Abstract
Background
The phase III KEYNOTE-913 study was conducted to evaluate the efficacy and safety of pembrolizumab as first-line therapy in patients with advanced Merkel cell carcinoma (MCC).
Objective
The aim was to report results from the primary analysis of KEYNOTE-913.
Patients and Methods
Patients with recurrent locally advanced or metastatic MCC received pembrolizumab 200 mg intravenously every 3 weeks for up to 35 treatments (~ 2 years). The primary end point was objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) by blinded independent central review (BICR). Secondary end points were duration of response (DOR) and progression-free survival (PFS) per RECIST v1.1 by BICR, overall survival (OS), and safety and tolerability.
Results
Fifty-five patients were treated with pembrolizumab. The median time from first dose to data cutoff (February 15, 2024) was 50.3 months (range 38.7–59.4). The ORR was 49% (95% confidence interval [CI] 35–63), with 12 complete responses and 15 partial responses. The median DOR was 39.8 months (range 4.8–52.5+), and the 24-month DOR rate was 69%. The median PFS was 9.3 months (95% CI 3–26), and the 24-month PFS rate was 39%. The median OS was 24.3 months (95% CI 12.4 to not reached), and the 24-month OS rate was 51%. Any-grade treatment-related adverse events (AEs) occurred in 38 patients (69%); 13 patients (24%) experienced grade 3–5 AEs. The most common treatment-related AEs were fatigue (n = 12 [22%]), pruritus (n = 12 [22%]), and lipase increase (n = 10 [18%]). One patient died of treatment-related Guillain-Barré syndrome.
Conclusions
Pembrolizumab provided durable antitumor activity and promising survival and had a manageable safety profile in patients with recurrent locally advanced or metastatic MCC, supporting its use in this population.
Trial Registration
Clinicaltrials.gov, NCT03783078.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00885-w.
Key Points
| KEYNOTE-913 evaluated first-line pembrolizumab in 55 patients with advanced Merkel cell carcinoma (MCC). |
| The objective response rate (ORR) was 49% (95% confidence interval 35–63), with 12 complete and 15 partial responses. |
| Median duration of response was 39.8 months, median progression-free survival was 9.3 months, and median overall survival was 24.3 months. |
| Treatment-related adverse events occurred in 69% of patients (grade 3–5 in 24%). |
| Pembrolizumab had durable antitumor activity and manageable safety in advanced MCC. |
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous malignancy with a high risk of recurrence and metastasis [1, 2]. The prognosis for patients with advanced MCC has historically been poor, with 5-year overall survival (OS) rates of 35% for patients with nodal disease and 14% for patients with distant metastasis [3]. However, the treatment paradigm has changed significantly in the past 5 years, with programmed cell death protein 1/ligand 1 (PD-1/L1) inhibitors replacing cytotoxic chemotherapy as the preferred treatment for recurrent locally advanced or metastatic MCC [4, 5].
The first PD-1/L1 inhibitor approved for the treatment of MCC was avelumab, which received accelerated approval from the US Food and Drug Administration in 2017 [6]. This approval was based on the results of part A of the phase II JAVELIN Merkel 200 study, which showed that avelumab was well tolerated and provided durable responses in patients with chemotherapy-refractory metastatic MCC [7]. The results of part B showed that avelumab was also effective in patients with treatment-naive metastatic MCC [8]. The PD-1 inhibitor pembrolizumab subsequently received accelerated approval for use in recurrent locally advanced or metastatic MCC in 2018 based on the results of the phase II Cancer Immunotherapy Trials Network (CITN)-09/KEYNOTE-017 trial [9, 10]. Results from the 3-year follow-up of this study showed pembrolizumab was associated with an objective response rate (ORR) of 58%; the median response duration was not reached, the median progression-free survival (PFS) was 16.8 months, and the median OS was not reached [11]. The PD-1 inhibitors nivolumab and retifanlimab have also been shown to be effective in MCC [12–14].
The phase III KEYNOTE-913 study (NCT03783078) was designed to evaluate further the efficacy and safety of pembrolizumab as first-line therapy in patients with recurrent locally advanced or metastatic MCC. Results from the primary analysis are reported.
