Abstract
Salt-induced stress poses a significant barrier to agricultural productivity by impeding crop growth. Presently, environmentalists are dedicated to safeguarding food security by enhancing agricultural yields in challenging environments. Biostimulants play a crucial role in mitigating abiotic stresses in crop production, and among these, plant essential oils (EOs) stand out as organic substances with diverse biological effects on living organisms. Among the natural promoters of plant growth, Rosmarinus officinalis L. essential oil (RoEO) has gained considerable attention. Although the manifold effects of essential oils (EOs) on plant growth have been extensively demonstrated, their impact on salt stress tolerance in durum wheat seedlings remains unexplored. This investigation was undertaken to evaluate the biostimulatory capabilities of RoEO on the durum wheat cultivar “Mahmoudi.” The effects of three RoEO concentrations (1, 2.5, and 5 ppm) on seed germination, growth establishment, and the induction of salt resistance under salinity conditions (150 mM NaCl) were tested. At 5 ppm, RoEO enhanced seedlings’ tolerance to salinity by improving growth and reducing membrane deterioration and oxidative stress-induced damage. The expression profile analyses of seven stress-related genes (TdNHX1, TdSOS1, TdSOD, TdCAT, TdGA20-ox1, TdNRT2.1, and TdGS) using RT-qPCR showed enhancement of several important genes in durum wheat seedlings treated with 5 ppm RoEO, even under control conditions, which may be related to salt stress tolerance. The results indicate that the application of RoEO suggests a possible alternative strategy to increase salt tolerance in durum wheat seedlings towards better growth quality, thus increasing ROS scavenging and activation of antioxidant defense.
Supplementary information
The online version contains supplementary material available at 10.1007/s00709-024-01965-8.
Keywords: Durum wheat, Rosemary essential oil, Biostimulant, Antioxidant enzymes, Salt stress
Introduction
Rising climate change has precipitated a myriad of environmental challenges, leading to a decline in crop productivity. Among these issues, salinity has emerged as a particularly detrimental abiotic stressor that impedes both seed germination and seedling growth (Xu et al. 2017). The impact of salt stress on cereal productivity significantly influences the pillars of sustainable food security and overall food production (Alkharabsheh et al. 2021). Wheat holds paramount importance as a global cereal crop, serving as a vital source of calories and protein essential for the world’s human population. It boasts the largest harvested area and ranks as the second-highest in terms of production rate among cereals. Wheat is subjected to a variety of abiotic stresses in the arid Mediterranean region, including high temperatures, drought, and salinity, which severely affect plant development as well as yield and quality losses (Laraus 2004). In the realm of agriculture, the escalating demand for sustainability underscores the imperative to transition from synthetic fertilizers to natural or biological alternatives (Tauler and Baraza 2015; Tahami et al. 2017). To meet this objective, biostimulants and bio-protectants have been put forth as promising agents with the potential to foster plant growth and enhance overall crop yields (Bulgari et al. 2019; Singh et al. 2020). Organic acids, amino acids, and compounds like humic acid, salicylic acid, and polyamines stand out as stimulants. Their application, whether applied to the soil or foliage, typically enhances various plant growth-related traits (Souri 2016; