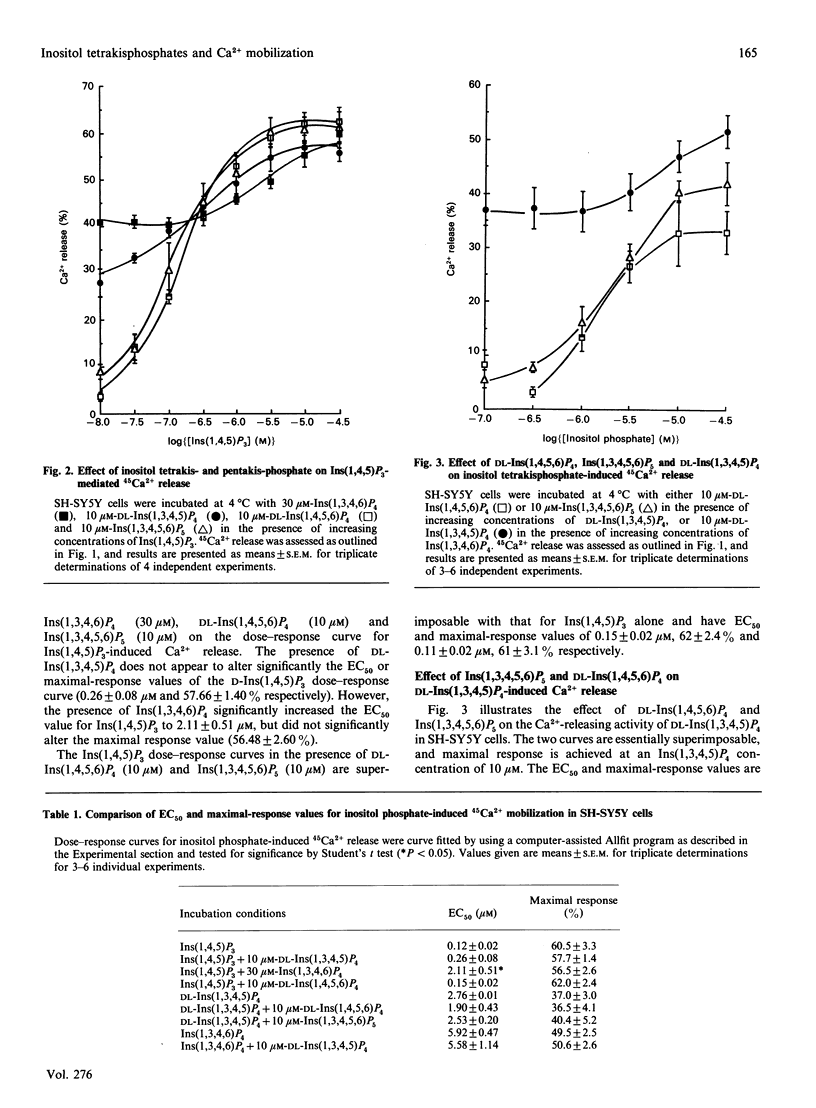
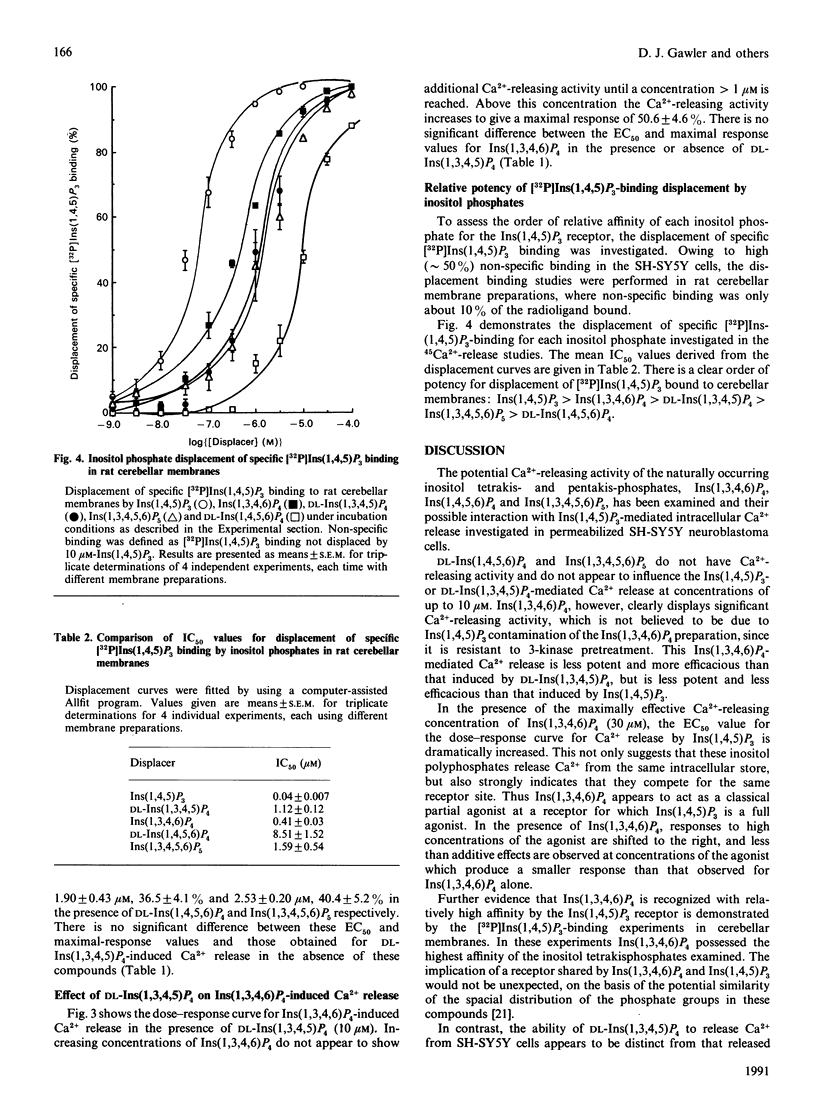
The potential Ca2(+)-releasing activity of the inositol tetrakisphosphates Ins(1,3,4,6)P4 and DL-Ins(1,4,5,6)P4 and the inositol pentakisphosphate Ins(1,3,4,5,6)P5 and their effect on Ins(1,4,5)P3- and DL-Ins (1,3,4,5)P4-mediated Ca2+ release were examined in permeabilized SH-SY5Y human neuroblastoma cells. Neither DL-Ins(1,4,5,6)P4 nor Ins(1,3,4,5,6)P5 exhibit Ca2(+)-releasing activity at concentrations up to 10 microM, but Ins(1,3,4,6)P4 releases Ca2+ dose-dependently, with an EC50 value (conen, giving half-maximal effect) of 5.92 +/- 0.47 microM. Maximal response by this tetrakisphosphate (49 +/- 2.5%) is significantly less than that seen with Ins(1,4,5)P3 (60 +/- 3%) and is achieved at a concentration of 30 microM. In the presence of this concentration of Ins(1,3,4,6)P4 the EC50 value for Ins(1,4,5)P3-mediated Ca2+ release increases from 0.12 +/- 0.02 microM to 2.11 +/- 0.51 microM, providing evidence that this naturally occurring inositol tetrakisphosphate may recognize and exhibit its Ca2(+)-releasing activity via the Ins(1,4,5)P3 receptor. DL-Ins(1,3,4,5)P4, however, at its maximally effective concentration (10 microM) does not significantly affect Ins(1,4,5)P3-mediated Ca2+ release, and therefore appears to mediate its Ca2(+)-mobilizing action through a receptor distinct from that for Ins(1,4,5)P3.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Baukal A. J., Hunyady L., Catt K. J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989 Aug 15;264(23):13605–13611. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Irvine R. F. Specific binding sites for [3H]inositol(1,3,4,5)tetrakisphosphate on membranes of HL-60 cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):680–685. doi: 10.1016/0006-291x(87)90421-9. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Hunyady L., Baukal A. J., Catt K. J. Inositol 1,3,4,5-tetrakisphosphate stimulates calcium release from bovine adrenal microsomes by a mechanism independent of the inositol 1,4,5-trisphosphate receptor. Biochem J. 1990 Jun 1;268(2):333–338. doi: 10.1042/bj2680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Williams G. H. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex. J Biol Chem. 1988 Jun 15;263(17):7940–7942. [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Gawler D. J., Potter B. V., Nahorski S. R. Inositol 1,3,4,5-tetrakisphosphate-induced release of intracellular Ca2+ in SH-SY5Y neuroblastoma cells. Biochem J. 1990 Dec 1;272(2):519–524. doi: 10.1042/bj2720519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol tetrakisphosphate mobilizes calcium from cerebellum microsomes. Mol Pharmacol. 1989 Sep;36(3):391–397. [PubMed] [Google Scholar]
- King W. G., Downes C. P., Prestwich G. D., Rittenhouse S. E. Ca2(+)-stimulatable and protein kinase C-inhibitable accumulation of inositol 1,3,4,6-tetrakisphosphate in human platelets. Biochem J. 1990 Aug 15;270(1):125–131. doi: 10.1042/bj2700125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Challiss R. A., Nahorski S. R. Accumulation and metabolism of Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in muscarinic-receptor-stimulated SH-SY5Y neuroblastoma cells. Biochem J. 1991 Feb 1;273(Pt 3):791–794. doi: 10.1042/bj2730791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell B. Inositol phosphates. Profusion and confusion. Nature. 1986 Jan 16;319(6050):176–177. doi: 10.1038/319176a0. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Maeda N., Mikoshiba K. Expressed cerebellar-type inositol 1,4,5-trisphosphate receptor, P400, has calcium release activity in a fibroblast L cell line. Neuron. 1990 Jul;5(1):11–18. doi: 10.1016/0896-6273(90)90029-f. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Willcocks A. L., Challiss R. A., Nahorski S. R. Characteristics of inositol 1,4,5-trisphosphate binding to rat cerebellar and bovine adrenal cortical membranes: evidence for the heterogeneity of binding sites. Eur J Pharmacol. 1990 Sep 18;189(2-3):185–193. doi: 10.1016/0922-4106(90)90022-p. [DOI] [PubMed] [Google Scholar]


