Abstract
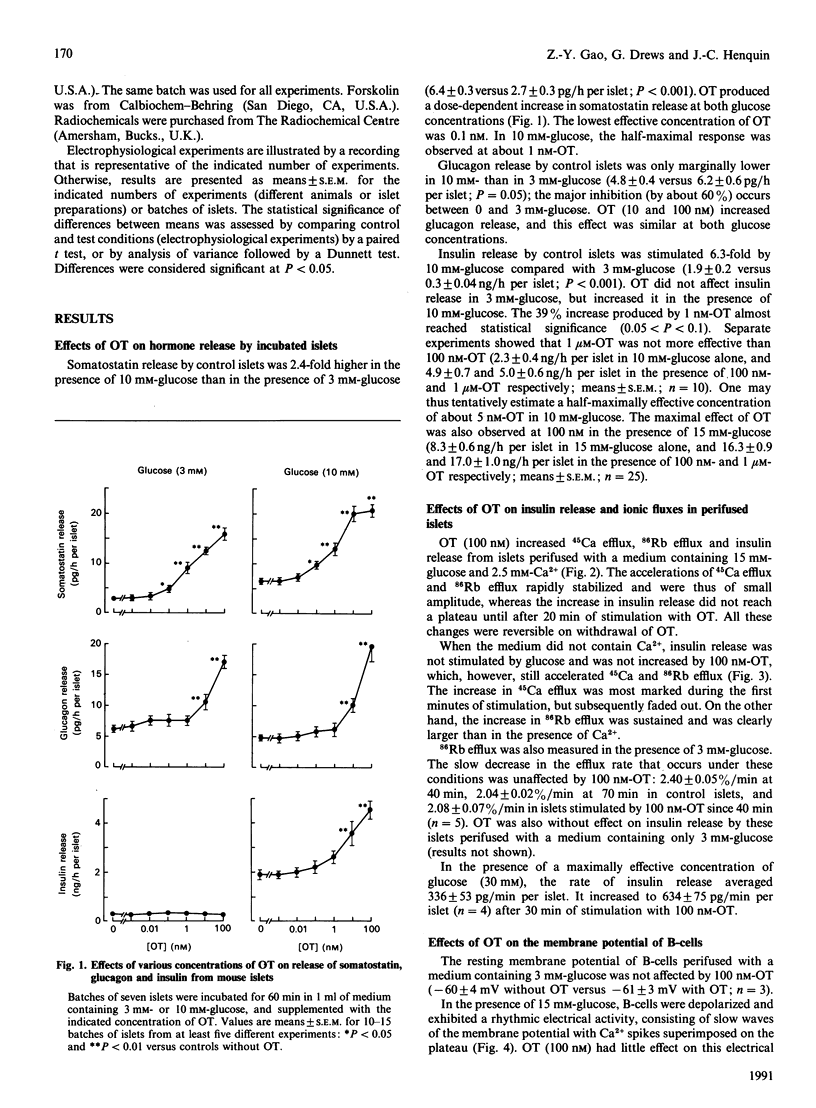
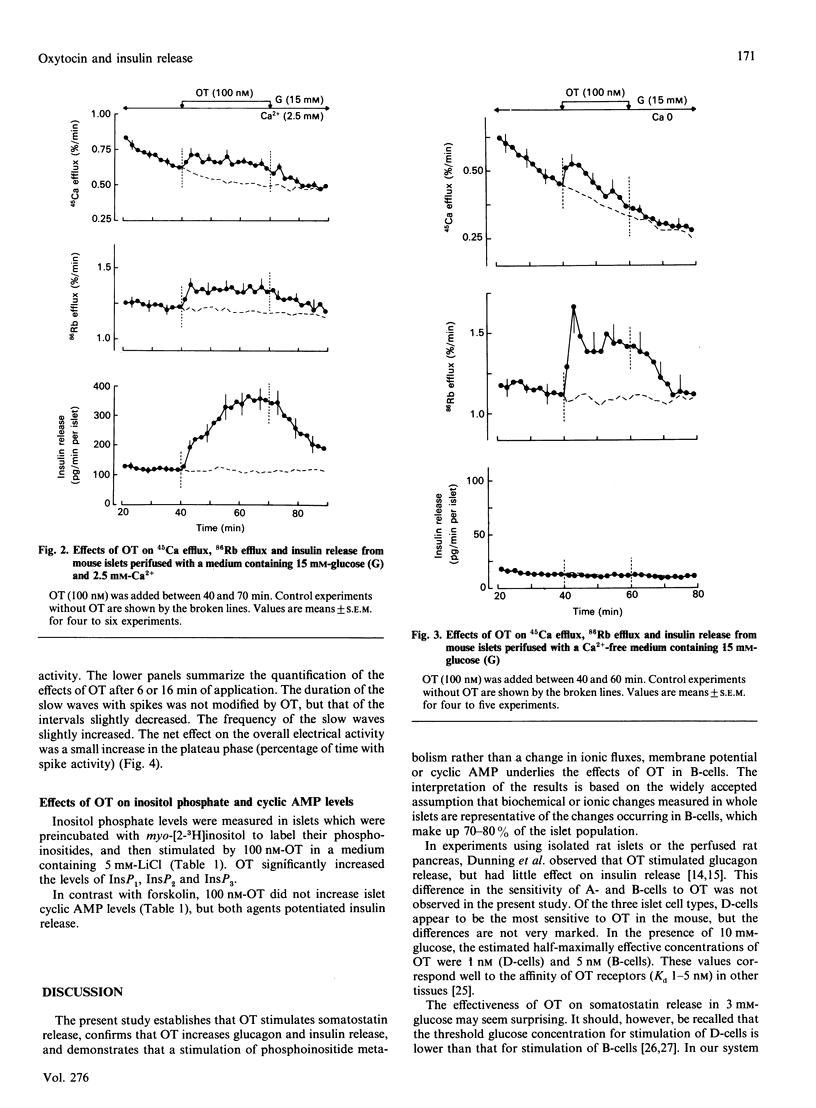
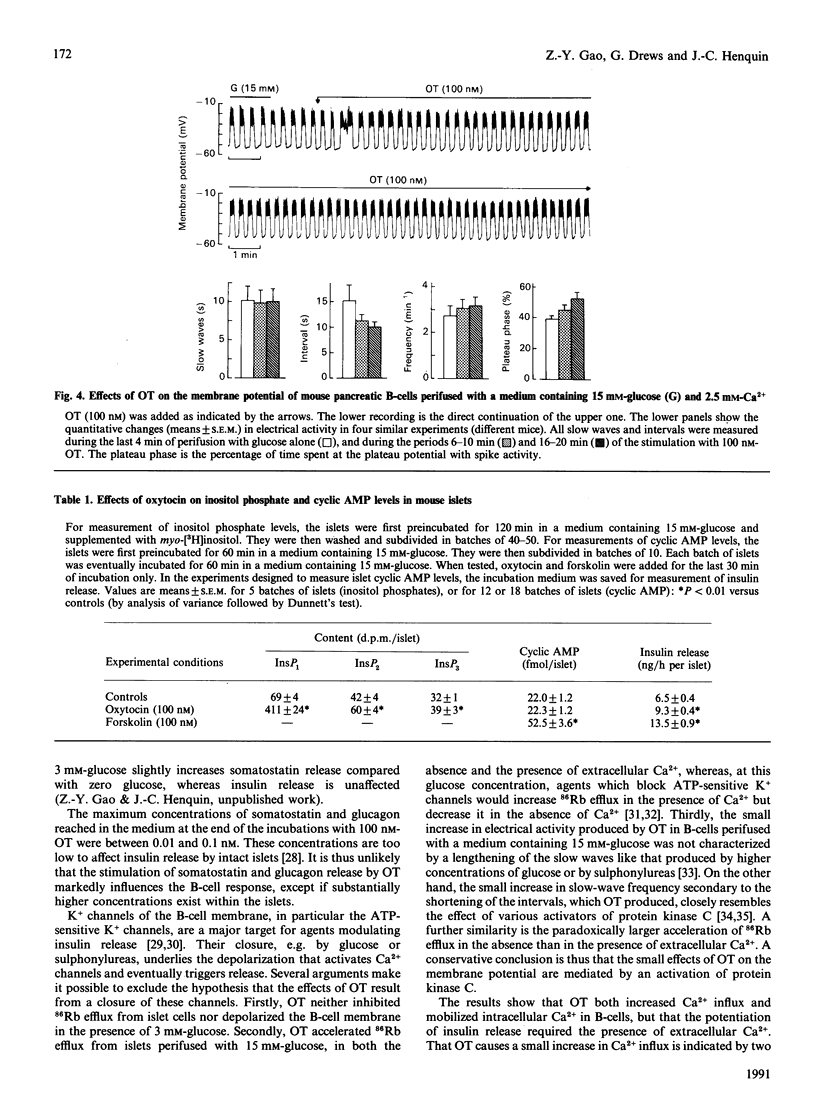
Oxytocin (OT) produced a dose-dependent increase in somatostatin, glucagon and insulin release by isolated mouse islets. A small effect on somatostatin release was observed with 0.1 nM-OT, but 1-10 nM-OT was required to affect A- and B- cells significantly. The effects of OT on somatostatin and glucagon release were similar in the presence of 3 mM- and 10 mM-glucose. No change in insulin release was produced by OT in 3 mM-glucose, but a stimulation was still observed in the presence of a maximally effective concentration of glucose (30 mM). The increase in insulin release produced by OT (in 15 mM-glucose) was accompanied by small accelerations of 86Rb and 45Ca efflux from islet cells. Omission of extracellular Ca2+ accentuated the effect of OT on 86Rb efflux, attenuated that on 45Ca efflux, and abolished that on release. OT never inhibited 86Rb efflux. It did not affect the resting potential of B-cells, but slightly increased the Ca2(+)-dependent electrical activity induced by 15 mM-glucose. OT did not affect cyclic AMP levels, but increased inositol phosphate levels in islet cells. It is suggested that the amplification of glucose-induced insulin release that OT produces is due to a stimulation of phosphoinositide metabolism, and presumably an activation of protein kinase C, rather than to a change in cyclic AMP levels or a direct action on the membrane potential. Since OT is present in the pancreas, it is possible that it exerts a neuropeptidergic control of the islet function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Hampshire J. Oxytocin infusion increases plasma insulin and glucagon levels and glucose production and uptake in the normal dog. Diabetes. 1981 Feb;30(2):112–114. doi: 10.2337/diab.30.2.112. [DOI] [PubMed] [Google Scholar]
- Amico J. A., Finn F. M., Haldar J. Oxytocin and vasopressin are present in human and rat pancreas. Am J Med Sci. 1988 Nov;296(5):303–307. doi: 10.1097/00000441-198811000-00003. [DOI] [PubMed] [Google Scholar]
