Abstract
The holostean fishes are the extant representatives of the primitive ray-finned fishes from which the present-day teleosts may have evolved. The primary structure of insulin from a holostean fish, the bowfin (Amia calva), was established as: A-chain: Gly-Ile-Val-Glu-Gln-Cys-Cys-Leu-Lys-Pro-Cys-Thr-Ile-Tyr-Glu-Met-Glu- Lys-Tyr-Cys-Asn B-chain: Ala-Ala-Ser-Gln-His-Leu-Cys-Gly-Ser-His-Leu-Val-Glu-Ala-Leu-Phe-Leu- Val-Cys-Gly-Glu-Ser-Gly-Phe-Phe-Tyr-Asn-Pro-Asn-Lys-Ser This amino acid sequence contains several substitutions (methionine at A16, phenylalanine at B16 and serine at B22) at sites that have been strongly conserved in other vertebrate species and that may be expected to influence biological activity. Consistent with this prediction, bowfin insulin was approx. 14-fold less potent than pig insulin in inhibiting the binding of [125I-Tyr-A14](human insulin) to transfected mouse NIH 3T3 cells expressing the human insulin receptor.
Full text
PDF
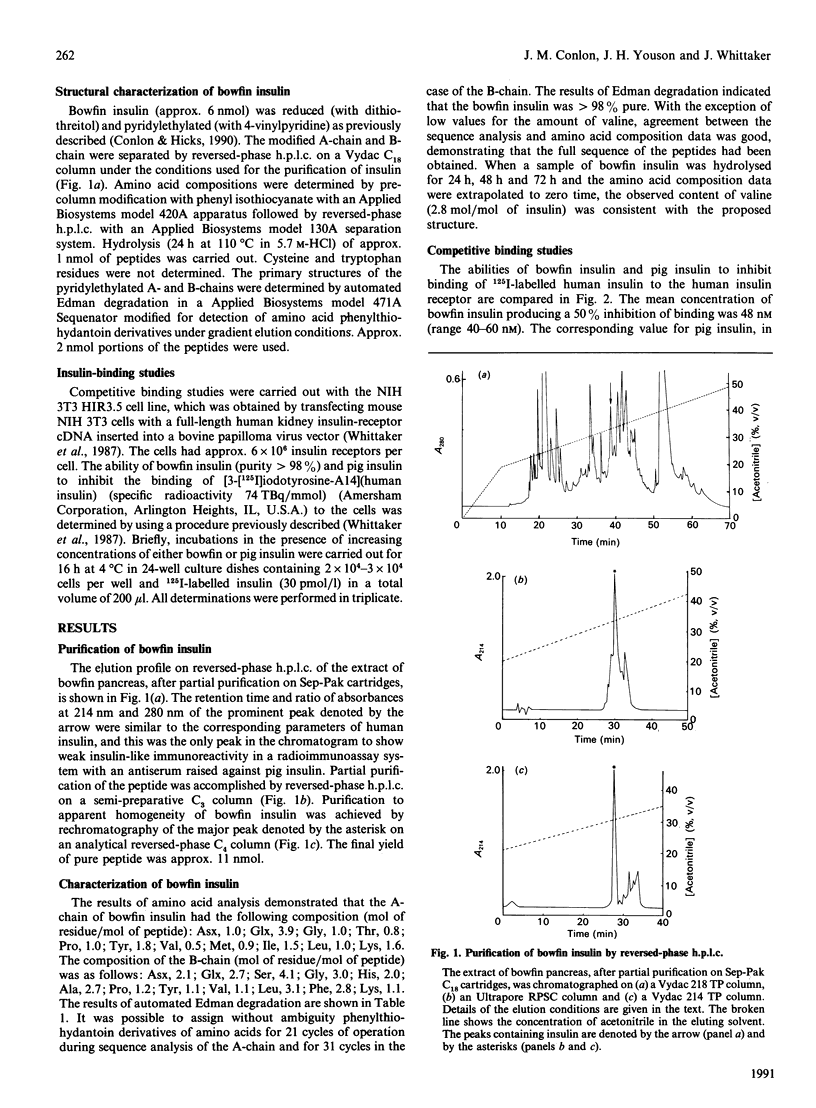
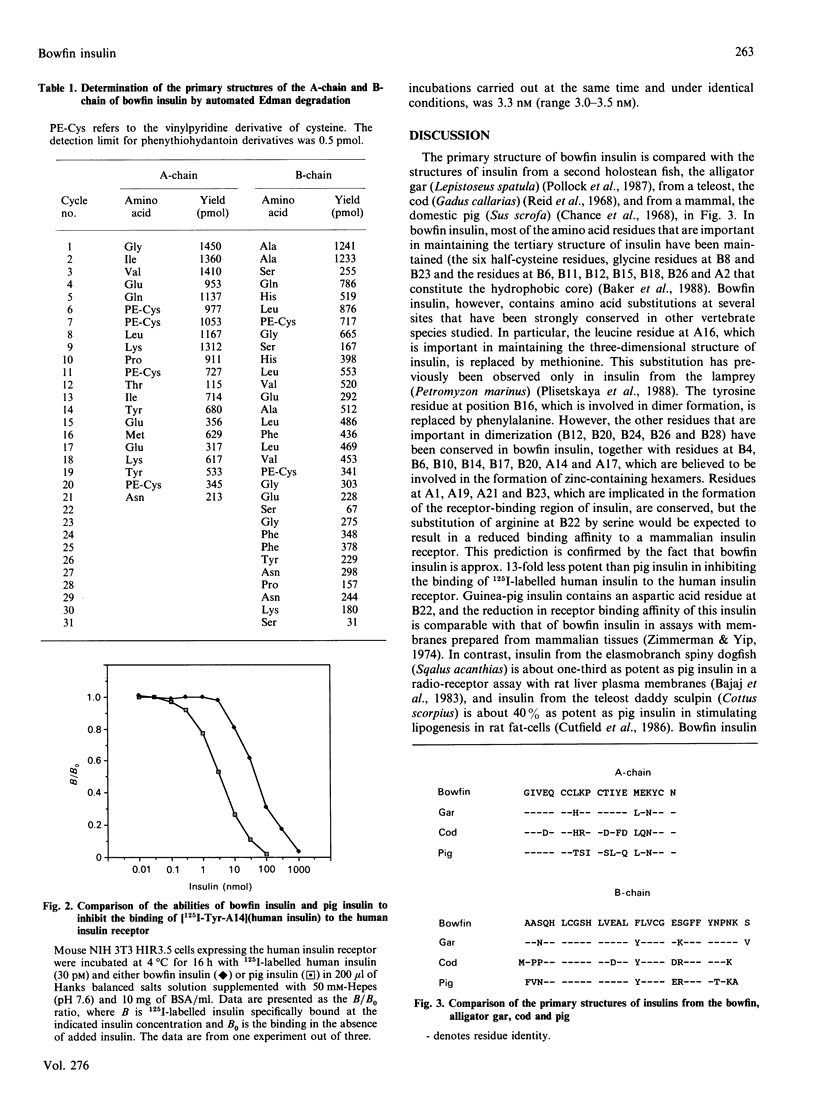

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. J., Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia. 1980 Nov;19(5):475–481. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- Bajaj M., Blundell T. L., Pitts J. E., Wood S. P., Tatnell M. A., Falkmer S., Emdin S. O., Gowan L. K., Crow H., Schwabe C. Dogfish insulin. Primary structure, conformation and biological properties of an elasmobranchial insulin. Eur J Biochem. 1983 Oct 3;135(3):535–542. doi: 10.1111/j.1432-1033.1983.tb07685.x. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 6;319(1195):369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Wood S. P. Is the evolution of insulin Darwinian or due to selectively neutral mutation? Nature. 1975 Sep 18;257(5523):197–203. doi: 10.1038/257197a0. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Bjenning C., Moon T. W., Youson J. H., Thim L. Neuropeptide Y-related peptides from the pancreas of a teleostean (eel), holostean (bowfin) and elasmobranch (skate) fish. Peptides. 1991 Mar-Apr;12(2):221–226. doi: 10.1016/0196-9781(91)90003-8. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Dafgård E., Falkmer S., Thim L. A glucagon-like peptide, structurally related to mammalian oxyntomodulin, from the pancreas of a holocephalan fish, Hydrolagus colliei. Biochem J. 1987 Aug 1;245(3):851–855. doi: 10.1042/bj2450851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J. M., Hicks J. W. Isolation and structural characterization of insulin, glucagon and somatostatin from the turtle, Pseudemys scripta. Peptides. 1990 May-Jun;11(3):461–466. doi: 10.1016/0196-9781(90)90043-5. [DOI] [PubMed] [Google Scholar]
- Cutfield J. F., Cutfield S. M., Carne A., Emdin S. O., Falkmer S. The isolation, purification and amino-acid sequence of insulin from the teleost fish Cottus scorpius (daddy sculpin). Eur J Biochem. 1986 Jul 1;158(1):117–123. doi: 10.1111/j.1432-1033.1986.tb09728.x. [DOI] [PubMed] [Google Scholar]
- Epple A., Brinn J. E., Jr Islet histophysiology: evolutionary correlations. Gen Comp Endocrinol. 1975 Nov;27(3):320–349. doi: 10.1016/0016-6480(75)90201-4. [DOI] [PubMed] [Google Scholar]
- Falkmer S., Wilson S. Comparative aspects of the immunology and biology of insulin. Diabetologia. 1967 Dec;3(6):519–528. doi: 10.1007/BF01213571. [DOI] [PubMed] [Google Scholar]
- Plisetskaya E. M., Pollock H. G., Elliott W. M., Youson J. H., Andrews P. C. Isolation and structure of lamprey (Petromyzon marinus) insulin. Gen Comp Endocrinol. 1988 Jan;69(1):46–55. doi: 10.1016/0016-6480(88)90051-2. [DOI] [PubMed] [Google Scholar]
- Pollock H. G., Kimmel J. R., Hamilton J. W., Rouse J. B., Ebner K. E., Lance V., Rawitch A. B. Isolation and structures of alligator gar (Lepisosteus spatula) insulin and pancreatic polypeptide. Gen Comp Endocrinol. 1987 Sep;67(3):375–382. doi: 10.1016/0016-6480(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Grant P. T., Youngson A. The sequence of amino acids in insulin isolated from islet tissue of the cod (Gadus callarias). Biochem J. 1968 Nov;110(2):289–296. doi: 10.1042/bj1100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. E., Yip C. C. Guinea pig insulin. I. Purification and physical properties. J Biol Chem. 1974 Jul 10;249(13):4021–4025. [PubMed] [Google Scholar]


