Abstract
Cannabidiol (CBD) is a non-psychotropic cannabinoid with multiple pharmacological properties. Cannabidiol has attracted growing attention in the cosmetic industry, with an increasing number of CBD-containing skincare products on the market in recent years. The aim of this review is to evaluate the current evidence on the use of CBD for cosmetic purposes. Following an overview of CBD and the endocannabinoid system in the skin, we summarize pre-clinical and clinical studies that address the potential of CBD in cosmetic dermatology. Available in vitro and in vivo evidence suggests that CBD has anti-oxidant, anti-inflammatory, moisturizing, anti-acne, wound-healing, and anti-aging properties. However, only a few clinical studies have been conducted on the use of CBD in the skin. In addition, there is a critical need to develop an efficient drug-delivery system for topical/transdermal application of CBD. Further research, including clinical and pharmacokinetic studies, are needed to fully evaluate the role of CBD in cosmetic dermatology.
Key Points
| Cannabidiol has potential anti-oxidant, anti-inflammatory, moisturizing, anti-acne, wound-healing, and anti-aging properties. |
| Clinical and pharmacokinetic studies are warranted to further evaluate the use of cannabidiol in cosmetic dermatology. |
Introduction
Cannabinoids are biologically active compounds that bind to the cannabinoid receptors in the human body. They are largely classified as phytocannabinoids (isolated from the plant Cannabis sativa), endocannabinoids (produced in the human body), and synthetic cannabinoids (chemically synthesized) [1]. Cannabidiol (CBD) is one of the most abundant phytocannabinoids in C. sativa along with delta-9-tetrahydrocannabinol [2, 3]. Unlike delta-9-tetrahydrocannabinol, CBD has no psychoactive effects and demonstrates good tolerability. Growing evidence also suggests that CBD has multiple pharmacological properties, including analgesic, neuroprotective, and immunomodulatory effects [4–6]. Therefore, the medical use of CBD has received significant attention in recent years [7].
Previous studies have shown that cannabinoid receptors are broadly expressed and have endogenous ligands in the skin, suggesting that the skin has its own endocannabinoid system (ECS) [8–10]. Indeed, skin diseases are considered an important indication for CBD. Thus far, preliminary clinical studies have investigated the efficacy of CBD in psoriasis, atopic dermatitis, seborrheic dermatitis, systemic sclerosis, epidermolysis bullosa, and pyoderma gangrenosum with overall positive results [11–15].
Moreover, CBD is gaining popularity in the cosmetic industry: an increasing number of CBD-containing skincare products have been on the market in recent years [16]. However, many of them make unsubstantiated claims about their benefit to the skin [17]. This situation prompts the need to evaluate the current evidence on the use of CBD for cosmetic purposes. In this study, we review the available data from pre-clinical and clinical studies to help understand the potential of CBD in cosmetic dermatology.
Cannabidiol (CBD) and the Endocannabinoid System (ECS) in the Skin
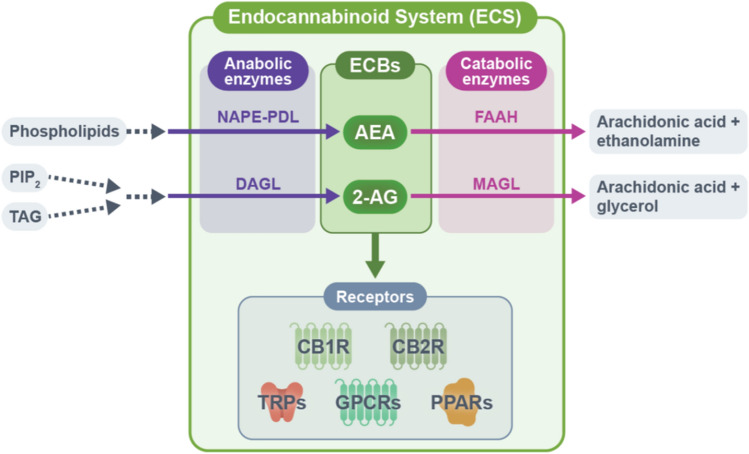
Researchers have identified two G protein-coupled receptors of cannabinoids: cannabinoid receptor 1 (CB1R) and cannabinoid receptor 2 (CB2R) [18, 19]. These receptors are differently expressed in various cell types in the skin, such as keratinocytes, melanocytes, sebocytes, and fibroblasts. Cannabinoid receptor 1 and cannabinoid receptor 2 are also present in cutaneous nerves and skin immune cells [20]. In addition, several orphan G protein-coupled receptors (GPR18, GPR55, and GPR119), transient receptor potential channels (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8, and TRPA1), and peroxisome proliferator-activated receptors (PPARα and PPARγ) that mediate the effects of cannabinoids are distributed in the skin [21–25]. The expression of N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), the major endocannabinoids, along with their anabolic and catabolic enzymes (e.g., N-acylphosphatidylethanolamine phospholipase D [NAPE-PLD], diacylglycerol lipases [DAGLα and DAGLβ], fatty acid amide hydrolase [FAAH], and monoacylglycerol lipase [MAGL]) are also found in the skin [20, 26–28]. These data support the notion that the skin has its own ECS consisting of endocannabinoids, cannabinoid receptors, and metabolic enzymes for endocannabinoids (Fig. 1) [8].
Fig. 1.
Key components of the endocannabinoid system (ECS). The ECS consists of endocannabinoids, endocannabinoid receptors, and anabolic and catabolic enzymes of the endocannabinoids. N-arachidonoylethanolamine (AEA) and N-arachidonoylethanolamine (2-AG) represent two major endocannabinoids in the human body. N-arachidonoylethanolamine is synthesized from phospholipids by several pathways, the major pathway involving the anabolic enzyme N-acylphosphatidylethanolamine phospholipase D (NAPE-PDL). N-arachidonoylethanolamine is mainly degraded to arachidonic acid and ethanolamine by fatty acid amide hydrolase (FAAH). The synthesis of 2-AG involves a signaling pathway starting from phosphatidylinositol-4,5-bisphosphate (PIP2) and triacylglycerol (TAG) and is mediated by diacylglycerol lipase (DAGL)-α and DAGLβ. N-arachidonoylethanolamine is mainly degraded to arachidonic acid and glycerol by monoacylglycerol lipase (MAGL). N-arachidonoylethanolamine and 2-AG exert diverse physiological actions through cannabinoid receptors including cannabinoid receptor 1 (CB1R), cannabinoid receptor 2 (CB2R), transient receptor potential channels (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8, and TRPA1), orphan G protein-coupled receptors [GPCRs] (GPR18, GPR55, and GPR119), and peroxisome proliferator-activated receptors (PPARα and PPARγ). ECB endocannabinoid, TRP transient receptor potential
Regarding the mechanisms of action of endocannabinoids, previous studies have indicated that AEA is a partial agonist at CB1R and a weak agonist at CB2R, while 2-AG is a full agonist at CB1R and CB2R [29–31]. In addition to these classical endocannabinoids, endocannabinoid-like compounds, such as N-palmitoylethanolamide (PEA) and oleoylethanolamide share the same synthesis and degradation enzymes as endocannabinoids and play an important role in the ECS [32–34]. Specifically, N-PEA enhances the physiological effects of AEA by serving as an alternative substrate for FAAH. This mechanism is known as the ‘entourage effect’ [35]. Although endocannabinoids and endocannabinoid-like compounds are lipophilic and require inter-cellular and intra-cellular carriers, their transporter system has not been well characterized [36].
The ECS has been shown to be closely involved in skin homeostasis through various mechanisms [37, 38]. For instance, CB1R activation by low concentrations of AEA (1 μM) inhibits the differentiation of two-dimensional cultured human keratinocytes [39]. N-arachidonoylethanolamine also prevents the up-regulation of several differentiation markers, including K1 and K10, in a CB1R-dependent manner [40]. In contrast, higher concentrations (3–30 μM) of AEA suppress the proliferation of primary human epidermal keratinocytes and induce their apoptosis through sequential activation of CB1R and TRPV1 [41]. Karsak et al. demonstrated in vivo that 2,4-dinitrofluorobenzene-induced allergic inflammation was exacerbated in CB1R and CB2R double knock-out mice, while inflammation was attenuated in FAAH knock-out mice with elevated AEA levels [42], indicating a protective role of the ECS in allergic inflammation in the skin. In addition, the suppressive role of the ECS in fibrosis was demonstrated in vivo, where bleomycin-induced dermal fibrosis was aggravated in CB2R knock-out mice, while a selective CB2R agonist attenuated these changes in bleomycin-treated wild-type mice [43]. These data demonstrate the diverse roles of the ECS in skin homeostasis. As CBD shares some receptors with endocannabinoids, it is important to consider the potential influence of CBD on the ECS in the skin. Specifically, the biological effects of CBD are primarily related to the ECS, but CBD does not bind to the orthostatic binding site of CB1R and CB2R with high affinity [38, 44]. Instead, CBD binds to the allosteric binding site of CB1R and CB2R, possibly acting as a negative allosteric modulator at these receptors [45, 46].
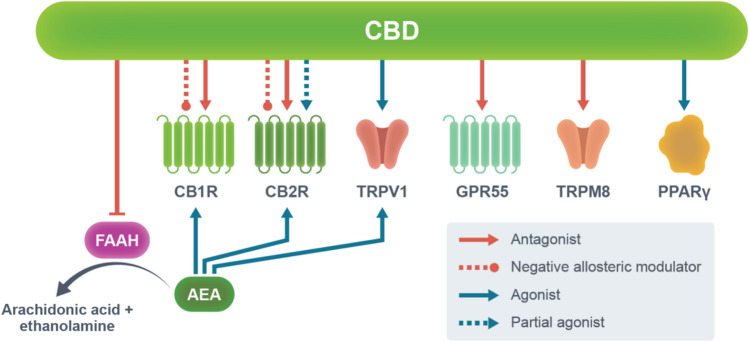
Additionally, CBD has been shown to act as an antagonist/inverse agonist at CB1R and CB2R, while it may also behave as a partial agonist at CB2R [46–48]. Another important role of CBD in the ECS is to target fatty acid-binding proteins that facilitate the transportation of AEA to its catabolic enzyme fatty acid amide hydrolase [49, 50]. This then leads to an increase in extracellular AEA levels, followed by the activation of CB1R, CB2R, and TRPV1 [51]. Cannabidiol is also known as an agonist of PPARγ [52] and TRPVs (TRPV1, TRPV2, TRPV3, TRPV4, and TRPA1) [49, 53, 54], and an antagonist of TRPM8 [55] and GPR55 [56] (Fig. 2; Table 1). However, the exact mechanisms of action of CBD are still elusive.
Fig. 2.
Main molecular targets of cannabidiol (CBD). The mechanisms of action of CBD primarily involve the endocannabinoid system. Unlike endocannabinoids, CBD does not bind to the orthostatic binding site of cannabinoid receptor 1 (CB1R) and cannabinoid receptor 2 (CB2R) with high affinity. Instead, CBD binds to the allosteric binding site of CB1R and CB2R, possibly acting as a negative allosteric modulator at these receptors. Cannabidiol also acts as an antagonist/inverse agonist at CB1R and CB2R, while it may also act as a partial agonist at CB2R. Importantly, CBD inhibits the activity of fatty acid amide hydrolase (FAAH), leading to an increase in extracellular N-arachidonoylethanolamine (AEA) levels and subsequent activation of CB1R, CB2R, and transient receptor potential cation channel subfamily V member 1 (TRPV1). Cannabidiol also acts as an agonist of transient receptor potential channels (TRPV1, TRPV2, TRPV3, TRPV4, and TRPA1) and peroxisome proliferator-activated receptor gamma (PPARγ), while acting as an antagonist of G protein-coupled receptor 55 (GPR55) and transient receptor potential cation channel subfamily M member 8 (TRPM8). However, the exact mechanisms of action of CBD remain elusive
Table 1.
Molecular targets of CBD in the skin
| Receptor | Affinity of CBD | Function of CBD |
|---|---|---|
| GPCR | ||
| CB1R | Ki = 3.3–4.9 mM | Inverse agonist/antagonist [47] |
| IC50 = 0.27–0.96 mM | Negative allosteric modulator [45] | |
| CB2R | Ki = 4.3 µM | Antagonist [46] |
| EC50 = 503 nM | Inverse agonist [47] | |
| IC50 = 3 nM | Negative allosteric modulator [46] | |
| PPARs | ||
| PPARα | N.D. | Partial agonist [153] |
| PPARγ | EC50 = 2010 nM | Agonist [52] |
| TRPs | ||
| TRPV1 | EC50 = 1000 nM | Agonist [53] |
| TRPV2 | EC50 = 1250 nM | Agonist [53] |
| TRPV3 | EC50 = 3700 nM | Agonist [54] |
| TRPV4 | EC50 = 800 nM | Agonist [54] |
| TRPA1 | EC50 = 110 nM | Agonist [53] |
| TRPM8 | IC50 = 160 nM | Antagonist [55] |
CB1R cannabinoid receptor type 1, CB2R cannabinoid receptor type 2, CBD cannabidiol, PPARα peroxisome proliferator-activated receptor alpha, PPARγ peroxisome proliferator- activated receptor gamma, TRPA1 transient receptor potential ankyrin 1, TRPM8 transient receptor potential cation channel 8, TRPV1 transient receptor potential vanilloid type 1, TRPV2 transient receptor potential vanilloid type 2, TRPV3 transient receptor potential vanilloid type 3, TRPV4 transient receptor potential vanilloid type 4
The Potential of CBD in Cosmetic Dermatology
Antioxidant Properties
Oxidative stress reflects an imbalance between reactive oxygen species generation and the antioxidant capacity of the body. The skin serves as a protective barrier to oxidative insults such as ultraviolet (UV) radiation and air pollution. Excessive oxidative stress causes cell damage, leading to chronic inflammation and aging in the skin [57, 58].
Several studies evaluated the antioxidant properties of CBD by using UV and hydrogen peroxide (H2O2) as oxidative stressors. Cannabidiol reduced cell death in keratinocytes and melanocytes following UV exposure [59]. Cannabidiol was also shown to attenuate the redox imbalance of UV-irradiated keratinocytes by reducing the generation of reactive oxygen species, enhancing the efficiency of the antioxidant thioredoxin system, and increasing vitamin A and E levels [60]. In addition, CBD accumulated within the cellular membrane of keratinocytes, preventing the increase in lipid peroxidation after the exposure to UV and H2O2 [61]. Moreover, CBD promoted the activation of nuclear factor erythroid 2-related factor 2, a master regulator of antioxidant response in keratinocytes [62]. Indeed, CBD was able to induce the antioxidant enzyme heme oxygenase 1, a major target gene of nuclear factor erythroid 2-related factor 2, in both in vitro and in vivo settings. This induction was attributed to the CBD-mediated degradation of the transcription factor BTB And CNC Homology 1 (BACH1), a negative regulator of heme oxygenase 1 [63]. In a study of two-dimensional and three-dimensional cultured fibroblasts, CBD up-regulated antioxidant PPAR-γ expression while suppressed proinflammatory nuclear factor-kappa B (NF-κB) expression [64]. Taken together, these data suggest that CBD plays a protective role against oxidative stress in the skin.
Anti-inflammatory Properties
Nuclear factor-kappa B is a ubiquitously expressed transcription factor that plays a key role in inflammation [65]. An in vitro study revealed that CBD interfered with NF-κB activity in tumor necrosis factor-alpha (TNF-α)-challenged keratinocytes, down-regulating NF-κB-dependent metalloproteinase-9 expression. Additionally, CBD attenuated the expression of 15 out of 26 TNF-α-induced genes in keratinocytes. Cannabidiol did not influence NF-κB activity or metalloproteinase-9 expression but attenuated the expression of 11 out of 16 up-regulated genes in TNF-α-challenged dermal fibroblasts [66]. In poly-(I:C)-stimulated keratinocytes, CBD up-regulated the expression of AEA and dose-dependently inhibited the expression of monocyte chemotactic protein-2 and inflammatory cytokines including interleukin (IL)-6, IL-8, and TNF-α. This inhibition was reversed by CB2R and TRPV1 antagonists, suggesting the involvement of ECS in anti-inflammatory activities of CBD [67].
A few studies have evaluated the anti-inflammatory properties of CBD in in vivo settings. In a carrageenan-induced inflammation rat model, 1% topical CBD gel significantly attenuated paw edema with reduced lymphocyte infiltration into the skin [68]. Similar results were obtained in carrageenan-induced mice that were treated with CBD-loaded ethosomal carriers to enhance its permeation and accumulation in the skin [69].