- Augert G., Exton J. H. Insulin and oxytocin effects on phosphoinositide metabolism in adipocytes. J Biol Chem. 1988 Mar 15;263(8):3600–3609. [PubMed] [Google Scholar]
- BURT R. L., LEAKE N. H., DANNENBURG W. N. SEX DIFFERENCES IN METABOLIC EFFECTS OF OXYTOCIN. Nature. 1964 Feb 22;201:829–830. doi: 10.1038/201829a0. [DOI] [PubMed] [Google Scholar]
- Balasse E., Rasio E. Mécanisme d'action de l'ocytocine sur la concentration plasmatique des acides gras libres (A.G.L.) chez le chien. Arch Int Pharmacodyn Ther. 1965 Oct;157(2):356–359. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bozem M., Nenquin M., Henquin J. C. The ionic, electrical, and secretory effects of protein kinase C activation in mouse pancreatic B-cells: studies with a phorbol ester. Endocrinology. 1987 Sep;121(3):1025–1033. doi: 10.1210/endo-121-3-1025. [DOI] [PubMed] [Google Scholar]
- Chiodera P., Coiro V., Camellini L., Rossi G., Pignatti D., Volpi R., Roti E. Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Horm Res. 1984;20(2):150–154. doi: 10.1159/000179988. [DOI] [PubMed] [Google Scholar]
- Dunning B. E., Moltz J. H., Fawcett C. P. Actions of neurohypophysial peptides on pancreatic hormone release. Am J Physiol. 1984 Jan;246(1 Pt 1):E108–E114. doi: 10.1152/ajpendo.1984.246.1.E108. [DOI] [PubMed] [Google Scholar]
- Dunning B. E., Moltz J. H., Fawcett C. P. Modulation of insulin and glucagon secretion from the perfused rat pancreas by the neurohypophysial hormones and by desamino-D-arginine vasopressin (DDAVP). Peptides. 1984 Sep-Oct;5(5):871–875. doi: 10.1016/0196-9781(84)90109-8. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Leat W. M., Sheldrick E. L., Stewart H. J. Stimulation of phosphoinositide hydrolysis by oxytocin and the mechanism by which oxytocin controls prostaglandin synthesis in the ovine endometrium. Biochem J. 1986 Aug 1;237(3):797–805. doi: 10.1042/bj2370797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z. Y., Drews G., Nenquin M., Plant T. D., Henquin J. C. Mechanisms of the stimulation of insulin release by arginine-vasopressin in normal mouse islets. J Biol Chem. 1990 Sep 15;265(26):15724–15730. [PubMed] [Google Scholar]
