Abstract
A replication-competent rhabdovirus-based vector expressing human immunodeficiency virus type 1 (HIV-1) Gag protein was characterized on human cell lines and analyzed for the induction of a cellular immune response in mice. We previously described a rabies virus (RV) vaccine strain-based vector expressing HIV-1 gp160. The recombinant RV was able to induce strong humoral and cellular immune responses against the HIV-1 envelope protein in mice (M. J. Schnell et al., Proc. Natl. Acad. Sci. USA 97:3544–3549, 2000; J. P. McGettigan et al., J. Virol. 75:4430–4434, 2001). Recent research suggests that the HIV-1 Gag protein is another important target for cell-mediated host immune defense. Here we show that HIV-1 Gag can efficiently be expressed by RV on both human and nonhuman cell lines. Infection of HeLa cells with recombinant RV expressing HIV-1 Gag resulted in efficient expression of HIV-1 precursor protein p55 as indicated by both immunostaining and Western blotting. Moreover, HIV-1 p24 antigen capture enzyme-linked immunosorbent assay and electron microscopy showed efficient release of HIV-1 virus-like particles in addition to bullet-shaped RV particles in the supernatants of the infected cells. To initially screen the immunogenicity of this new vaccine vector, BALB/c mice received a single vaccination with the recombinant RV expressing HIV-1 Gag. Immunized mice developed a vigorous CD8+ cytotoxic T-lymphocyte response against HIV-1 Gag. In addition, 26.8% of CD8+ T cells from mice immunized with RV expressing HIV-1 Gag produced gamma interferon after challenge with a recombinant vaccinia virus expressing HIV-1 Gag. These results further confirm and extend the potency of RV-based vectors as a potential HIV-1 vaccine.
Even though the requirements for protective immune responses against human immunodeficiency virus type 1 (HIV-1) are not well defined, growing evidence suggests that a CD8+ cytotoxic T-lymphocyte (CTL)-mediated immune response is critical in controlling HIV-1 infection (19, 35). This finding is based on several studies showing that exposed but uninfected individuals have HIV-1-specific CTLs without detectable antibodies (42, 43). In addition, data show a strong correlation between a high frequency of HIV-1-specific CTLs with a low HIV-1 viral titer and a slow disease progression in chronically HIV-1-infected individuals (24, 31). It also has been shown that CTL numbers decline in association with progression of AIDS (24). In addition, eliminating CD8+ lymphocytes from monkeys during chronic simian immunodeficiency virus (SIV) infection resulted in a rapid and marked increase in viremia, which was again suppressed coincident with the reappearance of SIV-specific CD8+ T cells (44). Taken together, these data suggest that it is important for a potential HIV-1 vaccine to induce a long-lasting and potent CTL response against HIV-1.
HIV-1 Gag is one of the most conserved proteins of HIV-1 (HIV Molecular Immunology Database, Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, N. Mex., 1999), and epitopes which are conserved among different HIV-1 clades have been described for individuals infected with HIV-1 (14, 27). These data suggest that HIV-1 Gag is a promising target for an HIV-1 vaccine. Today, a variety of approaches to generate an immune response against HIV-1 are in different stages of investigation, and recent approaches include recombinant proteins (17, 39, 48), peptides (5, 7, 34), naked DNA (3, 4, 9, 26, 36, 40, 50), and viral vectors (6, 12, 22, 30, 32, 33, 45). The induction of efficient CD8+ T-lymphocyte-mediated cellular immune responses requires the endogenous synthesis of the target protein, which can be achieved easily with recombinant, live viral vectors.
So far, the only effective method to protect macaques from SIV infection is the use of live, attenuated SIV. It has been shown that genetically attenuated SIV induces high titers of anti-SIV antibodies and CTL activity, which protected against subsequent challenge of the immunized animals with a pathogenic SIV strain (11). A major drawback with the use of attenuated-lentivirus vaccine approaches is the finding that even a nef-deleted SIV can give rise to an AIDS-like disease in both neonatal and adult macaques (reference 2 and references therein). Additional concerns regarding the use of attenuated lentiviruses arise from the recent finding that recombination of live, attenuated SIV with challenge virus in some cases results in an even more virulent strain (20). However, the overall results indicate that live viral vectors might be excellent candidates for an HIV-1 vaccine. A large number of recombinant viral vectors have been tested in the SIV macaque model system, but to date no protective immunity has been obtained, although some amelioration of disease course has been seen (6, 12, 33, 47). These results indicate that further research studies are needed to improve the immunogenicity of currently used viral vectors and further analyze the potency of new viral vectors in the SIV-macaque system.
We have been shown previously that strong HIV-1 gp160-specific CTLs can be elicited in mice after a single vaccination with an attenuated-rabies virus (RV) vaccine strain-based vector. In addition, these responses were stable for at least 135 days after immunization, and recombinant RVs expressing HIV-1 gp160 were able to induce cross-reactive CTLs against a variety of different HIV-1 envelope proteins. In this study we investigate the ability of a replication-competent RV vaccine strain-based vector expressing HIV-1 Gag to elicit a HIV-1 Gag-specific CTL response in mice. Our results indicate that a single inoculation results in a vigorous CTL response specific for HIV-1, which further suggests the study of RV-based vectors as a potential HIV-1 vaccine.
MATERIALS AND METHODS
Plasmid construction.
All PCRs were performed using high-fidelity Vent DNA polymerase (New England Biolabs) to minimize the introduction of sequence errors. pSBN was described previously (45) and was the target to introduce a new single restriction site (PacI; boldface) downstream of the RV G gene by site-directed mutagenesis (GeneEditor) using primer 5′-GTGAGACCAGACTGTAATTAATTAACGTCCTTTCAACGATCC-3′ as indicated by the manufacturer (Promega). The resulting plasmid was designated pSPBN. The gene encoding HIV-1NL4-3 Gag was amplified by PCR from pNL4-3 (1) using forward primer 5′-AAACTCGAGCGTACGACAATGGGTGCGAGAGCGTCAG-3′ containing a BsiWI site (boldface) and reverse primer 5′-AAAGCTAGCTTAATTAATCGCGATTATTGTGACGAGGGGTCG-3′ containing an NheI site (boldface). The PCR product was digested with BsiWI and NheI and cloned into pSPBN, which had been previously digested with BsiWI and NheI. The resulting plasmid was designated pSPBN-Gag.
Recovery of infectious RV from cDNA.
For experiments involving recovery of the recombinant RVs, the previously described RV recovery system was used (15, 45). Briefly, BSR-T7 cells (8), which stably express T7 RNA polymerase, were transfected with 5 μg of full-length RV cDNA in addition to plasmids encoding the RV N, P, L, and G proteins using a Ca2PO4 transfection kit (Stratagene), as instructed by the vendor. Three days posttransfection, supernatants were transferred onto fresh BSR cells, and infectious RV was detected 3 days later by immunostaining with fluorescein isothiocyante (FITC) against the RV N protein (Centocor Inc.).
Immunofluorescence microscopy.
BSR or HeLa cells were plated in six-well plates containing coverslips and infected at a multiplicity of infection (MOI) of 0.1 for 48 h. Cells were fixed with 80% acetone at 4°C for 30 min, incubated with a monoclonal human antibody directed against the HIV-1 p24 antigen (18) (diluted 1:50) for 1 h at room temperature and again washed three times with phosphate-buffered saline (PBS). After incubation for another 30 min with goat anti-human FITC (diluted 1:200; Jackson ImmunoReasearch), cells were washed three times with PBS and analyzed by fluorescence microscopy. A FITC-labeled antibody against RV N (Centocor Inc.) was used as described previously (16, 45).
HIV-1 p24 antigen capture ELISA.
HeLa cells were infected at a MOI of 5. Forty-eight hours later, supernatants were collected and cells were resuspended in lysing buffer (Triton X-100 in PBS–2-chloroacetamide). The supernatants and cell suspension were transferred to microcentrifuge tubes and spun for 2 min at 14,000 rpm to remove cell debris. The quantification of the Gag p24 protein in cell supernatants and lysates was performed using the p24 antigen enzyme-linked immunosorbent assay (ELISA), as described by the manufacturer (ZeptoMetrix, Inc.).
Electron microscopy.
HeLa cells were infected at a MOI of 1 for 48 h, fixed at room temperature in neutral buffered 2.5% glutaraldehyde, and gelled into warm agar. They were postfixed in 1% OsO4, dehydrated in graded ethanol and propylene oxide, and embedded in Spurr's epoxy. Thin sections were cut and stained with uranyl acetate and lead citrate and examined with a LEO EM10 electron microscope at 60 kV.
Western blotting.
HeLa cells were infected at a MOI of 2 for 24 h and resuspended in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 1× protease inhibitor cocktail [Sigma]) on ice for 5 min. The suspension was transferred to a microcentrifuge tube and spun for 1 min at 16,000 × g to remove cell debris. Proteins were separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF-Plus membrane (Osmonics, Minnetonka, Minn.). Blots were blocked for 1 h (5% dry milk powder in PBS [pH 7.4]). After being blocked, blots were washed twice using a 0.1% PBS–Tween 20 solution and incubated with a human anti-p24 antibody (diluted 1:500) or polyclonal rabbit anti-RV G antibody (16). Blots were then washed three times with 0.1% PBS-Tween. Secondary goat anti-human or goat anti-rabbit horseradish peroxidase-conjugated antibodies (diluted 1:25,000) (Jackson ImmunoResearch) were added, and blots were incubated overnight at 4°C. Blots were washed three times with 0.1% PBS–Tween and washed once with PBS (pH 7.4). Chemiluminescence analysis (NEN) was performed as instructed by the vendor.
Cytotoxicity assays.
Groups of five 6- to 8-week-old female BALB/c mice (Harlan) were inoculated intraperitoneally (i.p.) with 107 focus-forming units (FFU) of SPBN-Gag. To analyze the induction of a specific CTL response against HIV-1 Gag, spleens from two mice of each group were aseptically removed and combined and single-cell suspensions were prepared. Red blood cells were lysed with ACK lysing buffer (BioWhittaker), splenocytes were washed twice in RPMI-10 medium containing 10% fetal bovine serum and pulsed with 10 μg of the major histocompatibility complex (MHC) class I-restricted p24 peptide (amino acids AMQMLKETI)/ml, and 10% T-STIM (Collaborative Biomedical Products) was added as a source of interleukin-2 (IL-2). Cytolytic activity of cultured CTLs was measured by a 4-h assay with 51Cr-labeled P815 target cells. Target cells were prepared by incubating them with 10 μg of p24 peptide/ml–100 μCi of 51Cr for 2 h and washed twice and were added to effector cells at various effector-to-target cell ratios for 4 h. The percentage of specific 51Cr release was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was determined from supernatants of cells that were lysed by the addition of 5% Triton X-100. Spontaneous release was determined from target cells incubated without added effector cells.
Intracellular IFN-γ staining and flow cytometry analysis.
Groups of 6- to 8-week-old female BALB/c mice were inoculated i.p. with 107 FFU of recombinant RV expressing HIV-1 Gag (SPBN-Gag) or vector alone (SPBN). Nine weeks postimmunization, mice were challenged with vaccinia virus expressing HIV-1 gag (21) (vP1287) or an unrelated hepatitis C virus (HCV) protein. Five days later, spleens were removed, single-cell suspensions were prepared, and red blood cells were removed. Cells were cultured at 2 × 106 cells/ml with or without p24 peptide (AMQMLKETI) for 16 h and treated with GolgiPlug (PharMingen) to inhibit cytokine secretion from the Golgi apparatus for an additional 6 h. Cells were incubated with rat anti-mouse CD16-CD32 (1 μg/106 cells) (PharMingen) to inhibit nonspecific binding by the fluorescent antibodies. Cells were washed twice with staining buffer (PBS containing 1% fetal bovine serum) and then stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8 antibody (0.06 μg/106 cells) (Pharmingen) and washed twice in staining buffer. Cells were resuspended in 50 μl of staining buffer, 100 μl of fixation medium (Caltag Laboratories) was added, and cells were incubated for 15 min at room temperature. Fixed cells were washed three times with PBS and resuspended in 100 μl of permeabilization medium (Caltag Laboratories) containing 0.12 μg of FITC-conjugated rat anti-mouse gamma interferon (IFN-γ) monoclonal antibody (Pharmingen)/106 cells for 15 min at room temperature. Cells were washed three times with PBS and counted by flow cytometry. Surface (immunoglobulin 2a [IgG2a] K) and internal (IgG1) isotype-matched negative controls (Pharmingen) were analyzed as control samples.