Several in vivo studies examined the roles of CB1R and CB2R in the anti-inflammatory properties of CBD in the skin [70]. Leonti et al. showed that a CB1R receptor antagonist polyyne falcarinol aggravated histamine-induced urticarial lesions, suggesting that CB1R mediates the anti-inflammatory properties of CBD in the skin [71]. This is also supported by the attenuation of skin inflammation by a topical CB1R-specific agonist in an oxazolone-induced atopic dermatitis model [72]. However, the role of CB2R in the anti-inflammatory properties of CBD remains controversial. For instance, Oka et al. showed that a CB2R agonist suppressed 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mouse ears [73]. However, other studies demonstrated that a CB2R agonist increased allergic inflammation in mice [42, 74]. Overall, these results highlight the need to further explore the roles of CB1R and CB2R in the anti-inflammatory properties of CBD. Clinical evidence for anti-inflammatory properties of CBD is limited to a few studies of psoriasis, atopic dermatitis, and seborrheic dermatitis. The use of a shampoo containing 0.075% CBD significantly reduced the severity and symptoms of scalp inflammation in patients with mild-to-moderate scalp psoriasis (n = 22) and seborrheic dermatitis (n = 28) [13]. In another study, the use of 1% CBD-infused gel significantly improved the Eczema Area and Severity Index score and pruritus scales in patients with atopic dermatitis (n = 14) [75]. In a study of psoriasis (n = 5), atopic dermatitis (n = 5), and resulting scars (n = 10), CBD-enriched ointment significantly reduced the Psoriasis Area and Severity Index score in patients with psoriasis [11]. These clinical data, although preliminary, support the therapeutic effects of CBD for skin inflammation.
Moisturizing Properties
Dermal water content is an important biophysical parameter for the integrity of the skin. The loss of dermal water leads to reduced skin elasticity, wrinkle formation, and skin aging [76]. Ikarashi et al. showed that the topical application of CBD significantly increased the dermal water content in vivo. This increase was accompanied by an increase in aquaporin-3, an important channel for skin water retention [77]. Although aquaporin-3 expression has been shown to be up-regulated by PPARγ agonists, there was no change in the messenger RNA expression level of Pparg in mouse skin treated with CBD [78]. In addition, a recent study by Jang et al. demonstrated that CBD increased the expression of filaggrin and involucrin in both keratinocytes and epidermal equivalents [79]. The moisturizing properties of CBD were also supported by a clinical study reporting that the CBD-containing ointment significantly decreased the transepidermal water loss and improved skin hydration and elasticity (n = 20) [11], but the underlying mechanisms are unknown. Therefore, further studies are needed to understand the mechanisms of the moisturizing effects of CBD in the skin, including which receptors mediate the up-regulated expression of AQP-3, filaggrin, and involucrin by CBD.
Acne
Acne is a common skin condition characterized by increased sebum production and inflammation of the sebaceous glands. The expression of CB1R, CB2R, and major metabolic enzymes of endocannabinoids were detected in human sebaceous glands [41, 80, 81]. In addition, a previous study demonstrated that AEA promoted lipogenesis in human sebocytes at low concentrations while inducing apoptosis at high concentrations [82]. Therefore, the ECS is thought to play an important role in the homeostasis of the sebaceous gland.
Oláh et al. showed that CBD inhibited the lipogenic actions of various compounds, such as arachidonic acid and a mixture of linoleic acid and testosterone, in cultured sebocytes and skin organ culture. Cannabidiol was also able to suppress sebocyte proliferation by activating TRPV4 channels. TRPV4 activation interfered with the pro-lipogenic ERK1/2 MAPK pathway, leading to the down-regulation of nuclear receptor interacting protein-1 and subsequent inhibition of sebocyte lipogenesis. Additionally, CBD exerted anti-inflammatory actions via A2A adenosine receptor-dependent up-regulation of tribbles homolog 3 and inhibition of the NF-κB signaling pathway [83]. In addition to excessive sebaceous gland activity, a skin microbiome imbalance may mediate the pathogenesis of acne. In this context, CBD inhibited the expression of inflammatory cytokines (IL-6, IL-8, and TNF-α) in keratinocytes stimulated with extracellular vesicles of Cutibacterium acnes, whose overgrowth is associated with acne [84]. This inhibition was mediated by the activation of CB2R, enhanced by a TRPV1 antagonist, and accompanied by inactivation of the MAPK and NF-κB signaling pathways [85]. A recent study also demonstrated that CBD selectively killed a subset of Gram-negative bacteria, supporting the anti-microbial properties of CBD [86]. Regarding the concentration of CBD, Oláh et al. showed that CBD significantly reduced the overall proliferation of sebocytes at 1–10 μM doses, while higher doses of CBD (50 μM) resulted in apoptosis-driven cytotoxicity [83]. A recent study by Cohen et al. also examined the effect of CBD at different concentrations (0.3125–10 µg/mL) on C. acnes growth and demonstrated that concentrations of 5 and 10 μg/mL of CBD specifically attenuated C. acnes growth [87]. These results suggest that there are optimal concentrations of CBD for the treatment of acne. In addition, other non-psychotropic phytocannabinoids including cannabigerol and cannabidivarin have also been suggested to serve as anti-acne agents [88, 89].
Following the results of experimental studies, a 5% formulation of CBD (BTX 1503) is under clinical evaluation. In this open-label, single-arm, 28-day evaluation, 23 patients with moderate-to-severe acne were treated with a twice-daily application of BTX 1503 to the entire face. The results have not been published yet, but preliminary data showed that BTX 1503 was safe and well tolerated [90].
Wound Healing
Wound healing is a complex process consisting of the following three overlapping processes: inflammatory reaction, cell proliferation, and tissue remodeling [91]. A recent study demonstrated in vitro that CBD improved wound healing in both healthy and stress-induced premature senescent dermal fibroblasts [92]. In another study, researchers developed a CBD-containing alginate-based hydrogel for wound healing. The hydrogel successfully scavenged free radicals and decreased the expression of TNF-α, IL-6, and IL-1β in umbilical vein endothelial cells. In in vivo experiments, the hydrogel enhanced the wound-healing process by controlling the inflammatory infiltration, promoting the collagen deposition, and facilitating the formation of blood vessels [93]. In clinical settings, Chelliah et al. reported three pediatric cases of epidermolysis bullosa, in which topical CBD improved wound healing [14]. Further studies are clearly needed to evaluate the effects of CBD on wound healing.
Skin Aging
Skin aging is a degenerative phenomenon resulting from continuous exposure to intrinsic (e.g., age, genetics, and hormones) and extrinsic factors (e.g., UV, air pollution, and tobacco smoking). It causes undesirable appearance changes such as laxity, xerosis, lentigines, and coarse wrinkles [94, 95]. As the population ages, there is a growing demand for anti-aging products in cosmetic dermatology [96].
A previous study reported that CB1R knock-out mice showed atrophy of the subdermal fat layer along with early-onset memory impairment, suggesting the role of the endocannabinoid system in anti-aging [88]. Researchers also demonstrated that CBD attenuated the up-regulation of multiple cellular senescence biomarkers including β-galactosidase and cyclin D1 in dermal fibroblasts following the exposure to H2O2 [92]. The same group further evaluated the anti-aging properties of CBD in combination with nutrient signaling regulators such as metformin, resveratrol, and rapamycin [97]. They found that CBD combined with triacetylresveratrol significantly increased viability, reduced metabolic dysfunction, and attenuated nuclear eccentricity of senescent fibroblasts. Another group assessed the anti-aging properties of a CBD-containing and eicosapentaenoic acid-containing gel in in vitro, ex vivo, and clinical settings [98]. Cannabidiol significantly inhibited the UV-induced secretion of IL-8 and prostaglandin E2 (PGE2), the two major inflammatory agents associated with photo-aging, from keratinocytes. Additionally, this inhibition was potentiated by eicosapentaenoic acid. In ex vivo skin culture, a mixture of CBD and eicosapentaenoic acid enhanced the extracellular matrix remodeling with reduced IL-8 and PGE2 following UV exposure. In a clinical evaluation, a CBD and PEA mixture reduced crow’s feet wrinkle area and volume, fine line wrinkle volume, and age-dependent subepidermal low-echogenic band in 33 women aged 45–65 years. Collectively, these data support the rejuvenating properties of CBD in the skin, which may be more potent when combined with other biologically active compounds.
Skin Malignancy
In the aging population, potential therapeutic use of CBD for malignancy also attracts growing attention [70]. For instance, Laborada and Cohen reported a case of cutaneous squamous cell carcinoma and lichen simplex chronicus that were both successfully treated with a topical application of CBD [99]. Consistently, several phytocannabinoids, endocannabinoids, and synthetic cannabinoids were demonstrated to decrease the growth of non-melanoma skin cancer and melanoma through cannabinoid receptor-dependent and receptor-independent pathways [100, 101]. However, Zheng et al. demonstrated that mice deficient in CB1R and CB2R had significantly lower rates of UVB-induced inflammation and skin carcinogenesis compared to wild-type mice [102]. Therefore, therapeutic potential of CBD for skin cancer warrants further investigation.
Clinical Studies on the Use of CBD in the Skin
Currently, there are five ongoing clinical trials using CBD in dermatology: one for acne vulgaris (NCT06362889), one for atopic dermatitis (NCT06022874), one for severe pruritis (NCT06435299), and two for scar healing (NCT05650697, NCT06129591). NCT06362889 is a phase I study to evaluate the safety and efficacy of microneedling with CBD and hempseed oil for acne vulgaris [103]. NCT06022874 is an observational study to evaluate the potential therapeutic effects of topical CBD products for atopic dermatitis [104]. NCT06435299 is a phase III study to evaluate the safety and efficacy of CBD oil for severe pruritus [105]. NCT05650697 and NCT06129591 are phase I studies to evaluate the effects of topical CBD oil on paramedian forehead flap scar healing [106, 107].
Delivery System of CBD to the Skin
There are two CBD-based products approved by the US Food and Drug Administration to date: (1) Sativex®, a combination of CBD and delta-9-tetrahydrocannabinol available as an oromucosal spray and approved for muscle spasticity in multiple sclerosis [108, 109] and (2) Epidolex®, a CBD extract available as an oral solution and approved for Lennox–Gastaut syndrome and Dravet syndrome in individuals aged 2 years and older [110, 111]. Other delivery routes, including nasal [112], sublingual [113], and inhalational [114] administration have also been explored for CBD. For dermatological and cosmetic applications, topical and transdermal routes are considered ideal to achieve high local concentrations with minimal systemic side effects. Moreover, these routes can avoid the problems of the conventional oral route, such as acid degradation and first-pass metabolism [115, 116]. However, delivering the highly lipophilic CBD through the stratum corneum into deeper skin layers is somewhat challenging [117].
The use of chemical penetration enhancers is a popular approach to improve the transdermal drug delivery. They modify the intercellular lipid domain of the stratum corneum by fluidization, polarity alteration, lipid extraction, and/or phase separation, temporally disrupting the major barricade of the skin [118]. Indeed, researchers reported the enhanced transdermal delivery of CBD in combination with boswellic acid [119], hyaluronic acid (with silicone oil) [120], and volatile oils including emu oil and eucalyptus oil [121]. The use of argan oil [122], essential oil [123], mineral oil [124], sunflower oil [125], hydroxy acids [126], non-ionic surfactants [127], and monohydric alcohol solution [128] was also explored for various formulations of cannabinoids. Another strategy for efficient transdermal delivery is to encapsulate drugs into nanoparticulate carriers that deliver them to the desired depth. In addition to ethosomes, which were shown to facilitate the transdermal delivery of CBD [129], several nanoparticulate carriers such as liposomes, niosomes, and lipid nanoparticles might provide a potential transdermal delivery system for CBD [130–133]. In addition, physical enhancement strategies might be used for CBD, alone or in combination with the passive delivery systems mentioned above. These strategies include microneedles, electroporation, iontophoresis, magnetophoresis, laser ablation, and ultrasound transport [134–137]. In summary, although the skin represents a viable route for the delivery of CBD, especially for cosmetic indications, further studies are needed to explore the emerging delivery systems and conquer its low penetration into the skin.
Adverse Effects
The adverse effects (AEs) of oral CBD have been extensively reviewed in the literature [138–141]. In a recent systematic review of 12 randomized controlled trials involving 745 participants, AEs associated with oral CBD were mild or modest in nine studies [138]. The most common AEs with an incidence of ≥10% included gastrointestinal symptoms (59.5%), somnolence (16.7%), loss of appetite (16.5%), hypertransaminasemia (12.8%), and fatigue (11.4%). Serious AEs included hypertransaminasemia with serum alanine aminotransferase/aspartate aminotransferase levels three times higher than the upper limit of normal (6.4%), seizures (1.3%), and rash (1.1%). All serious AEs were reported in three studies in which CBD was administered as an add-on therapy to anticonvulsant medications. As CBD and some anticonvulsants are metabolized by the same cytochrome P450 enzymes [142, 143], the authors partially attributed these AEs to the potential interactions between CBD and concomitant medications.
There have been no systematic reviews focusing on the AEs of topical/transdermal CBD, but the available limited evidence suggests that it is safe and well tolerated [144]. In a clinical study of topical CBD for digital ulcers in systemic sclerosis, 7 of 25 patients (28%) reported mild AEs of itch and perilesional erythema, but none of the patients discontinued the CBD treatment [145]. No patients experienced severe AEs. In particular, no alteration of the perilesional skin was observed on a physical examination. Other clinical studies of topical/transdermal CBD showed that there were no AEs associated with the CBD treatment [11–13].
Overall, CBD seems to have a favorable safety and tolerability profile, especially in topical/transdermal formulations. Given the increasing global demand for CBD in cosmetic dermatology, the safety of CBD should be further investigated in clinical trials with larger sample sizes, multiple doses, and different delivery systems.
Discussion
In this review, we provided an overview of the literature on the potential of CBD for cosmetic purposes (Figs. 3 and 4; Table 2). Accumulating pre-clinical data and emerging clinical evidence support the therapeutic potential of CBD in acne, wounds, dry skin, oxidation, inflammation, and skin aging. Available evidence also suggests that CBD is safe and well tolerated. While topical/transdermal drug formulations are desirable for CBD, delivering the highly lipophilic compound into deeper skin layers is challenging. New strategies and technologies should be further explored for the efficient delivery of CBD via the skin.
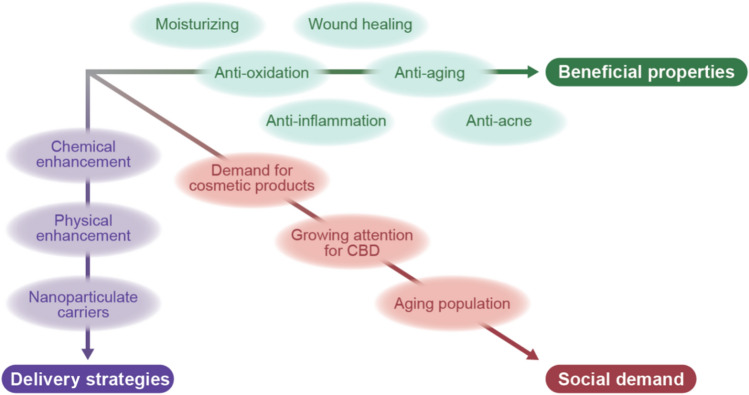
Fig. 3.
Three strategic axes for the use of cannabidiol (CBD) in cosmetic dermatology. The multi-faceted beneficial roles of CBD in the skin, the development of delivery technologies, and the increasing social demand would prompt the use of CBD in cosmetic dermatology
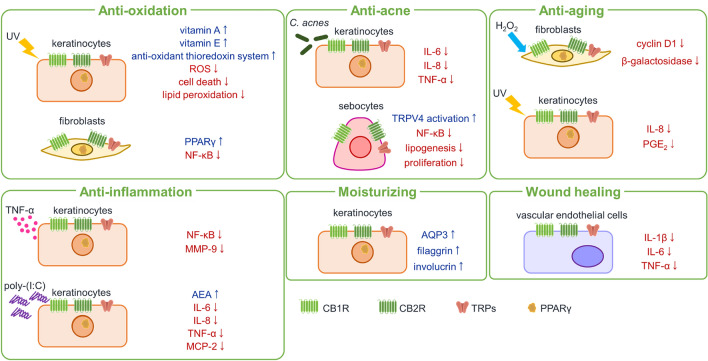
Fig. 4.
Schematic of the anti-oxidant, anti-inflammatory, moisturizing, anti-acne, wound-healing, and anti-aging actions of cannabidiol in the skin. AEA N-arachidonoylethanolamine, AQP3 aquaporin 3, CB1R cannabinoid receptor 1, CB2R cannabinoid receptor 2, C. acnes Cutibacterium acnes, H2O2 hydrogen peroxide, IL interleukin, MCP-2 monocyte chemotactic protein-2, MMP-9, metalloproteinase-9, NF-κB nuclear factor-kappa B, PGE2 prostaglandin E2, PPARγ peroxisome proliferator-activated receptor gamma, ROS reactive oxygen species, TNF-α tumor necrosis factor-alpha, TRP transient receptor potential, TRPV4 transient receptor potential cation channel subfamily V member 4, UV ultraviolet
Table 2.
Summary of studies investigating the cosmetic properties of CBD
| Evidence | Model/setting | Concentration/dose | Result | Biological context | References |
|---|---|---|---|---|---|
| In vitro | Human keratinocytes (PCS 200-013) and melanocytes (PCS-200-011), exposed to UVB (60 mJ/cm2) | 4 µM | ↑ Cell viability in a dose-dependent manner in both keratinocytes and melanocytes; CBD did not show absorption in the UVB spectra | Oxidative stress (UV) | [59] |
| In vitro | Human keratinocytes, exposed to UVA (30 J/cm2) and UVB (60 mJ/cm2) | 4 µM | ↓ ROS; ↑ thioredoxin-dependent system, vitamins A and E | Oxidative stress (UV) | [60] |
| In vitro | Human keratinocytes (CDD 1102 KERTr), exposed to UVB (60 mJ/cm2) and H2O2 (200 µM) | 4 µM | ↓ Lipid peroxidation, LDH release; ↑ PUFA; CBD penetrated keratinocytes and accumulated within the cellular membrane | Oxidative stress (UV, H2O2) | [61] |
| In vitro | Human keratinocytes (CDD 1102 KERTr), exposed to UVA (30 J/cm2) and UVB (60 mJ/cm2) | 1 µM | ↑ NRF-2 and HOMX1 expression; ↓ NF-κB pathway | Oxidative stress (UV) | [62] |
| In vitro/in vivo | NHEK, HaCaT cells in vitro; BALB/cByJRj mice in vivo | 10 µM in vitro; 0.1–10% in vivo (topically, 1/day for 5 days) | ↑ HOMX1 expression through ↓ BACH1 in vitro; ↑ HOMX1 in vivo | Oxidative stress (endogenous peroxidase) | [63] |
| In vitro | 2D and 3D culture models of fibroblasts (CCD-25Sk), exposed to UVA (30 J/cm2) and UVB (60 mJ/cm2) | 4 µM | ↓ NF-κB pathway; ↑ PPARγ expression; CBD reduced the collagen degradation in both 2D and 3D fibroblast models | Oxidant stress (UV) | [64] |
| In vitro | HaCaT and HDF cells (PCS-201-012), treated with TNF-α (10 ng/mL) | 0.05–5 µM in HaCaT cells; 0.1–2.5 µM in HDF cells | In HaCaT cells: ↓ NF-κB, MMP-9; CBD down-regulated 15 out of 26 TNF-α-induced genes. In HDF cells: CBD down-regulated 11 out of 16 TNF-α-induced genes with no inhibition of NF-κB. | Inflammation (TNF-α) | [66] |
| In vitro | HaCaT cells, stimulated with poly-(I:C) [100 µg/mL] | 1–20 µM | ↑ AEA; ↓ MCP-2, IL-6, IL-8, and TNF-α in a CB2R-dependent and TRPV1-dependent manner | Inflammation (poly-[I:C]) | [67] |
| In vivo | Sprague-Dawley rats, carrageenan induction (0.1 mL, 1% w/v in saline, injected into the plantar of the right hind paw) | 1% gel | ↓ Paw edema, lymphocytic inflammation | Inflammation (carrageenan) | [68] |
| In vivo | CD1 nude mice, carrageenan induction | Ethosomal formulation (3% w/w CBD and 40% w/w EtOH in a carbomer gel) | ↓ Paw edema; transdermal absorption; detection in the plasma | Inflammation (carrageenan) | [69] |
| Clinical | Psoriasis (n = 5), atopic dermatitis (n = 5), and resulting scars (n = 10) | CBD-containing ointment (2/day for 3 months) | ↓ PASI in patients with psoriasis; ↓ number of papules and pustules in dermatological patients; CBD improved TEWL, hydration, and elasticity in all patients | Inflammation (psoriasis, atopic dermatitis); dry skin | [11] |
| Clinical | Mild-to-moderate scalp psoriasis (n = 22) and seborrheic dermatitis (n = 28) | 0.075% CBD in shampoo (2/day for 2 weeks) | ↓ Scores for arborizing vessels, twisted capillaries, and scales; ↓ scores for erythema and scaling; ↓ scores for itching and burning | Inflammation (psoriasis, seborrheic dermatitis) | [13] |
| Clinical | Atopic dermatitis (n = 16) | 1% CBD-infused gel (2/day for 2 weeks) | ↓ EASI score, VAS-Pruritus and 5-D Pruritus scales | Inflammation (atopic dermatitis) | [75] |
| In vivo | HR-1 mice | 1% CBD solution (2/day for 14 days) | ↑ Dermal water contents, AQP3 expression; CBD did not affect the expression levels of loricrin, filaggrin, and other moisturizing factors | Dry skin | [78] |
| In vitro | Human sebocytes (SZ95) | 1–10 µM | ↓ Lipogenic actions of arachidonic acid and a mixture of linoleic acid and testosterone; ↓ proliferation of sebocytes via TRPV4 activation, ERK1/2 MAPK pathway, and down-regulation of NRIP1; ↓ inflammation via the A2A adenosine receptor-dependent up-regulation of TRIB3 and inhibition of NF-κB | Acne | [154] |
| In vitro | NHEK, stimulated with Cutibacterium acnes-derived extracellular vesicles | 0.5–2 µM | ↓ IL-6, IL-8, and TNF-α expression via CB2R activation; ↓ MAPK and NF-κB signaling pathway | Acne | [85] |
| Clinical | Moderate-to-severe acne (n = 23) | 5% CBD formulation (2/day for 28 days) | Safe and well tolerated; the results have not been published | Acne | [90] |
| In vitro | Normal fibroblasts and SIPS fibroblasts (CCD-1064Sk and-1135Sk) | 2 µM | ↑ Wound healing in both healthy and SIPS fibroblasts; inhibits the change in nuclear architecture in both healthy and SIPS fibroblasts; ↓ β-galactosidase activity in SIPS fibroblasts but not in normal fibroblasts; ↓ cyclin D1 expression in normal fibroblast exposed to H2O2 |
Wound healing Aging |
[92] |
| In vitro/in vivo | HUVECs, mouse embryonic fibroblast cells of NIH 3T3 (in vivo); skin defect model for acute wound using Sprague Dawley rats (male, 8–10 weeks old) | Hydrogel dressing (CBD/Alg@Zn) | Scavenged DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals, ↓ inflammatory response (in vitro); facilitates the wound-healing process by ↓ inflammatory infiltration, ↑ collagen deposition, ↑ granulation tissue, and ↑ blood vessel formation (in vivo) | Wound healing; anti-oxidant stress | [148] |
| Clinical | Epidermolysis bullosa (n = 3) | Topical CBD | ↑ Wound healing | Wound healing | [14] |
| In vitro | SIPS fibroblasts (CCD-1064Sk) | 2 µM with or without rapamycin, metformin, or TRSV | CBD combined with TRSV up-regulated the viability of skin fibroblasts, wound-healing functional activity; ↓ metabolic dysfunction and nuclear eccentricity | Aging (oxidative stress) | [97] |
| In vitro/ex vivo/clinical | HaCaT, UVB exposure (60 mJ/cm2, in vitro); human skin organ culture, UVB exposure (350 mJ/cm2, ex vivo); female aged 45–65 years, n = 34 (clinical) | 10 µg/mL (in vitro); 0.1% (ex vivo and clinical) | CBD inhibited the secretion of PGE2 and IL-8 following UV exposure; PEA potentiated the inhibitory activity of CBD on PGE2 and IL-8 secretion (in vitro); ↑ ECM remodeling following UV exposure (ex vivo); ↓ crow’s feet wrinkle area and volume, fine line wrinkle volume, and age-dependent subepidermal low-echogenic band (clinical) | Aging (UV) | [98] |
2D two-dimensional, 3D three-dimensional, BACH1 BTB And CNC Homology 1, CBD cannabidiol, EASI Eczema Area and Severity Index, ECM extracellular matrix, HDF human dermal fibroblasts, HMOX1 heme oxygenase 1 gene, HUVEC human umbilical vein endothelial cell, LDH lactate dehydrogenase, NF-κB nuclear factor-kappa B, NHEK normal human epidermal keratinocytes, NRF-2 nuclear factor erythroid 2-like 2, NRIP1 nuclear receptor interacting protein-1, PASI Psoriasis Area and Severity Index, PUFA polyunsaturated fatty acid, PGE2 prostaglandin E2, ROS reactive oxygen species, SIPS stress-induced premature senescence, TEWL transepidermal water loss, TRIB3 tribbles homolog 3, TRSV triacetylresveratrol, UVA ultraviolet A, UVB ultraviolet B, VAS visual analog scale, ↑ increased, ↓ decreased
Previous studies have suggested that CBD is beneficial in the treatment of various diseases that range from infection and malignancy to autoimmune and degenerative disorders [6, 38, 146, 147]. The studies that we reviewed have shown that CBD also plays a protective role against oxidative and inflammatory stress [11, 13, 59–64, 66–69, 75, 148], which is not only responsive for a number of pathological conditions, but also relevant to healthy people, especially in terms of aging [149–151]. Indeed, CBD has been demonstrated to attenuate the age-related changes in keratinocytes and fibroblasts in vitro and human skin in vivo [92, 97, 98]. Given the aging population and the growing market of cosmetic products, the anti-aging properties of CBD in the skin will continue to draw attention [152]. However, the current evidence for the beneficial properties of CBD in the skin is largely limited to pre-clinical studies. Further clinical evidence, ideally from randomized controlled trials, should be accumulated in the future.