- Garcia M. C., Hermans M. P., Henquin J. C. Glucose-, calcium- and concentration-dependence of acetylcholine stimulation of insulin release and ionic fluxes in mouse islets. Biochem J. 1988 Aug 15;254(1):211–218. doi: 10.1042/bj2540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. P., Trimble E. R., Wollheim C. B., Renold A. E., Miller R. E. Glucose and cyclic AMP as stimulators of somatostatin and insulin secretion from the isolated, perfused rat pancreas: a quantitative study. Diabetes. 1981 Jan;30(1):40–44. doi: 10.2337/diab.30.1.40. [DOI] [PubMed] [Google Scholar]
- HEIDENREICH O., KOOK Y., REUS E. [Quantitative studies on the hyperglycemic effect of synthetic oxytocin and vasopressin and on the appearance of this hormone effect during physiological conditions]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1962;243:136–147. [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Rapid stimulation by vasopressin, oxytocin and angiotensin II of glycogen degradation in hepatocyte suspensions. Biochem J. 1978 May 15;172(2):311–317. doi: 10.1042/bj1720311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Effects of trifluoperazine and pimozide on stimulus-secretion coupling in pancreatic B-cells. Suggestion for a role of calmodulin? Biochem J. 1981 Jun 15;196(3):771–780. doi: 10.1042/bj1960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hii C. S., Stutchfield J., Howell S. L. Enhancement of glucagon secretion from isolated rat islets of Langerhans by phorbol 12-myristate 13-acetate. Biochem J. 1986 Jan 1;233(1):287–289. doi: 10.1042/bj2330287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S. Mechanisms of action of vasopressin and vasopressin antagonists. Kidney Int Suppl. 1988 Oct;26:S38–S42. [PubMed] [Google Scholar]
- Kaneto A., Kosaka K. Stimulation of glucagon secretion by oxytocin. Endocrinology. 1970 Aug;87(2):439–444. doi: 10.1210/endo-87-2-439. [DOI] [PubMed] [Google Scholar]