Tetramer analysis of splenic lymphocytes and peripheral blood.
Groups of 6- to 8-week-old female BALB/c mice were inoculated i.p. with 107 FFU of recombinant RV expressing HIV-1 Gag (SPBN-Gag). Eleven weeks postimmunization, mice were challenged with vaccinia virus expressing HIV-1 Gag (vP1287) or an unrelated hepatitis C virus (HCV) protein. Five days later, spleens were removed and single-cell suspensions were prepared and purified over Lympholyte-M (CedarLane Laboratories). Cells (3 × 106) were washed twice in fluorescence-activated cell sorter (FACS) buffer (PBS supplemented with 2% fetal bovine serum) and incubated with rat anti-mouse CD16-CD32 (1 μg per 106 cells; Fc Block; Pharmingen)–33 μg of unconjugated streptavidin (Molecular Probes)/ml for 1 h on ice. Cells were washed twice with FACS buffer and incubated with 2 μl of the tetramer (H-2K(d)/AMQMLKETI) from the HIV-1 Gag p24 protein and 2 μg of FITC-conjugated rat anti-mouse CD8 antibody (Caltag)/ml for 30 min at room temperature with occasional agitation. Cells were washed three times with FACS buffer and resuspended in 300 μl of PBS containing 2% paraformaldehyde and analyzed by FACScan. Similarly, peripheral blood was collected from SPBN-Gag-immunized and vaccinia virus-challenged mice and incubated with 2 μl of the tetramer–2 μg of FITC-conjugated rat anti-mouse CD8 antibody (Caltag)/ml for 30 min at room temperature with occasional agitation. Cells were washed three times with FACS buffer and resuspended in 300 μl PBS containing 2% paraformaldehyde and analyzed by FACScan.
RESULTS
Construction of replication-competent RV expressing HIV-1 Gag protein.
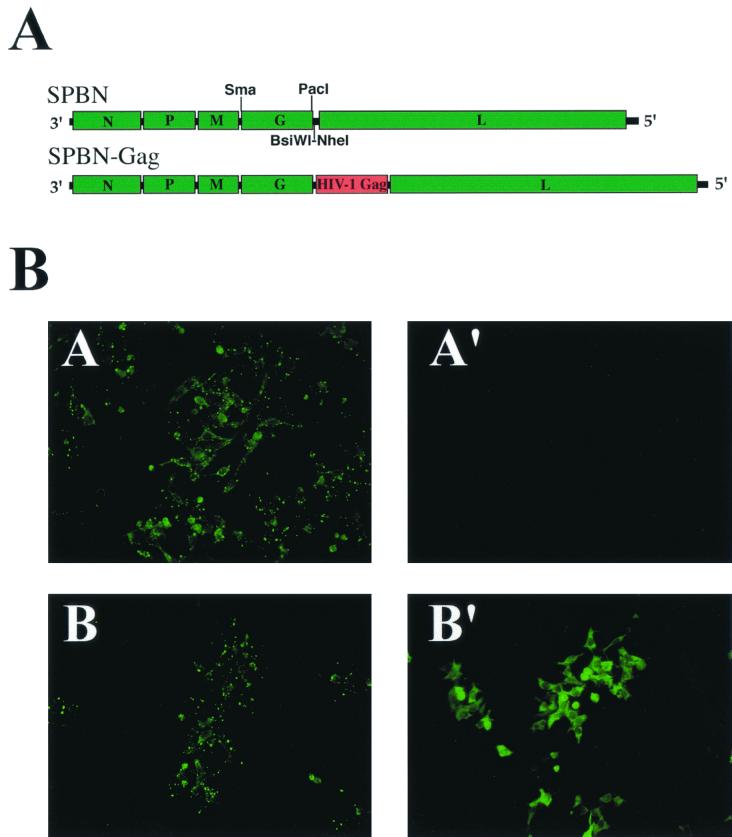
Recent research shows that foreign proteins such as HIV-1 gp160 are stably expressed by RV-based vaccine vectors (45). Moreover, long-lasting and vigorous humoral and cellular immune responses against the expressed HIV-1 envelope proteins in vaccinated mice were observed (45). Another important target for a vaccine against HIV-1 is HIV-1 Gag, one of the most conserved proteins of HIV-1. We reported previously the construction of a recombinant RV vaccine-based vector SBN that contains a synthetic RV transcription unit and two single restriction sites downstream of the glycoprotein (G) gene (45). Site-directed mutagenesis and a PCR strategy were used to introduce a new single PacI restriction site downstream of the coding region for the RV glycoprotein (G) gene into the pSBN cDNA, resulting in pSPBN (Fig. 1A). To construct a recombinant RV expressing HIV-1 Gag, the HIV-1NL4-3 gag coding region was amplified by PCR and cloned into pSPBN using the BsiWI and NheI sites. The resulting plasmid was designated pSPBN-Gag (Fig. 1A). Recombinant replication-competent RVs SPBN and SPBN-Gag were recovered by transfection of BSR cells stably expressing the T7 RNA polymerase with plasmids encoding the RV N, P, and L proteins along with a plasmid coding for the respective RV full-length antigenomic RNA. Three days after transfection, supernatants of transfected cells were transferred to fresh cells and 3 days later were analyzed by indirect immunofluorescence microscopy for the presence of infectious RVs. A positive signal for RV nucleoprotein confirmed the successful recovery of recombinant RVs SPBN and SPBN-Gag (data not shown). In contrast to the previously recovered recombinant RVs expressing the HIV-1 envelope protein (16), SPBN and SPBN-Gag grew to titers similar to those for the parental vector, SBN, which were at least 2 × 108.
FIG. 1.
Construction of recombinant RVs and expression of HIV-1 Gag from RV. (A) RV vaccine strain-based expression vector (top; SPBN). The HIV-1NL4-3 gag coding region was amplified by PCR and cloned into SPBN using the BsiWI and NheI sites. The resulting plasmid was designated pSPBN. (B) Expression of HIV-1 Gag from recombinant SPBN-Gag. HeLa cells were infected at a MOI of 0.1 with SPBN (A and A′) or SPBN-Gag (B and B′) and analyzed by immunofluorescence microscopy with an antibody directed against RV N (A and B) or HIV-1 Gag (A′ and B′) 48 h after infection.
Expression of HIV-1 Gag protein in infected cells.