The efficient topical/transdermal delivery is another important aspect of the development of clinically viable CBD products [69, 112, 133]. Because of the lipophilicity of CBD, its transcutaneous permeation is highly limited without the aid of delivery strategies [117]. Data from other cannabinoids support the potential use of new technologies, including chemical enhancement, physical enhancement, and nanoparticulate carriers [112, 120, 121, 127, 128], but there is limited literature available on these new technologies for CBD. In future work, emerging drug-delivery technologies should be rigorously explored for CBD, which would facilitate the efficient and convenient application of CBD via the skin.
Conclusions
Available evidence suggests that CBD has multiple beneficial properties in cosmetic dermatology, including anti-oxidant, anti-inflammatory, and anti-aging effects. Indeed, the skin is an ideal delivery route for CBD, enabling high local concentrations while minimizing systemic side effects. Given its highly lipophilic nature, delivering CBD through the stratum corneum into deeper skin layers requires specialized delivery systems, which are still under research and development. Therefore, efficient delivery systems as well as beneficial properties of CBD should be rigorously investigated in the future. In addition, as the biological effects of CBD are primarily mediated through cannabinoid receptors, the potential effects of CBD on the ECS need to be evaluated. Ideally, these studies should be performed not only in preclinical experiments but also in clinical trials.
Nonetheless, the aging population and the growing demand for cosmetic products, together with the increasing attention to CBD, enhance the social demand for the use of CBD for cosmetic purposes. Further accumulation of clinical data, along with the development of efficient topical/transdermal delivery systems, would provide a bright future for CBD in cosmetic dermatology.
Acknowledgements
We appreciate Ms. Maiko Matsuda, VESPER Studio Inc., Tokyo, Japan, for her contribution of illustrating skills.
Funding
Open Access funding provided by The University of Tokyo.
Declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Takemichi Fukasawa and Ayumi Yoshizaki belong to the Social Cooperation Program, Department of Clinical Cannabinoid Research, supported by the Japan Cosmetic Association and Japan Federation of Medium & Small Enterprise Organizations. Ai Kuzumi, Asako Yoshizaki-Ogawa, Takemichi Fukasawa, and Shinichi Sato have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: AK, AY, SS; methodology: AK; literature search: AK, TF, AY-O; project administration: AY; supervision: AY, SS; writing (original draft): AK; writing (review and editing): AY, SS. All authors read and approved the final manuscript.
References
- 1.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechoulam R, Shvo Y. Hashish I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–8. [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Gaoni Y. A total synthesis of DL-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965;87:3273–5. [DOI] [PubMed] [Google Scholar]
- 4.Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21:8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos AC, Fogaça MV, Scarante FF, Joca SRL, Sales AJ, Gomes FV, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol. 2017;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz AI, Nguyen LC, Oumeslakht L, Bensussan A, Ben MS. Cannabinoids as immune system modulators: cannabidiol potential therapeutic approaches and limitations. Cannabis Cannabinoid Res. 2023;8:254–69. [DOI] [PubMed] [Google Scholar]
- 7.Kuzumi A, Yamashita T, Fukasawa T, Yoshizaki-Ogawa A, Sato S, Yoshizaki A. Cannabinoids for the treatment of autoimmune and inflammatory skin diseases: a systematic review. Exp Dermatol. 2024;33: e15064. [DOI] [PubMed] [Google Scholar]
- 8.Tóth K, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system. Molecules. 2019;24:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao K, Stewart C, Grant-Kels JM. Cannabis and the skin. Clin Dermatol. 2021;39:784–95. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli G, Magnavacca A, Fumagalli M, DellʼAgli M, Piazza S, Sangiovanni E. Cannabis sativa and skin health: dissecting the role of phytocannabinoids. Planta Med. 2022;88:492–506. [DOI] [PubMed] [Google Scholar]
- 11.Palmieri B, Laurino C, Vadalà M. A therapeutic effect of CBD-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170:e93–9. [DOI] [PubMed] [Google Scholar]
- 12.Maghfour J, Rietcheck HR, Rundle CW, Runion TM, Jafri ZA, Dercon S, et al. An observational study of the application of a topical cannabinoid gel on sensitive dry skin. J Drugs Dermatol. 2020;19:1204–8. [DOI] [PubMed] [Google Scholar]
- 13.Vincenzi C, Tosti A. Efficacy and tolerability of a shampoo containing broad-spectrum cannabidiol in the treatment of scalp inflammation in patients with mild to moderate scalp psoriasis or seborrheic dermatitis. Skin Appendage Disord. 2020;6:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018;35:e224–7. [DOI] [PubMed] [Google Scholar]
- 15.Maida V, Corban J. Topical medical cannabis: a new treatment for wound pain: three cases of pyoderma gangrenosum. J Pain Symptom Manag. 2017;54:732–6. [DOI] [PubMed] [Google Scholar]
- 16.Jhawar N, Schoenberg E, Wang JV, Saedi N. The growing trend of cannabidiol in skincare products. Clin Dermatol. 2019;37:279–81. [DOI] [PubMed] [Google Scholar]
- 17.Lim M, Kirchhof MG. Dermatology-related uses of medical cannabis promoted by dispensaries in Canada, Europe, and the United States. J Cutan Med Surg. 2019;23:178–84. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. [DOI] [PubMed] [Google Scholar]
- 19.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. [DOI] [PubMed] [Google Scholar]
- 20.Baswan SM, Klosner AE, Glynn K, Rajgopal A, Malik K, Yim S, et al. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin Cosmet Investig Dermatol. 2020;13:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–21. [DOI] [PubMed] [Google Scholar]
- 22.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol Rev. 2010;62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales P, Reggio PH. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Prog Chem Org Nat Prod. 2017;103:103–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rio CD, Millan E, Garcia V, Appendino G, DeMesa J, Munoz E. The endocannabinoid system of the skin: a potential approach for the treatment of skin disorders. Biochem Pharmacol. 2018;157:122–33. [DOI] [PubMed] [Google Scholar]
- 26.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. [DOI] [PubMed] [Google Scholar]
- 27.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. [DOI] [PubMed] [Google Scholar]
- 28.Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- 30.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor: structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794–801. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor: comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–12. [DOI] [PubMed] [Google Scholar]
- 32.Lambert DM, DiPaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochim Biophys Acta. 1999;1440:266–74. [DOI] [PubMed] [Google Scholar]
- 33.Maccarrone M, Attinà M, Cartoni A, Bari M, Finazzi-Agrò A. Gas chromatography-mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J Neurochem. 2001;76:594–601. [DOI] [PubMed] [Google Scholar]
- 34.Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. 2017;174:1349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, et al. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks DH, Friedman A. The therapeutic potential of cannabinoids in dermatology. Skin Therapy Lett. 2018;23:1–5. [PubMed] [Google Scholar]
- 38.Peng J, Fan M, An C, Ni F, Huang W, Luo J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin Pharmacol Toxicol. 2022;130:439–56. [DOI] [PubMed] [Google Scholar]
- 39.Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, et al. The endocannabinoid system in human keratinocytes: evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem. 2003;278:33896–903. [DOI] [PubMed] [Google Scholar]
- 40.Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J Biol Chem. 2008;283:6005–12. [DOI] [PubMed] [Google Scholar]
- 41.Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011;131:1095–104. [DOI] [PubMed] [Google Scholar]
- 42.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–7. [DOI] [PubMed] [Google Scholar]
- 43.Akhmetshina A, Dees C, Busch N, Beer J, Sarter K, Zwerina J, et al. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum. 2009;60:1129–36. [DOI] [PubMed] [Google Scholar]
- 44.McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol. 2017;8:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176:1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin Drug Discov. 2013;8:509–22. [DOI] [PubMed] [Google Scholar]
- 52.Granja AG, Carrillo-Salinas F, Pagani A, Gómez-Cañas M, Negri R, Navarrete C, et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharmacol. 2012;7:1002–16. [DOI] [PubMed] [Google Scholar]
- 53.De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf). 2012;204:255–66. [DOI] [PubMed] [Google Scholar]
- 55.De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–15. [DOI] [PubMed] [Google Scholar]
- 56.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Liu Y, Zhao Z, Qiu J. Oxidative stress in the skin: impact and related protection. Int J Cosmet Sci. 2021;43:495–509. [DOI] [PubMed] [Google Scholar]
- 59.Gohad P, McCoy J, Wambier C, Kovacevic M, Situm M, Stanimirovic A, et al. Novel cannabidiol sunscreen protects keratinocytes and melanocytes against ultraviolet B radiation. J Cosmet Dermatol. 2020;20:1350–2. [DOI] [PubMed] [Google Scholar]
- 60.Jarocka-Karpowicz I, Biernacki M, Wroński A, Gęgotek A, Skrzydlewska E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules. 2020;10:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atalay S, Dobrzyńska I, Gęgotek A, Skrzydlewska E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020;36: 101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jastrząb A, Gęgotek A, Skrzydlewska E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells. 2019;8:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casares L, García V, Garrido-Rodríguez M, Millán E, Collado JA, García-Martín A, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28: 101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gęgotek A, Atalay S, Domingues P, Skrzydlewska E. The differences in the proteome profile of cannabidiol-treated skin fibroblasts following UVA or UVB irradiation in 2D and 3D cell cultures. Cells. 2019;8:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sangiovanni E, Fumagalli M, Pacchetti B, Piazza S, Magnavacca A, Khalilpour S, et al. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res. 2019;33:2083–93. [DOI] [PubMed] [Google Scholar]
- 67.Petrosino S, Verde R, Vaia M, Allarà M, Iuvone T, Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther. 2018;365:652–63. [DOI] [PubMed] [Google Scholar]
- 68.Bunman S, Muengtaweepongsa S, Piyayotai D, Charlermroj R, Kanjana K, Kaew-Amdee S, et al. Analgesic and anti-inflammatory effects of 1% topical cannabidiol gel in animal models. Cannabis Cannabinoid Res. 2023;9:740–50. [DOI] [PubMed] [Google Scholar]
- 69.Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J Control Release. 2003;93:377–87. [DOI] [PubMed] [Google Scholar]
- 70.Milando R, Friedman A. Cannabinoids: potential role in inflammatory and neoplastic skin diseases. Am J Clin Dermatol. 2019;20:167–80. [DOI] [PubMed] [Google Scholar]
- 71.Leonti M, Casu L, Raduner S, Cottiglia F, Floris C, Altmann KH, et al. Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro-allergic effects in skin. Biochem Pharmacol. 2010;79:1815–26. [DOI] [PubMed] [Google Scholar]
- 72.Kim HJ, Kim B, Park BM, Jeon JE, Lee SH, Mann S, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:e401–8. [DOI] [PubMed] [Google Scholar]
- 73.Oka S, Wakui J, Gokoh M, Kishimoto S, Sugiura T. Suppression by WIN55212-2, a cannabinoid receptor agonist, of inflammatory reactions in mouse ear: Interference with the actions of an endogenous ligand, 2-arachidonoylglycerol. Eur J Pharmacol. 2006;538:154–62. [DOI] [PubMed] [Google Scholar]
- 74.Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB2 receptor-mediated response and efficacy of cannabinoid CB2 receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520:164–71. [DOI] [PubMed] [Google Scholar]
- 75.Maghfour J, Rundle CW, Rietcheck HR, Dercon S, Lio P, Mamo A, et al. Assessing the effects of topical cannabidiol in patients with atopic dermatitis. Dermatol Online J. 2021;27:13030/qt8h50k2vs. [PubMed] [Google Scholar]
- 76.Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6:75–82. [DOI] [PubMed] [Google Scholar]
- 77.Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002;277:46616–21. [DOI] [PubMed] [Google Scholar]
- 78.Ikarashi N, Shiseki M, Yoshida R, Tabata K, Kimura R, Watanabe T, et al. Cannabidiol application increases cutaneous aquaporin-3 and exerts a skin moisturizing effect. Pharmaceuticals (Basel). 2021;14:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jang YS, Jeong S, Kim AR, Mok BR, Son SJ, Ryu JS, et al. Cannabidiol mediates epidermal terminal differentiation and redox homeostasis through aryl hydrocarbon receptor (AhR)-dependent signaling. J Dermatol Sci. 2023;109:61–70. [DOI] [PubMed] [Google Scholar]
- 80.Czifra G, Szöllősi AG, Tóth BI, Demaude J, Bouez C, Breton L, et al. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J Invest Dermatol. 2012;132:1967–76. [DOI] [PubMed] [Google Scholar]
- 81.Szöllősi AG, Oláh A, Bíró T, Tóth BI. Recent advances in the endocrinology of the sebaceous gland. Dermatoendocrinol. 2017;9: e1361576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobrosi N, Tóth BI, Nagy G, Dózsa A, Géczy T, Nagy L, et al. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008;22:3685–95. [DOI] [PubMed] [Google Scholar]
- 83.Oláh A, Tóth BI, Borbíró I, Sugawara K, Szöllõsi AG, Czifra G, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:F1000 Faculty Rev-1953. [DOI] [PMC free article] [PubMed]
- 85.Jiang Z, Jin S, Fan X, Cao K, Liu Y, Wang X, et al. Cannabidiol inhibits inflammation induced by Cutibacterium acnes-derived extracellular vesicles via activation of CB2 receptor in keratinocytes. J Inflamm Res. 2022;15:4573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blaskovich MAT, Kavanagh AM, Elliott AG, Zhang B, Ramu S, Amado M, et al. The antimicrobial potential of cannabidiol. Commun Biol. 2021;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen G, Jakus J, Baroud S, Gvirtz R, Rozenblat S. Development of an effective acne treatment based on CBD and herbal extracts: preliminary in vitro, ex vivo, and clinical evaluation. Evid Based Complement Alternat Med. 2023;2023:4474255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen PR. Therapeutic and cosmetic uses of cannabis: cannabinoids for acne treatment and skin rejuvenation. Skinmed. 2021;19:45–7. [PubMed] [Google Scholar]
- 89.Ramer R, Hinz B. Cannabinoid compounds as a pharmacotherapeutic option for the treatment of non-cancer skin diseases. Cells. 2022;11:4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spleman L, Sinclair R, Freeman M, Davis M, Gebauer K. 1061 The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180. [Google Scholar]
- 91.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. [DOI] [PubMed] [Google Scholar]
- 92.Gerasymchuk M, Robinson GI, Groves A, Haselhorst L, Nandakumar S, Stahl C, et al. Phytocannabinoids stimulate rejuvenation and prevent cellular senescence in human dermal fibroblasts. Cells. 2022;11:3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Z, Qi J, Hu L, Ouyang D, Wang H, Sun Q, et al. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater Adv. 2022;134: 112560. [DOI] [PubMed] [Google Scholar]
- 94.Russell-Goldman E, Murphy GF. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020;190:1356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krutmann J, Schikowski T, Morita A, Berneburg M. Environmentally-induced (extrinsic) skin aging: exposomal factors and underlying mechanisms. J Invest Dermatol. 2021;141:1096–103. [DOI] [PubMed] [Google Scholar]
- 96.Omatsu J, Yamashita T, Mori T, Osuji Y, Kawanabe R, Kuzumi A, et al. Neuromuscular electrical stimulation for facial wrinkles and sagging: the 8-week prospective, split-face, controlled trial in Asians. J Cosmet Dermatol. 2024. 10.1111/jocd.16403. [DOI] [PubMed] [Google Scholar]
- 97.Gerasymchuk M, Robinson GI, Kovalchuk O, Kovalchuk I. The effects of nutrient signaling regulators in combination with phytocannabinoids on the senescence-associated phenotype in human dermal fibroblasts. Int J Mol Sci. 2022;23:8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen G, Jakus J, Portillo M, Gvirtz R, Ogen-Shtern N, Silberstein E, et al. In vitro, ex vivo, and clinical evaluation of anti-aging gel containing EPA and CBD. J Cosmet Dermatol. 2023;22:3047–57. [DOI] [PubMed] [Google Scholar]
- 99.Laborada J, Cohen PR. Cutaneous squamous cell carcinoma and lichen simplex chronicus successfully treated with topical cannabinoid oil: a case report and summary of cannabinoids in dermatology. Cureus. 2022;14: e23850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blázquez C, Carracedo A, Barrado L, Real PJ, Fernández-Luna JL, Velasco G, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–5. [DOI] [PubMed] [Google Scholar]
- 101.Glodde N, Jakobs M, Bald T, Tüting T, Gaffal E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35–40. [DOI] [PubMed] [Google Scholar]
- 102.Zheng D, Bode AM, Zhao Q, Cho YY, Zhu F, Ma WY, et al. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008;68:3992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evaluating microneedling with CBD and hempseed oil for acne vulgaris safety and efficacy. Available from: https://clinicaltrials.gov/study/NCT06362889. Accessed 7 Jul 2024.