- Knudtzon J. Acute effects of oxytocin and vasopressin on plasma levels of glucagon, insulin and glucose in rabbits. Horm Metab Res. 1983;15(2):103–104. doi: 10.1055/s-2007-1018641. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Agnost V. L., Catalano R. M., Schröder H., Waldman S. A., Bennett B. M., Tuan J. J., Murad F. Atrial natriuretic peptide, oxytocin, and vasopressin increase guanosine 3',5'-monophosphate in LLC-PK1 kidney epithelial cells. Endocrinology. 1988 Apr;122(4):1478–1485. doi: 10.1210/endo-122-4-1478. [DOI] [PubMed] [Google Scholar]
- MIRSKY I. A. Effect of oxytocin on plasma free fatty acids of non-diabetic and diabetic dogs. Proc Soc Exp Biol Med. 1962 May;110:42–44. doi: 10.3181/00379727-110-27416. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Lett. 1986 May 26;201(1):9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- McDonald J. K., Greiner F., Wood J. G., Noe B. D. Oxytocin-like immunoreactive nerves are associated with insulin-containing cells in pancreatic islets of anglerfish (Lophius americanus). Cell Tissue Res. 1987 Jul;249(1):7–12. doi: 10.1007/BF00215412. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Muchmore D. B., Little S. A., de Haën C. A dual mechanism of action of ocytocin in rat epididymal fat cells. J Biol Chem. 1981 Jan 10;256(1):365–372. [PubMed] [Google Scholar]
- Paolisso G., Sgambato S., Passariello N., Torella R., Giugliano D., Mignano S., Varricchio M., D'Onofrio F. Pharmacological doses of oxytocin affect plasma hormone levels modulating glucose homeostasis in normal man. Horm Res. 1988;30(1):10–16. doi: 10.1159/000181018. [DOI] [PubMed] [Google Scholar]
- Persaud S. J., Jones P. M., Sugden D., Howell S. L. The role of protein kinase C in cholinergic stimulation of insulin secretion from rat islets of Langerhans. Biochem J. 1989 Dec 15;264(3):753–758. doi: 10.1042/bj2640753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G., Schuit F. C., in't Veld P. A., Maes E., Hooghe-Peters E. L., Van de Winkel M., Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985 Sep;117(3):824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- Richardson S. B., Eyler N., Twente S., Monaco M., Altszuler N., Gibson M. Effects of vasopressin on insulin secretion and inositol phosphate production in a hamster beta cell line (HIT). Endocrinology. 1990 Feb;126(2):1047–1052. doi: 10.1210/endo-126-2-1047. [DOI] [PubMed] [Google Scholar]