Materials and Methods
Study Design and Patients
KEYNOTE-913 was a multicenter, single-arm, open-label, phase III study conducted at 22 sites in Australia, Canada, France, Italy, New Zealand, Spain, Sweden, and the United States. Eligible patients were aged 12 years or older and had histologically confirmed MCC that was locoregional and recurred following standard locoregional therapy with surgery or radiation therapy, or both, and was not amenable to local therapy or was metastatic (stage IV per American Joint Committee on Cancer, 8th edition, guidelines). Patients were required to have at least one measurable lesion per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1), have an Eastern Cooperative Oncology Group performance status of 0 or 1, and have adequate organ function. Patients could not have received previous treatment for advanced or metastatic disease, with the following exceptions: previous intra-tumoral therapy or previous neoadjuvant or adjuvant systemic chemotherapy if concluded at least 3 months before study treatment initiation. Previous therapy with an anti–PD-1/L1 or anti–cytotoxic T lymphocyte antigen-4 agent was not permitted. Patients with known active central nervous system metastases or carcinomatous meningitis, an active autoimmune disease, or an active infection requiring systemic therapy were excluded.
The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. All patients provided written informed consent.
Treatment
All patients received intravenous pembrolizumab 200 mg (or 2 mg/kg up to a maximum of 200 mg for pediatric patients) every 3 weeks for up to 35 cycles (approximately 2 years), or until documented progressive disease (PD), unacceptable toxicity, or investigator decision to discontinue. Patients who achieved an investigator-determined complete response (CR) could stop treatment if they had received at least eight administrations of pembrolizumab and had received at least two administrations beyond the date that CR was declared. Patients could continue to receive pembrolizumab beyond PD per RECIST v1.1 at the investigator’s discretion if they were continuing to derive benefit and tolerating treatment.
Assessments and Outcomes
Tumor imaging by computed tomography or magnetic resonance imaging was performed at baseline, every 12 weeks until week 54, and every 24 weeks thereafter. Adverse events (AEs) were recorded throughout treatment and for 30 days after treatment end (90 days for serious AEs or 30 days if the patient started new anticancer therapy). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Treatment-related AEs were determined by the investigator to be related to study treatment. Immune-mediated AEs and infusion reactions were based on a list of terms prepared by the sponsor and included AEs regardless of attribution to study treatment by the investigator. Patients were contacted every 12 weeks after completion or discontinuation of study treatment to assess survival status.
The primary end point was ORR per RECIST v1.1 by blinded independent central review (BICR). Secondary end points were duration of response (DOR) and PFS per RECIST v1.1 by BICR, OS, and safety and tolerability. For assessment of ORR, DOR, and PFS, RECIST v1.1 was modified to allow assessment of a maximum of ten target lesions in total and a maximum of five target lesions per organ.
Statistical Analysis
Enrollment of 50 participants was planned. With an ORR of 56%, this sample size would provide a 95% confidence interval (CI) for the true ORR of approximately ± 15%. No power analyses or statistical comparisons were performed for this study. Efficacy and safety were assessed in all patients who received at least one dose of study treatment. For ORR, the Clopper-Pearson method was used to calculate 95% exact binomial CIs. DOR, PFS, and OS were estimated using the Kaplan-Meier method. Safety was summarized descriptively. Statistical analyses were performed using SAS, version 9.4.
Results
Patients
Of 71 patients screened between February 25, 2019, and November 17, 2020, 55 were enrolled and received at least one dose of pembrolizumab. At the time of the data cutoff (February 15, 2024), the median time from the first dose of pembrolizumab was 50.3 months (range 38.7–59.4); 13 patients had completed treatment and 42 had discontinued treatment (Fig. 1). The most common reasons for discontinuation were PD (n = 20) and AEs (n = 18). The median age was 74 years (range 38–91), 43 patients (78%) were ≥ 65 years of age, 31 patients (56%) were male, 39 (71%) were White, and 32 (58%) had an Eastern Cooperative Oncology Group performance status of 0 (Table 1). Most patients had stage IV disease (n = 45 [82%]). Median baseline sum of target lesions was 75 mm (range 12–324), 38 patients (69%) had undergone previous surgery for MCC, and 19 patients (35%) had received previous radiation therapy for MCC. No pediatric patients were enrolled.
Fig. 1.
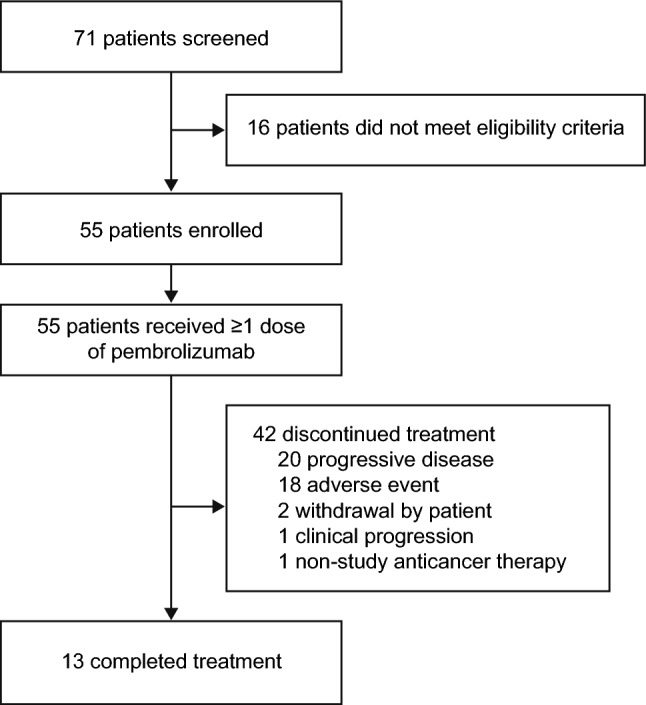
Trial profile
Table 1.
Baseline demographic and disease characteristics
| All patients (n = 55) | |
|---|---|
| Age, years | |
| Median (range) | 74 (38–91) |
| ≥ 65 years | 43 (78) |
| Sex | |
| Male | 31 (56) |
| Female | 24 (44) |
| Race | |
| White | 39 (71) |
| Not reported | 16 (29) |
| ECOG PS | |
| 0 | 32 (58) |
| 1 | 23 (42) |
| Cancer stage | |
| IIIA | 3 (5) |
| IIIB | 7 (13) |
| IV | 45 (82) |
| Baseline tumor size, median (range), mm | 75 (12–324) |
| Previous surgery for MCC | |
| No | 17 (31) |
| Yes | 38 (69) |
| Previous radiation therapy for MCC | |
| No | 36 (65) |
| Yes | 19 (35) |
Data are n (%) unless otherwise specified
ECOG PS Eastern Cooperative Oncology Group performance status, MCC Merkel cell carcinoma
Efficacy
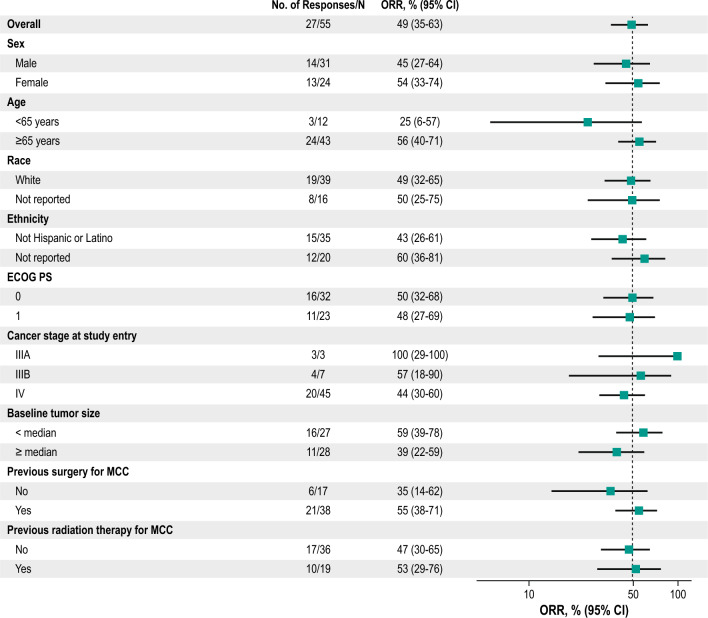
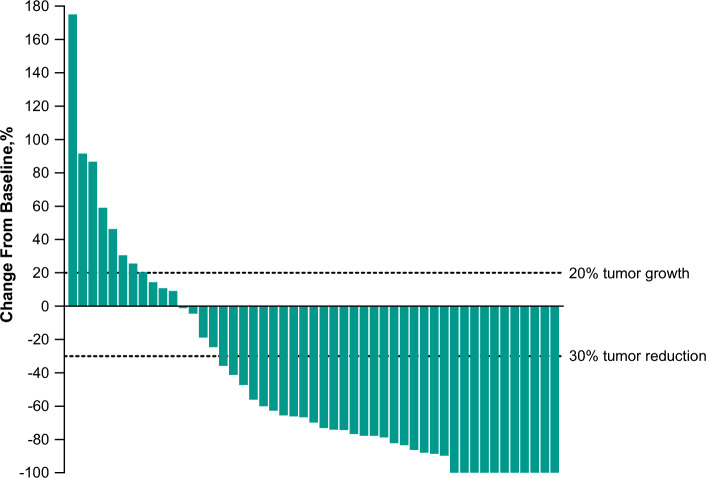
Twenty-seven of 55 enrolled patients had a response (12 CRs and 15 partial responses [PRs]) for an ORR of 49% (95% CI 35–63) (Table 2). The treatment effect of pembrolizumab was generally consistent across subgroups (Fig. 2). Of the 49 patients with at least one postbaseline assessment of the sum of target lesions, 38 (78%) experienced any decrease from baseline in the sum of target lesions and 31 (63%) had a decrease of more than 50% (Fig. 3 and Supplemental Fig. S1, see the electronic supplementary material).
Table 2.
Summary of confirmed objective response per RECIST v1.1 by BICR
| All patients (n = 55) | |
|---|---|
| Objective response rate, n (% [95% CI]) | 27 (49 [35–63]) |
| Best overall response, n (%) | |
| Complete response | 12 (22) |
| Partial response | 15 (27) |
| Stable disease | 4 (7) |
| Progressive disease | 16 (29) |
| Nonevaluablea | 2 (4) |
| No assessmentb | 6 (11) |
BICR blinded independent central review, CI confidence interval, RECIST v1.1 Response Evaluation Criteria in Solid Tumors, version 1.1
aIncludes patients who had postbaseline assessments available but were not evaluable
bIncludes patients who had a baseline assessment but no postbaseline assessment at data cutoff
Fig. 2.
Forest plot of ORR in subgroups per RECIST v1.1 by BICR. BICR blinded independent central review, CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, MCC Merkel cell carcinoma, ORR objective response rate, RECIST v1.1 Response Evaluation Criteria in Solid Tumors, version 1.1
Fig. 3.
Best percentage change from baseline in the sum of target lesions per RECIST v1.1 by BICRa. BICR blinded independent central review, RECIST v1.1 Response Evaluation Criteria in Solid Tumors, version 1.1. aIncludes all patients with at least 1 postbaseline assessment
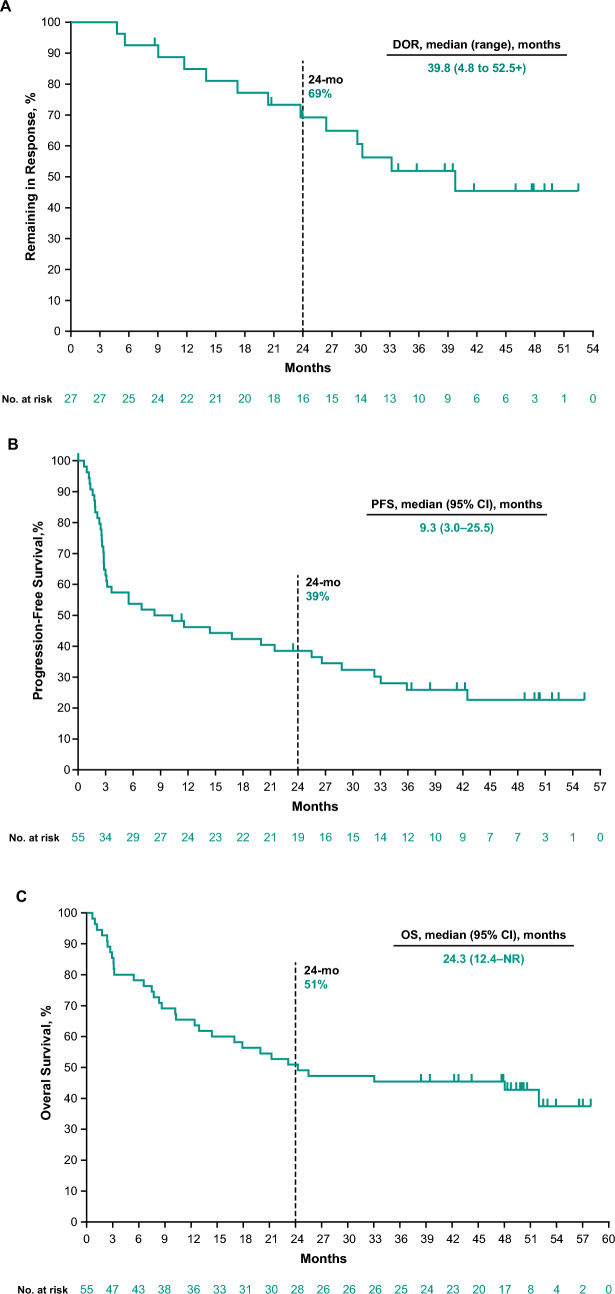
The median DOR in the overall population was 39.8 months (range 4.8–52.5+ months), and the estimated proportion of responders remaining in response at 24 months was 69% (Fig. 4A). At the time of the data cutoff, 40 events (73%) of PD or death had occurred. The median PFS was 9.3 months (95% CI 3–26), and the estimated 24-month PFS rate was 39% (Fig. 4B). With 32 deaths (58%), the median OS was 24.3 months (95% CI 12.4 to not reached), and the estimated 24-month OS rate was 51% (Fig. 4C).
Fig. 4.
Kaplan-Meier estimates of DOR per RECIST v1.1 by BICR (A), PFS per RECIST v1.1 by BICR (B), and OS (C). BICR blinded independent central review, CI confidence interval, DOR duration of response, mo months, NR not reached, OS overall survival, PFS progression-free survival, RECIST v1.1 Response Evaluation Criteria in Solid Tumors, version 1.1
Safety
Patients received a median of seven doses of pembrolizumab (range 1–35). Treatment-related AEs occurred in 38 patients (69%), including 13 (24%) with grade 3–5 treatment-related AEs (Table 3). The most common treatment-related AEs of any grade (≥ 15%) were fatigue (n = 12 [22%]), pruritus (n = 12 [22%]), lipase increase (n = 10 [18%]), increased alanine aminotransferase (n = 8 [15%]), and asthenia (n = 8 [15%]). The only grade 3–5 treatment-related AE that occurred in more than one patient was lipase increase (n = 5 [9%]). Eleven patients (20%) discontinued pembrolizumab because of treatment-related AEs. One patient (2%) died of an AE that was considered treatment-related (Guillain-Barré syndrome). This patient had a history of ataxia and peripheral neuropathy and developed Guillain-Barré syndrome 35 days after starting treatment with pembrolizumab. Despite treatment with systemic corticosteroids and intravenous immunoglobulin, the patient’s condition deteriorated, and the patient died.
Table 3.
Treatment-related adverse events
| All patients (n = 55) | ||
|---|---|---|
| Treatment-related adverse event summary | ||
| Any | 38 (69) | |
| Grade 3–5 | 13 (24) | |
| Seriousa | 7 (13) | |
| Led to discontinuation | 11 (20) | |
| Led to death | 1 (2)b | |
| Treatment-related adverse events with incidence ≥ 5% | Any grade | Grade 3/4 |
|---|---|---|
| Fatigue | 12 (22) | 1 (2) |
| Pruritus | 12 (22) | 0 |
| Lipase increased | 10 (18) | 5 (9) |
| Asthenia | 8 (15) | 1 (2) |
| ALT increased | 8 (15) | 0 |
| AST increased | 7 (13) | 1 (2) |
| Arthralgia | 7 (13) | 0 |
| Amylase increased | 5 (9) | 0 |
| Rash | 5 (9) | 0 |
| Diarrhea | 4 (7) | 0 |
| Dysgeusia | 4 (7) | 0 |
| Eczema | 4 (7) | 0 |
| Blood CPK increased | 3 (5) | 0 |
| Dry mouth | 3 (5) | 0 |
| Headache | 3 (5) | 0 |
| Hyperthyroidism | 3 (5) | 0 |
| Hypothyroidism | 3 (5) | 0 |
| Muscular weakness | 3 (5) | 1 (2) |
| Nausea | 3 (5) | 0 |
Data are n (%)
Treatment-related adverse events were determined by the investigator to be related to study treatment
ALT alanine aminotransferase, AST aspartate aminotransferase, CPK creatinine phosphokinase
aAny adverse event that results in death, is life threatening, results in persistent or significant disability or incapacity, results in or prolongs an existing inpatient hospitalization, results in a congenital anomaly or birth defect, or any other important medical event
bOne patient died of Guillain-Barré syndrome and this was considered related to treatment
Immune-mediated AEs occurred in 14 patients (25%), including three (5%) with grade 3–5 events (one grade 3 colitis, one grade 3 encephalitis, and one grade 5 Guillain-Barré syndrome) (Supplementary Table 1, see the electronic supplementary material). No infusion reactions were observed. Immune-mediated AEs occurring in two or more patients included hyperthyroidism (n = 3 [5%]), hypothyroidism (n = 3 [5%]), colitis (n = 2 [4%]), hypophysitis (n = 2 [4%]), and pneumonitis (n = 2 [4%]).
Discussion
The results of KEYNOTE-913 showed that pembrolizumab provides durable antitumor activity, promising survival, and manageable safety in patients with recurrent locally advanced or metastatic MCC. The ORR was 49%, with 12 patients achieving CR and 15 patients achieving PR. The treatment effect of pembrolizumab also was generally consistent across subgroups, including by previous surgery or radiation therapy. Most patients (78%) experienced a decrease in tumor burden, and 63% had a reduction of more than 50%. Responses were durable, and the median DOR was 39.8 months; an estimated 69% of responders remained in response at 24 months. Survival was also promising, with a 24-month PFS rate of 39% and a 24-month OS rate of 51%. At a median follow-up of 50.3 months, the median OS in the current analysis was 24.3 months, which compares favorably with the OS of 9.5–12.0 months previously reported for metastatic MCC before the introduction of PD-1/PD-L1 inhibitors [15–18].
A benefit with pembrolizumab in patients with previously untreated advanced MCC was also reported in the phase II CITN-09/KEYNOTE-017 study [9, 11, 19]. At a similar median follow-up (14.9 months), the ORR in CITN-09/KEYNOTE-017 was 56% (95% CI 41.3–70.0), the 24-month PFS rate was 48.3%, and the 24-month OS rate was 68.7% [19]. Longer-term analysis of CITN-09/KEYNOTE-017 (median follow-up 31.8 months) showed that most responses with pembrolizumab were durable, with an estimated 72.7% of responders continuing in response at 36 months [11]. Notably, responses were observed with pembrolizumab in CITN-09/KEYNOTE-017 regardless of whether patients had positive or negative results for Merkel cell polyomavirus [9, 11]. Viral status was not collected in the current study.
The results of the current analysis and CITN-09/KEYNOTE-017 add to the body of evidence demonstrating that PD-1/L1 inhibitors are effective as first-line treatments for MCC. This was initially demonstrated with avelumab in part B of the JAVELIN Merkel 200 study. The ORR in this study was 39.7% (95% CI 30.7–49.2), the 12-month PFS rate was 31%, and the 12-month OS rate was 60% [8]. The PD-1 inhibitor retifanlimab has also been shown to have efficacy as first-line treatment in patients with advanced MCC, with an ORR of 52% reported in the PODIUM-201 study [14, 20]. The data available for first-line nivolumab in advanced MCC are currently limited. Among the 14 patients with treatment-naive advanced MCC included in the CheckMate 358 study, the ORR was 71% (95% CI 42–92) [12]. Promising results were reported for the cohort of patients with resectable MCC, with neoadjuvant nivolumab inducing pathologic CR and radiographic tumor regression in approximately half of treated patients [13]. Several studies have also indicated that immunotherapies are effective in the real-world setting. In a review of real-world studies evaluating the efficacy and safety of avelumab in patients with MCC, ORRs ranged from 29.1% to 72.1% [21]. A retrospective study that included 73 patients with previously untreated advanced MCC reported an ORR of 75.3% with first-line avelumab [22]. The SPEAR-Merkel study reported a real-world response rate of 64.3% with avelumab, 61.5% with non-avelumab immunotherapy, and 42.5% with chemotherapy among adult patients with previously untreated locally advanced or metastatic MCC [23].
The safety profile of pembrolizumab was generally consistent with that observed previously for pembrolizumab monotherapy [24]. In the current analysis, 69% of patients had at least one treatment-related AE, with 24% experiencing a grade 3–5 event. These findings are similar to those of a large pooled analysis of 1567 patients who received pembrolizumab monotherapy for advanced melanoma, in which any-grade treatment-related AEs occurred in 80.7% of patients, and grade 3/4 events occurred in 17.7% of patients [24]. The results of this analysis are also similar to those observed with pembrolizumab in the CITN-09/KEYNOTE-017 study (any-grade treatment-related AEs, 96%; grade 3–5, 28%), with avelumab in part B of JAVELIN Merkel 200 (any-grade treatment-related AEs, 81%; grade ≥ 3, 18.1%), and with nivolumab in the total advanced MCC population of CheckMate 358 (treatment-naive and experienced patients; any-grade treatment-related AEs, 68%; grade 3/4, 20%) [8, 12, 19].
The primary limitation of KEYNOTE-913 is the open-label, single-arm design of the study. As a result, only indirect comparison can be made between pembrolizumab and historical outcomes or other studies evaluating anti–PD-1/L1 therapies. Patient numbers are also relatively small, especially when considering outcomes in subgroups, but this is largely unavoidable when investigating a rare cancer such as MCC. An additional limitation was that Merkel cell polyomavirus and PD-L1 status were not collected in the study.
Conclusions
The results of the phase III KEYNOTE-913 study showed that pembrolizumab provided durable antitumor activity, promising survival, and manageable safety in patients with recurrent locally advanced or metastatic MCC. These findings support the use of pembrolizumab as first-line therapy for advanced or metastatic MCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD). The authors thank the participants and their families for participating in the study and all investigators and site personnel. Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Holly C. Cappelli, PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Declarations
Funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder participated in study design, data analysis and interpretation, and manuscript writing, and maintained the study database. All authors had full access to the data and had final responsibility for the decision to submit the manuscript for publication.
Conflict of interest
Laurent Mortier has received consulting fees and travel support from MSD, BMS, Novartis, and Pierre Fabre. Lisa Villabona, Marie Beylot-Barry, Francesca Spada, Michel De Pontville, and Enrique Espinosa declare that they have no conflicts of interest that might be relevant to the contents of this article. Ben Lawrence has received honoraria and travel support from MSD. Ana Arance has received research funding from MSD, honoraria from MSD and Merck Serono, and travel support from MSD and BMS. Marcus O. Butler has received research funding from Merck, Takara Bio, and Novartis; consulting fees from Bristol Myers Squibb, Merck, Novartis, Adaptimmune, Immunocore, GlaxoSmithKline, Sanofi, Sun Pharma, Instil Bio, IOVANCE, Pfizer, Ideaya Bio, Medison, LaRoche Possey, and Regeneron; and honoraria from Sanofi, Bristol Myers Squibb, Merck, Pfizer, and Novartis; and has participated on a safety review committee for Adaptimmune and GlaxoSmithKline. Philippe Saiag has received research funding and consulting fees from Merck Serono. Mahtab Samimi has received research funding from SOTIO and travel support from BMS and MSD, has participated on advisory boards for Merck and Pfizer, and is chair of EADV. Paolo A. Ascierto has received research support from Bristol Myers Squibb, Roche-Genentech, Pfizer, and Sanofi; consulting fees from Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Sanofi, Sandoz, Italfarmaco, Nektar, Pfizer, Lunaphore, Medicenna, Bio-Al Health, ValoTx, RepImmune, and Bayer; and travel support from Pfizer, Bio-AI Health, and RepImmune; and has participated on advisory boards for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, AstraZeneca, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Oncosec, Nouscom, Seagen, iTeos, and Erasca. Michele Maio has received consulting fees, honoraria, and travel support from, and has participated in advisory boards for, BMS, Roche, MSD, Merck, Sanofi, GSK, AstraZeneca, Pierre Fabre, Alfasigma, Eli Lilly, Amgen, Sciclone, Incyte, and Ionctura; and owns stock in Epigen and Teravance. Alfonso Berrocal has received consulting fees from MSD and BMS and honoraria and travel support from MSD. Jaume Capdevila has received research funding from Novartis, Pfizer, AstraZeneca, Advanced Accelerator Applications, Eisai, Amgen, and Bayer; and consulting fees from Novartis, Pfizer, Ipsen, Exelixis, Bayer, Eisai, Advanced Accelerator Applications, Amgen, Sanofi, Lilly, Huchinson Pharma, ITM, Advanz, Merck Serono, Esteve, and Roche. Max Levin has received lecturing fees from Bristol Myers Squibb, Roche, and Merck Sharp & Dohme. Debasmita Das is an employee of Merck & Co., Inc., Rahway, NJ, USA. Clemens Krepler is an employee of, and owns stock in, Merck & Co., Inc., Rahway, NJ, USA. Dmitri Grebennik is an employee of Merck & Co., Inc., Rahway, NJ, USA. Vanna Chiarion-Sileni has received travel support from Pierre-Fabre and Sanofi; has participated on advisory boards for Pierre-Fabre and Merck Sharp & Dohme.
Ethics approval
The study protocol and all amendments were approved by the appropriate institutional review board or ethics committee at each center (Table S2, see the electronic supplementary material). The study was conducted in accordance with the protocol, Good Clinical Practice Guidelines, and the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Code availability
Not applicable.
Author contributions
Acquisition of the data: LM, LV, BL, MOB, MB-B, PS, MS, FS, MD, MM, AB, EE, ML, DD, VCS. Analysis of the data: LM, PS, DD, CK, DG. Interpretation of the results: LM, LV, BL, AA, MB-B, PS, PAA, MM, JC, ML, DD, CK, DG, VCS. Drafting of the manuscript: LM, PS, DD. Critically reviewing or revising the manuscript for important intellectual content: LM, LV, BL, AA, MOB, MB-B, PS, MS, PAA, FS, MD, MM, AB, EE, JC, ML, DD, CK, DG, VCS.
References
- 1.Mcevoy AM, Lachance K, Hippe DS, Cahill K, Moshiri Y, Lewis CW, et al. Recurrence and mortality risk of Merkel cell carcinoma by cancer stage and time from diagnosis. JAMA Dermatol. 2022;158(4):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis CW, Qazi J, Hippe DS, Lachance K, Thomas H, Cook MM, et al. Patterns of distant metastases in 215 Merkel cell carcinoma patients: implications for prognosis and surveillance. Cancer Med. 2020;9(4):1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCaprio JA. Molecular pathogenesis of Merkel cell carcinoma. Annu Rev Pathol. 2021;16:69–91. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Doolittle-Amieva C, Moshiri Y, Akaike T, Parvathaneni U, Bhatia S, et al. How we treat Merkel cell carcinoma: within and beyond current guidelines. Future Oncol. 2021;17(11):1363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker M, Cordes L, Brownell I. Avelumab: a new standard for treating metastatic merkel cell carcinoma. Expert Rev Anticancer Ther. 2018;18(4):319–26. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, Dangelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Angelo SP, Lebbé C, Mortier L, Brohl AS, Fazio N, Grob JJ, et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer. 2021;9(7):e002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford D, Demko S, Jin S, Mishra-Kalyani P, Beckles AR, Goldberg KB, et al. FDA accelerated approval of pembrolizumab for recurrent locally advanced or metastatic Merkel cell carcinoma. Oncologist. 2020;25(7):e1077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer. 2021;9(4):e002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Bhatia S, Hollebecque A, Awada A, Boer JPD, Kudchadkar RR, et al. Abstract CT074: non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (nivo) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC). Cancer Res. 2017;77(13_Supplement):CT074. [Google Scholar]
- 13.Topalian SL, Bhatia S, Amin A, Kudchadkar RR, Sharfman WH, Lebbé C, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the checkmate 358 trial. J Clin Oncol. 2020;38(22):2476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grignani G, Rutkowski P, Lebbe C, Guida M, Gaudy Marqueste C, De Braud FGM, et al. Updated results from POD1UM-201: a phase II study of retifanlimab in patients with advanced or metastatic Merkel cell carcinoma (MCC). Ann Oncol. 2023;34:S686. [Google Scholar]
- 15.Miller NJ, Bhatia S, Parvathaneni U, Iyer JG, Nghiem P. Emerging and mechanism-based therapies for recurrent or metastatic Merkel cell carcinoma. Curr Treat Options Oncol. 2013;14(2):249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steuten L, Garmo V, Phatak H, Sullivan SD, Nghiem P, Ramsey SD. Treatment patterns, overall survival, and total healthcare costs of advanced Merkel cell carcinoma in the USA. Appl Health Econ Health Policy. 2019;17(5):733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, Anderson A, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13(19):1699–710. [DOI] [PubMed] [Google Scholar]
- 19.Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food & Drug Administration. FDA grants accelerated approval toretifanlimab-dlwr for metastatic or recurrent locally advanced Merkel cellcarcinoma. Silver Spring, MD: U.S. Food & Drug Administration; 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-retifanlimab-dlwr-metastatic-or-recurrent-locally-advanced-merkel.
- 21.Lohray R, Verma KK, Wang LL, Haynes D, Lewis DJ. Avelumab for advanced Merkel cell carcinoma: global real-world data on patient response and survival. Pragmat Obs Res. 2023;14:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia S, Nghiem P, Veeranki SP, Vanegas A, Lachance K, Tachiki L, et al. Real-world clinical outcomes with avelumab in patients with Merkel cell carcinoma treated in the USA: a multicenter chart review study. J Immunother Cancer. 2022;10(8):e004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhanegaonkar A, Liu FX, Boyd M, Fulcher N, Kim R, Krulewicz S, et al. Real-world clinical outcomes in patients with locally advanced or metastatic Merkel cell carcinoma treated in U.S. oncology clinical practices: results from SPEAR-Merkel. Oncologist. 2021;26(9):e1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert C, Hwu WJ, Hamid O, Ribas A, Weber JS, Daud AI, et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur J Cancer. 2021;144:182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.