- 104.The therapeutic effects of topical cannabidiol (CBD) products for atopic dermatitis. Available from: https://clinicaltrials.gov/study/NCT06022874. Accessed 7 Jul 2024.
- 105.Efficacy and tolerance of cannabidiol in patients with severe pruritus: a multicenter, double-blind, randomized, placebo-controlled study (CANNABITCH). Available from: https://clinicaltrials.gov/study/NCT06435299. Accessed 7 Jul 2024.
- 106.CBD effects on forehead split scar healing. Available from: https://clinicaltrials.gov/study/NCT05650697. Accessed 7 Jul 2024.
- 107.CBD Oil-Scar healing study. Available from: https://clinicaltrials.gov/study/NCT06129591?term=NCT06129591&rank=1. Accessed 7 Jul 2024.
- 108.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;7:607–15. [DOI] [PubMed] [Google Scholar]
- 109.Mullard A. FDA approves first Marijuana-derived product. Nat Rev Drug Discov. 2018;17:534. [DOI] [PubMed] [Google Scholar]
- 110.Rubin R. The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA. 2018;320:1227–9. [DOI] [PubMed] [Google Scholar]
- 111.Sekar K, Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Res. 2019;8:F1000 Faculty Rev-234. [DOI] [PMC free article] [PubMed]
- 112.Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36:1088–97. [DOI] [PubMed] [Google Scholar]
- 113.Mannila J, Järvinen T, Järvinen K, Jarho P. Precipitation complexation method produces cannabidiol/beta-cyclodextrin inclusion complex suitable for sublingual administration of cannabidiol. J Pharm Sci. 2007;96:312–9. [DOI] [PubMed] [Google Scholar]
- 114.Spindle TR, Cone EJ, Goffi E, Weerts EM, Mitchell JM, Winecker RE, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211: 107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–60. [DOI] [PubMed] [Google Scholar]
- 116.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stinchcomb AL, Valiveti S, Hammell DC, Ramsey DR. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol. 2004;56:291–7. [DOI] [PubMed] [Google Scholar]
- 118.Barry BW. Breaching the skin’s barrier to drugs. Nat Biotechnol. 2004;22:165–7. [DOI] [PubMed] [Google Scholar]
- 119.Skalicky J, Husek J, Hofbauerova J, Dittrich M. A composition for the treatment of inflammatory diseases comprising boswellic acids and cannabidiol. Available from: https://patents.google.com/patent/EP2444081A1/en. Accessed 7 Jul 2024.
- 120.Jackson DK, Hyatt K. Silicone and hyaluronic acid (HLA) delivery systems for products by sustainable processes for medical uses including wound management. Available from: https://patents.google.com/patent/US20130184354A1/en. Accessed 7 Jul 2024.
- 121.Lowe GA, Lowe V. Composition of cannabinoids, odorous volatile compounds, and emu oil for topical application, and a method for cannabinoid transdermal delivery. Available from: https://patents.google.com/patent/US9526752B1/en. Accessed 7 Jul 2024.
- 122.Shemanski ME. Formulations of argan oil and cannabidiol. Available from: https://patents.google.com/patent/WO2017160923A1/en. Accessed 7 Jul 2024.
- 123.Kado J. Topical formulation for binding to dermatological cannabinoid receptors. Available from: https://patents.google.com/patent/WO2018236897A1/en. Accessed 7 Jul 2024.
- 124.Cristobal W. Transcutaneous application of marijuana. Available from: https://patents.google.com/patent/US6132762A/en. Accessed 7 Jul 2024.
- 125.Maida V, Corban J. Topical medical cannabis: a new treatment for wound pain—three cases of pyoderma gangrenosum. J Pain Symptom Manage. 2017;54:732–6. [DOI] [PubMed] [Google Scholar]
- 126.Ghalili B, McGovern K. Topical compositions comprising hydroxy acids and cannabinoids for skin care. Available from: https://patents.google.com/patent/US20170042791A1/en. Accessed 7 Jul 2024.
- 127.Kuhrts E. Water-soluble phytocannabinoid formulations. Available from: https://patents.google.com/patent/US9907823B1/en. Accessed 7 Jul 2024.
- 128.Wallace WH. Method of relieving analgesia and reducing inflamation using a cannabinoid delivery topical liniment. Available from: https://patents.google.com/patent/US6949582B1/en. Accessed 7 Jul 2024.
- 129.Franzè S, Ricci C, Del Favero E, Rama F, Casiraghi A, Cilurzo F. Micelles-in-liposome systems obtained by proliposomal approach for cannabidiol delivery: Structural features and skin penetration. Mol Pharm. 2023;20:3393–402. [DOI] [PubMed] [Google Scholar]
- 130.Touitou E, Godin B, Weiss C. Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Dev Res. 2000;50:406–15. [Google Scholar]
- 131.Benson HA. Transfersomes for transdermal drug delivery. Expert Opin Drug Deliv. 2006;3:727–37. [DOI] [PubMed] [Google Scholar]
- 132.Rajan R, Jose S, Mukund VP, Vasudevan DT. Transferosomes: a vesicular transdermal delivery system for enhanced drug permeation. J Adv Pharm Technol Res. 2011;2:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeb A, Arif ST, Malik M, Shah FA, Din FU, Qureshi OS, et al. Potential of nanoparticulate carriers for improved drug delivery via skin. J Pharm Invest. 2019;49:485–517. [Google Scholar]
- 134.Rawat S, Vengurlekar S, Rakesh B, Jain S, Srikarti G. Transdermal delivery by iontophoresis. Indian J Pharm Sci. 2008;70:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152:330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tanwar H, Sachdeva R. Transdermal drug delivery system: a review. Int J Pharm Sci Res. 2016;7:2274. [Google Scholar]
- 137.Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS. Getting drugs across biological barriers. Adv Mater. 2017;29:1606596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Souza JDR, Pacheco JC, Rossi GN, de-Paulo BO, Zuardi AW, Guimarães FS, et al. Adverse effects of oral cannabidiol: an updated systematic review of randomized controlled trials (2020–2022). Pharmaceutics. 2022;14:2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dos Santos RG, Guimarães FS, Crippa JAS, Hallak JEC, Rossi GN, Rocha JM, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16:517–26. [DOI] [PubMed] [Google Scholar]
- 140.Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Crippa AS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–49. [DOI] [PubMed] [Google Scholar]
- 141.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–70. [DOI] [PubMed] [Google Scholar]
- 143.Zendulka O, Dovrtelova G, Nosková K, Turjap M, Sulcova A, Hanus L, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17:206–26. [DOI] [PubMed] [Google Scholar]
- 144.Sivesind TE, Maghfour J, Rietcheck H, Kamel K, Malik AS, Dellavalle RP. Cannabinoids for the treatment of dermatologic conditions. JID Innov. 2022;2: 100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spinella A, de Pinto M, Baraldi C, Galluzzo C, Testoni S, Lumetti F, et al. Topical cannabidiol in the treatment of digital ulcers in patients with scleroderma: comparative analysis and literature review. Adv Skin Wound Care. 2023;36:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bilbao A, Spanagel R. Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 2022;20:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ni B, Liu Y, Dai M, Zhao J, Liang Y, Yang X, et al. The role of cannabidiol in aging. Biomed Pharmacother. 2023;165: 115074. [DOI] [PubMed] [Google Scholar]
- 148.Styrczewska M, Kostyn A, Kulma A, Majkowska-Skrobek G, Augustyniak D, Prescha A, et al. Flax fiber hydrophobic extract inhibits human skin cells inflammation and causes remodeling of extracellular matrix and wound closure activation. Biomed Res Int. 2015;2015: 862391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–7. [DOI] [PubMed] [Google Scholar]
- 150.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129: 110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferreira MS, Magalhães MC, Oliveira R, Sousa-Lobo JM, Almeida IF. Trends in the use of botanicals in anti-aging cosmetics. Molecules. 2021;26:3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martins AM, Gomes AL, Vilas Boas I, Marto J, Ribeiro HM. Cannabis-based products for the treatment of skin inflammatory diseases: a timely review. Pharmaceuticals. 2022;15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oláh A, Markovics A, Szabó-Papp J, Szabó PT, Stott C, Zouboulis CC, et al. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp Dermatol. 2016;25:701–7. [DOI] [PubMed] [Google Scholar]