- Schrey M. P., Read A. M., Steer P. J. Stimulation of phospholipid hydrolysis and arachidonic acid mobilization in human uterine decidua cells by phorbol ester. Biochem J. 1987 Sep 15;246(3):705–713. doi: 10.1042/bj2460705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson R. L., Elde R. P. Dissociation of glucose stimulation of somatostatin and insulin release from glucose inhibition of glucagon release in the isolated perfused rat pancreas. Diabetes. 1983 Jun;32(6):561–567. doi: 10.2337/diab.32.6.561. [DOI] [PubMed] [Google Scholar]
- Stock S., Fastbom J., Björkstrand E., Ungerstedt U., Uvnäs-Moberg K. Effects of oxytocin on in vivo release of insulin and glucagon studied by microdialysis in the rat pancreas and autoradiographic evidence for [3H]oxytocin binding sites within the islets of Langerhans. Regul Pept. 1990 Aug 21;30(1):1–13. doi: 10.1016/0167-0115(90)90130-o. [DOI] [PubMed] [Google Scholar]
- Stock S., Uvnäs-Moberg K. L-vasopressin inhibits oxytocin-induced increases of plasma levels of insulin conscious dogs. Acta Physiol Scand. 1987 May;130(1):55–61. doi: 10.1111/j.1748-1716.1987.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Sugano K., Park J., Soll A., Yamada T. Phorbol esters stimulate somatostatin release from cultured cells. Am J Physiol. 1986 May;250(5 Pt 1):G686–G690. doi: 10.1152/ajpgi.1986.250.5.G686. [DOI] [PubMed] [Google Scholar]
- Vilhardt H., Krarup T., Holst J. J., Bie P. The mechanism of the effect of oxytocin on plasma concentrations of glucose, insulin and glucagon in conscious dogs. J Endocrinol. 1986 Feb;108(2):293–298. doi: 10.1677/joe.0.1080293. [DOI] [PubMed] [Google Scholar]
- Zhao X., Gorewit R. C. Inositol-phosphate response to oxytocin stimulation in dispersed bovine mammary cells. Neuropeptides. 1987 Oct;10(3):227–233. doi: 10.1016/0143-4179(87)90072-2. [DOI] [PubMed] [Google Scholar]