To ensure expression of HIV-1 Gag from the recombinant RV, HeLa cells were infected with SPBN or SPBN-Gag at a MOI of 0.1 and the infected cells were analyzed by immunofluorescence 48 h after infection. As shown in Fig. 1B, expression of the RV nucleoprotein (N) was detected in cells infected with SPBN and SPBN-Gag (Fig. 1B, A and B), whereas expression of HIV-1 Gag could only be detected in SPBN-Gag-infected cells (Fig. 1B, B′).
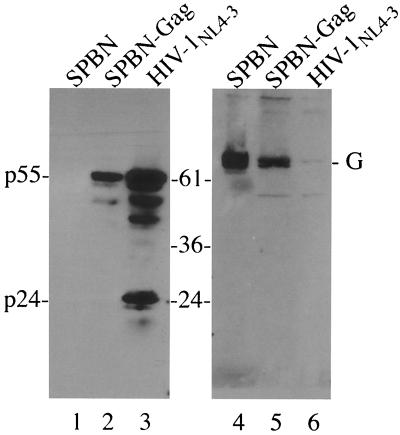
In the next step we analyzed cell lysates from HeLa cells infected with SPBN or SPBN-Gag by SDS-PAGE followed by immunoblotting with a human monoclonal antibody directed against HIV-1 capsid protein p24 (Fig. 2, lanes 1 to 3) or a polyclonal rabbit antibody against RV G (Fig. 2, lanes 4 to 6). A strong signal identified a protein at the expected size for HIV-1 Gag precursor p55 in the case of SPBN-Gag-infected cells (Fig. 2, lane 2), whereas no HIV-1 Gag protein was detected in cell lysates of SPBN-infected cells (Fig. 2, lane 1). In cell lysates from control cells infected with HIV-1 multiple protein bands were detected; these bands represent proteolytic cleavage products of the HIV-1 p55 precursor protein (Fig. 2, lane 3). Because SPBN-Gag does not contain the HIV-1 protease, the smaller protein bands detected in SPBN-Gag-infected cells are probably derived from early termination and/or internal initiation of p55 translation.
FIG. 2.
Western blot analysis of recombinant RVs expressing HIV-1 Gag. HeLa cells were infected at a MOI of 5 with SPBN or SPBN-Gag and lysed 24 h later. Proteins were separated by SDS-PAGE and analyzed by Western blotting. An antibody directed against HIV-1 p24 antigen detected a prominent band at the expected size for HIV-1 (lane 2); no signal was detected for SPBN-infected control cells (lane 1). Lysates from HIV-1NL4-3-infected SupT1 cells served as a control (lane 3). A antibody directed against RV G confirmed the infection of the cells with RV (lanes 4 and 6).
Infection of human cells with recombinant RV expressing HIV-1 Gag results in the release of immature HIV-1 VLPs.
To quantify expression of HIV-1 Gag by recombinant RV SPBN-Gag, cells were infected with SPBN or SPBN-Gag at a MOI of 1 and cell culture supernatants and cell lysates were analyzed 48 h later by a p24 antigen capture ELISA. The results, shown in Table 1, indicate the efficient production and release of HIV-1 p55 in the range of 2 to 4.5 ng/ml for both BSR and HeLa cells infected with SPBN-Gag. No p24 antigen was detected on control cells infected with RV expression vector SPBN (Table 1). These results show that RV-based vectors are able to efficiently produce HIV-1 Gag in human and nonhuman cell lines and also confirm the previous finding that RV-based vectors are able to replicate efficiently in cell lines derived from humans (29), an important requirement for an HIV-1 vaccine vector. These results were also confirmed for human and rhesus monkey peripheral blood mononuclear cells (PBMC; data not shown).
TABLE 1.
Quantification of HIV-1 Gag in lysates and supernatants from SPBN- or SPBN-Gag-infected cellsa
| Cell line, sample type | Gag level (ng/ml) due to infection with:
|
|
|---|---|---|
| SPBN-Gag | SPBN | |
| HeLa, lysates | 2.1 | <0.1 |
| HeLa, supernatants | 4.5 | <0.1 |
| BSR, lysates | 2.0 | <0.1 |
| BSR, supernatants | 1.9 | <0.1 |
BSR and HeLa cells were infected with SPBN or SPBN-Gag for 48 h, and p24 antigen was quantified using a p24 antigen ELISA.
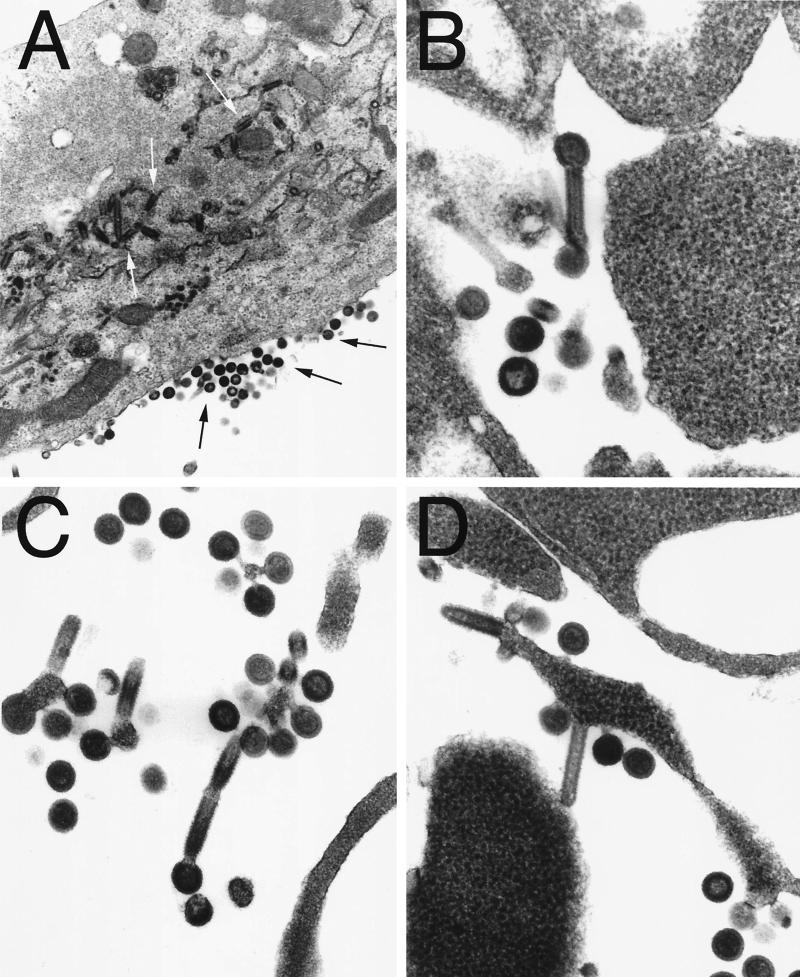
RV infection does not cause a cytopathic effect on most cells lines, and we therefore speculated that the majority of the detected HIV-1 Gag was due to HIV-1 virus-like particles (VLPs) rather than free p55 from lysed cells. To study the generation of the HIV-1 VLPs, HeLa cells were infected at a MOI of 1 for 48 h. Cells were fixed at room temperature in neutral buffered 2.5% glutaraldehyde and gelled into warm agar. These cells were postfixed in 1% OsO4, dehydrated in graded ethanol-propylene oxide, and embedded in Spurr's epoxy. Thin sections were cut and stained with uranyl acetate and lead citrate and examined. The results shown in Fig. 3A indicated high production of RV and immature HIV-1 VLPs, both on the plasma membrane and in cytoplasmic vacuoles. It is interesting that some HIV-1 VLPs apparently are budding from the ends of RV particles (Fig. 3B and C), indicating the simultaneous budding of large amounts of RV virions and HIV-1 VLPs at the same location of the host cell membrane.
FIG. 3.
Evaluation of recombinant RV expressing HIV-1 Gag by electron microscopy. HeLa cells were infected with SPBN-Gag at a MOI of 1 for 48 h. Cells were fixed at room temperature in neutral buffered 2.5% glutaraldehyde and gelled into warm agar. The cells were postfixed in 1% OsO4, dehydrated in graded ethanol-propylene oxide, and embedded in Spurr's epoxy. Thin sections were cut and stained with uranyl acetate and lead citrate and examined with a LEO EM10 electron microscope at 60 kV. The figure shows large numbers of bullet-shaped RV particles (A, white arrows) and late-budding and immature HIV-1 particles (A, black arrows) both on the plasma membrane and in cytoplasmic vacuoles Magnification: ×43,000 (A) and ×131,000 (B to D).
Induction of HIV-1 Gag-specific CTL responses in mice immunized with SPBN-Gag.
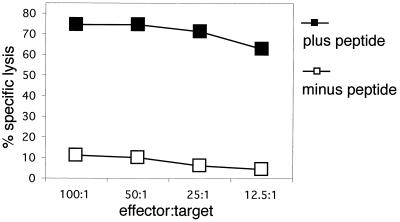
First, we addressed the question of whether the recombinant RV expressing HIV-1 Gag was able to induce a cellular immune response in mice. Our previous results with recombinant RV expressing HIV-1 gp160 indicated that RV-based vectors were able to induce vigorous, long-lasting CTL responses after a single inoculation. To analyze if this was also the case for HIV-1 Gag, BALB/c mice were vaccinated once i.p. with 107 FFU of SPBN-Gag. Six weeks postimmunization, three mice were sacrificed and splenocytes were pooled and stimulated with naive mouse splenocytes that were pulsed with 10 μg of MHC class I-restricted p24 peptide/ml for 7 days and cytolytic activity was measured by a standard chromium release assy. As can been observed in Fig. 4, a strong cytotoxic response only against P815 target cells pulsed with the p24 peptide and not against control cells without the p24 peptide can be detected. The same results were achieved in three independent experiments. Of note, the specific lysis still reached 70% at an effector-to-target ratio of 12.5:1, confirming that priming with recombinant RVs is an excellent strategy for a potential HIV-1 vaccine. Because RV replicates efficiently in both human and nonhuman primate cells, similar responses may be obtained in vaccinees other then mice. Even though the p24 peptide is MHC class I restricted, we decided to reconfirm that cytotoxic activity was mediated by CD8+ T cells. In vitro p24-restimulated splenocyte cultures of SPBN-Gag-immunized mice were divided into CD8+ and CD8− cells. As expected, the CD8+-depleted cultures showed no activity while the CD8+ T-cell-enriched and unprocessed cultures showed high specific lysis. In addition, the CD8+ T-cell-enriched population was enriched in lytic units (data not shown).
FIG. 4.
HIV-1-specific CTLs after a single immunization with SPBN-Gag. BALB/c mice were inoculated i.p. with 107 FFU of recombinant RV expressing HIV-1 Gag. Three weeks postimmunization, splenocytes from three mice were pooled and stimulated with the p24 peptide (AMQMLKETI). Cytolytic activity of cultured CTLs was measured after 7 days. The target cells (P815) were pulsed with the p24 peptide (plus peptide) or were not pulsed (minus peptide).
Flow cytometry analysis of IFN-γ-producing cells.
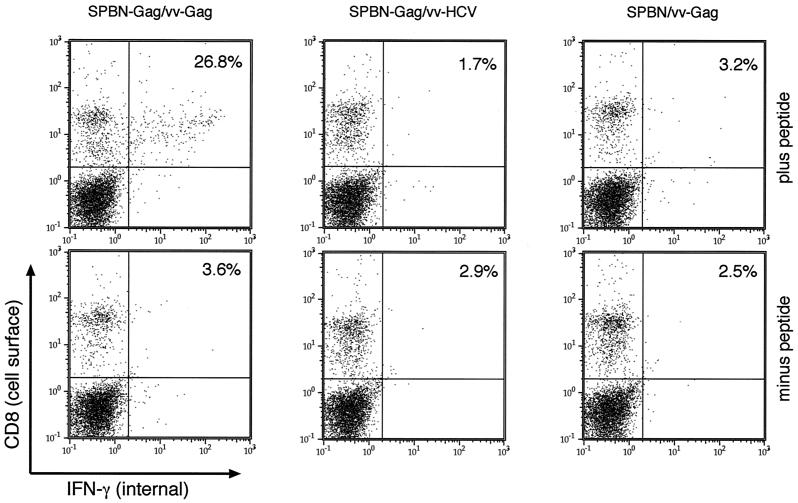
It has been shown clearly that CD8+ lymphocytes play an important role in controlling HIV-1 infection. The results described above indicated that an RV-based vector expressing HIV-1 p55 is able to induce a potent, HIV-1 Gag-specific memory response, which was indicated by functional CTLs. In the next step, we analyzed if successful priming with SPBN-Gag can also be detected in vivo. To analyze this in a more quantitative manner, the percentages of IFN-γ CD8+ T cells after challenge with a recombinant vaccinia virus expressing HIV-1 Gag (vv-Gag) were determined. In contrast to HIV-1, vaccinia virus replicates well in mice and was described previously as a suitable challenge vector to analyze priming against HIV-1 Gag by vaccine vectors (38, 52). Groups of 10 BALB/c mice were immunized with SPBN-Gag or SPBN as a vector control. Nine weeks after immunization, mice were challenged with 107 PFU of vv-Gag or a recombinant vaccinia virus expressing the structural proteins of HCV as a control (vv-HCV). Five days after the challenge infection, two mice in each group were sacrificed and spleens were removed. To determine the number of HIV-1 Gag-specific T cells expressing IFN-γ, splenocytes were cultured with or without p24 peptide for 24 h followed by immunostaining with two antibodies directed against murine IFN-γ or CD8. The flow cytometry analysis is shown in Fig. 5. We observed a high number of IFN-γ-secreting cells (2.7% of the total splenocytes and 26.8% of the total CD8+ T cells) 5 days after the challenge with vv-Gag in spleens of SPBN-Gag-immunized mice. As expected, control animals primed with SPBN and challenged with vv-Gag showed only a low number of IFN-γ-secreting CD8+ T cells, confirming that the high numbers of IFN-γ-secreting CD8+ T cells were due to the SPBN-Gag priming and not to the vv-Gag challenge.
FIG. 5.
Induction of HIV-1 Gag-specific IFN-γ-producing CD8+ T cells in BALB/c mice after a single immunization with SPBN-Gag. Groups of 6- to 8-week-old female BALB/c mice were inoculated i.p. with 107 FFU of recombinant RV expressing HIV-1 Gag (SPBN-Gag) or vector alone (SPBN). Nine weeks postimmunization, mice were challenged with recombinant vaccinia virus expressing HIV-1 Gag (vv-Gag) or the HCV structural protein (vv-HCV). Five days later, spleens were removed and cells were stimulated in vitro with or without the p24 peptide (AMQMLKETI) for 16 h as described in Materials and Methods. Cells were stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8a antibody and a FITC-conjugated rat anti-mouse IFN-γ. The number in each panel indicates the percentage of CD8+ T cells secreting IFN-γ.
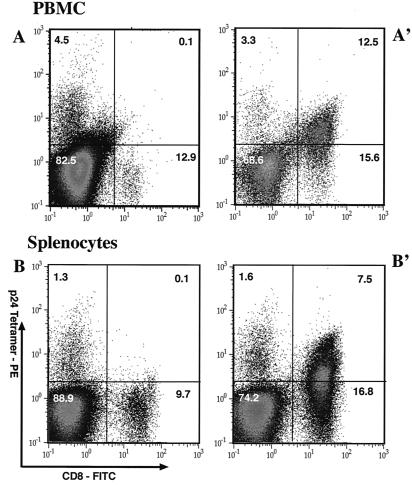
The high number of CD8+ T cells after challenge with vv-Gag but not with vv-HCV was also confirmed by staining with the Kd-p24 tetramer specific for the immunodominant HIV-1 Gag epitope (AMQMLKETI) recognized in H-2d mice. It was found that 30.8 or 44.5% of the CD8+ T cells from splenocytes or peripheral blood mononuclear cells (PBMCs), respectively, of SPBN-Gag-immunized mice were tetramer positive after challenge with vv-Gag whereas only a low number (0.8%) of the CD8+ T cells were detected after the challenge with vv-HCV (Fig. 6).
FIG. 6.
Staining of PBMCs or splenocytes with the Kd-AMQMLKETI MHC peptide tetrameric complex. Mice were immunized as described for Fig. 5, and 11 weeks postimmunization they were challenged with recombinant vaccinia virus expressing the HCV structural protein (A and B) or HIV-1 Gag (A′ and B′). Five days later, PBMC (A and A′) or splenocytes (B and B′) were isolated and cells were stained with FITC-conjugated rat anti-mouse CD8 antibody and the Kd-AMQMLKETI MHC peptide tetrameric complex. PE, phycoerythrin.
DISCUSSION
We described herein the biochemical characterization and immunogenicity of recombinant RV expressing the HIV-1 Gag protein. Our analyses shows that RV-based vectors stably express the HIV-1 Gag p55 precursor protein, which is identical in size to Gag expressed by HIV-1. Infection of human cells with the recombinant RV expressing HIV-1 Gag resulted in a large quantity of immature HIV-1 VLPs in addition to bullet-shaped RV particles. The expression of HIV-1 Gag and production of VLPs have been shown previously with other expression systems such as viral vectors and naked DNA (23, 41, 52). HIV-1-derived VLPs have been demonstrated to be immunogenic and are able to induce both cellular and humoral responses (13, 49). One advantage of the RV-based system is that RV grows very efficiently on human cells without killing the infected cells (46, 51); this results in the long-term production of VLPs. Compared to vaccines based on DNA, the advantage of replication-competent viral vectors such as RV is the spread of the viral vector and therefore expression of the antigen in a large number of cells.
We previously showed that RV-based vectors expressing the HIV-1 envelope protein are able to induce strong HIV-1 envelope protein-specific CTLs in the mouse model (28). In contrast to other vaccine approaches (10, 41), a single inoculation with recombinant RV expressing HIV-1 gp160 was sufficient to prime the immune system for a long-term memory response. Moreover, RVs expressing gp160 from one HIV-1 strain induced HIV-1 envelope-specific CTLs that were able to cross-react efficiently with envelope proteins from other HIV-1 strains, including primary viral isolates (28).
We were able to confirm and expand the results observed for HIV-1 gp160 with another important target for an HIV-1 vaccine, namely, HIV-1 Gag. Research results indicated that Gag-specific CD8+ T cells are important in controlling virus load during acute infection (25). In addition, recent research showed that multiple inoculations with a DNA vaccine encoding HIV-1 Gag in addition to human interleukin-2 protected rhesus macaques from developing an AIDS-like disease (4). Our data presented here indicate that RV-based vectors expressing HIV-1 Gag might be able to induce similar responses without the need for multiple inoculations. Another advantage of a rhabdovirus-based vector over DNA-based vaccines is that no modification of the antigen-encoding sequence(s) is required. Recent data indicate that numerous modifications, for example, of HIV-1 gag, are required to achieve sufficient expression and immunogenicity in mice (37, 38, 52). Because RV exclusively replicates in the cytoplasm of the infected cells, expressing the Gag-encoding RNA is independent from the HIV-1 Rev and Rev-responsive elements. This is also advantageous considering the dramatic variability of the HIV-1 genome, which may require vaccine vectors expressing HIV-1 antigens from different strains or clades (HIV Molecular Immunology Database, Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, N.Mex., 1999).
Although RV is an attractive candidate vector for an HIV-1 vaccine, safety, as with every live-virus vector, is also the major concern for the use of RV. Of note, the RV vector utilized in our studies is based on an RV vaccine strain successfully applied for oral immunization of wild animals for more than 10 years in Europe. It is completely apathogenic after peripheral application in a variety of animals, including chimpanzees (Report of the Fourth W. H. O. Consultation on Oral Immunization of Dogs against Rabies [W. H. O./Rab.Res./93.42], 1993). In addition, preliminary data from our laboratory and other laboratories indicate that it is possible to construct an RV expression vector which does not cause the disease rabies even after direct inoculation into the brains of mice (B. Dietzschold and M. J. Schnell, unpublished data).
In summary, the data presented here and previous to this article indicate that RV-based vectors are excellent vaccine vehicles to induce strong humoral and cellular immune responses against HIV-1 Gag and gp160 in the mouse model (28). Of note, further studies are required to determine if the strong immune response against HIV-1 Gag and gp160 induced by RV-based vectors in mice can also achieved in rhesus monkeys. We are optimistic that our preliminary data support this idea because recombinant RVs expressing the SIV envelope protein are able to replicate efficiently in human (16) and rhesus monkey PBMC (J. P. McGettigan and M. J. Schnell, unpublished data). The finding that chimpanzees orally immunized with an RV vaccine strain seroconvert against RV proteins after a single inoculation (Report of the Fourth W. H. O. Consultation on Oral Immunization of Dogs against Rabies [W. H. O./Rab.Res./93.42], 1993) also indicates that RV-based vectors have the potency to induce mucosal immunity. Taken together, these data emphasize the need for prompt testing of RV-based vaccine vectors in the SIV-rhesus macaque model system.
ACKNOWLEDGMENTS
The human monoclonal antibody directed against p24 (1), plasmid pNL4-3 encoding an infectious clone of HIV-1NL4-3 (18), and recombinant vaccinia virus vP1287 expressing HIV-1 Gag (21) were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The H-2K(d)/AMQMLKETI tetramer was synthesized by the National Institute of Allergy and Infectious Diseases MHC Tetramer Core Facility, Atlanta, Ga. We thank Catherine Siler for excellent technical assistance.
This study was supported by NIH grant AI44340, AmfAR grant 02697-28-RGV, and internal Thomas Jefferson University funds to M.J.S and the Center for Human Virology.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 3.Bagarazzi M L, Boyer J D, Javadian M A, Chattergoon M A, Shah A R, Cohen A D, Bennett M K, Ciccarelli R B, Ugen K E, Weiner D B. Systemic and mucosal immunity is elicited after both intramuscular and intravaginal delivery of human immunodeficiency virus type 1 DNA plasmid vaccines to pregnant chimpanzees J. Infect Dis. 1999;180:1351–1355. doi: 10.1086/314978. [DOI] [PubMed] [Google Scholar]
- 4.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Liftom M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov I M, Ahlers J D, Brandwein B Y, Earl P, Kelsall B L, Moss B, Strober W, Berzofsky J A. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12 J. Clin Investig. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 7.Berzofsky J A, Ahlers J D, Derby M A, Pendleton C D, Arichi T, Belyakov I M. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol Rev. 1999;170:151–172. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz U J, Finke S, Conzelmann K K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter J. Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, Borsetti A, Belli R, Akerblom L, Corrias F, Butto S, Heeney J, Verani P, Titti F, Ensoli B. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 10.Caley J J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Fredlinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 12.Davis N L, Caley I J, Brown K W, Betts M R, Irlbeck D M, McGrath K M, Connell M J, Montefiori D C, Frelinger J A, Swanstrom R, Johnson P R, Johnston R E. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles J. Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deml L, Schirmbeck R, Reimann J, Wolf H, Wagner R. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology. 1997;235:26–39. doi: 10.1006/viro.1997.8668. [DOI] [PubMed] [Google Scholar]
- 14.Durali D, Morvan J, Letourneur F, Schmitt D, Guegan N, Dalod M, Saragosti S, Sicard D, Levy J P, Gomard E. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients J. Virol. 1998;72:3547–3553. doi: 10.1128/jvi.72.5.3547-3553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finke S, Conzelmann K K. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus J. Virol. 1999;73:3818–3825. doi: 10.1128/jvi.73.5.3818-3825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley H D, McGettigan J P, Siler C A, Dietzschold B, Schnell M J. A recombinant rabies virus expressing vesicular stomatitis virus glycoprotein fails to protect against rabies virus infection. Proc Natl Acad Sci USA. 2000;97:14680–14685. doi: 10.1073/pnas.011510698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goebel F D, Mannhalter J W, Belshe R B, Eibl M M, Grob P J, de Gruttola V, Griffiths P D, Erfle V, Kunschak M, Engl W. Recombinant gp160 as a therapeutic vaccine for HIV-infection: results of a large randomized, controlled trial. European Multinational IMMUNO AIDS Vaccine Study Group. AIDS. 1999;13:1461–1468. doi: 10.1097/00002030-199908200-00004. [DOI] [PubMed] [Google Scholar]
- 18.Gorny M K, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 20.Gundlach B R, Lewis M G, Sopper S, Schnell T, Sodroski J, Dittmer U, Stahl-Hennig C, Uberla K. Evidence for recombination of live, attenuated immunodeficiency virus vaccine with challenge virus to a more virulent strain. J Virol. 2000;74:3537–3542. doi: 10.1128/jvi.74.8.3537-3542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurgo C, Guo H G, Franchini G, Aldovini A, Collalti E, Farrell K, Wong-Staal F, Gallo R C, Reitz M S., Jr Envelope sequences of two new United States HIV-1 isolates. Virology. 1988;164:531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- 22.Johnson J E, Schnell M J, Buonocore L, Rose J K. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J Virol. 1997;71:5060–5068. doi: 10.1128/jvi.71.7.5060-5068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karacostas V, Nagashima K, Gonda M A, Moss B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc Natl Acad Sci USA. 1989;86:8964–8967. doi: 10.1073/pnas.86.22.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu S, Wyatt R, Richmond J F, Mustafa F, Wang S, Weng J, Montefiori D C, Sodroski J, Robinson H L. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res Hum Retroviruses. 1998;14:151–155. doi: 10.1089/aid.1998.14.151. [DOI] [PubMed] [Google Scholar]
- 27.McAdam S, Kaleebu P, Krausa P, Goulder P, French N, Collin B, Blanchard T, Whitworth J, McMichael A, Gotch F. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS. 1998;12:571–579. doi: 10.1097/00002030-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 28.McGettigan J P, Foley H D, Belyakov I M, Berzofsky J A, Pomerantz R J, Schnell M J. Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelope protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelope proteins from different HIV-1 isolates. J Virol. 2001;75:4430–4434. doi: 10.1128/JVI.75.9.4430-4434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto K, Hooper D C, Spitsin S, Koprowski H, Dietzschold B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol. 1999;73:510–518. doi: 10.1128/jvi.73.1.510-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossman S P, Bex F, Berglund P, Arthos J, O'Neil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljestrom P, Hoover E A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 32.Natuk R J, Chanda P K, Lubeck M D, Davis A R, Wilhelm J, Hjorth R, Wade M S, Bhat B M, Mizutani S, Lee S, et al. Adenovirus-human immunodeficiency virus (HIV) envelope recombinant vaccines elicit high-titered HIV-neutralizing antibodies in the dog model. Proc Natl Acad Sci USA. 1992;89:7777–7781. doi: 10.1073/pnas.89.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto L A, Berzofsky J A, Fowke K R, Little R F, Merced-Galindez F, Humphrey R, Ahlers J, Dunlop N, Cohen R B, Steinberg S M, Nara P, Shearer G M, Yarchoan R. HIV-specific immunity following immunization with HIV synthetic envelope peptides in asymptomatic HIV-infected patients. AIDS. 1999;13:2003–2012. doi: 10.1097/00002030-199910220-00002. [DOI] [PubMed] [Google Scholar]
- 35.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putkonen P, Quesada-Rolander M, Leandersson A C, Schwartz S, Thorstensson R, Okuda K, Wahren B, Hinkula J. Immune responses but no protection against SHIV by gene-gun delivery of HIV-1 DNA followed by recombinant subunit protein boosts. Virology. 1998;250:293–301. doi: 10.1006/viro.1998.9379. [DOI] [PubMed] [Google Scholar]
- 37.Qiu J-T, Liu B, Tian C, Pavlakis G N, Yu X-F. Enhancement of primary and secondary cellular immune responses against human immunodeficiency virus type 1 Gag by using DNA expression vectors that target Gag antigen to the secretory pathway. J Virol. 2000;74:5997–6005. doi: 10.1128/jvi.74.13.5997-6005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu J T, Song R, Dettenhofer M, Tian C, August T, Felber B K, Pavlakis G N, Yu X F. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73:9145–9152. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinnan G V, Jr, Zhang P F, Fu D W, Dong M, Alter H J. Expression and characterization of HIV type 1 envelope protein associated with a broadly reactive neutralizing antibody response. AIDS Res Hum Retroviruses. 1999;15:561–570. doi: 10.1089/088922299311088. [DOI] [PubMed] [Google Scholar]
- 40.Robinson H L. DNA vaccines for immunodeficiency viruses. AIDS. 1997;11:S109–S119. [PubMed] [Google Scholar]
- 41.Rose N F, Roberts A, Buonocore L, Rose J K. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J Virol. 2000;74:10903–10910. doi: 10.1128/jvi.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. . (Erratum, 1:598.) [DOI] [PubMed] [Google Scholar]
- 43.Rowland-Jones S L, Dong T, Dorrell L, Ogg G, Hansasuta P, Krausa P, Kimani J, Sabally S, Ariyoshi K, Oyugi J, MacDonald K S, Bwayo J, Whittle H, Plummer F A, McMichael A J. Broadly cross-reactive HIV-specific cytotoxic T-lymphocytes in highly exposed persistently seronegative donors. Immunol Lett. 1999;66:9–14. doi: 10.1016/s0165-2478(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 45.Schnell M J, Foley H D, Siler C A, McGettigan J P, Dietzschold B, Pomerantz R J. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci USA. 2000;97:3544–3549. doi: 10.1073/pnas.050589197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedwick W D, Wiktor T J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis, and other ribonucleic acid viruses in BHK-21/13.S agarose suspensions. J Virol. 1967;1:1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanCott T C, Mascola J R, Loomis-Price L D, Sinangil F, Zitomersky N, McNeil J, Robb M L, Birx D L, Barnett S. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J Virol. 1999;73:4640–4650. doi: 10.1128/jvi.73.6.4640-4650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner R, Teeuwsen V J, Deml L, Notka F, Haaksma A G, Jhagjhoorsingh S S, Niphuis H, Wolf H, Heeney J L. Cytotoxic T cells and neutralizing antibodies induced in rhesus monkeys by virus-like particle HIV vaccines in the absence of protection from SHIV infection. Virology. 1998;245:65–74. doi: 10.1006/viro.1998.9104. [DOI] [PubMed] [Google Scholar]
- 50.Weiner D B, Kennedy R C. Genetic vaccines. Sci Am. 1999;281:50–57. doi: 10.1038/scientificamerican0799-50. [DOI] [PubMed] [Google Scholar]
- 51.Yoshino K, Taniguchi S, Arai K. Autointerference of rabies virus in chick embryo fibroblasts Proc. Soc Exp Biol Med. 1966;123:387–392. doi: 10.3181/00379727-123-31496. [DOI] [PubMed] [Google Scholar]
- 52.zur Megede J, Chen M C, Doe B, Schaefer M, Greer C E, Selby M, Otten G R, Barnett S W. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J Virol. 2000;74:2628–2635. doi: 10.1128/jvi.74.6.2628-2635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]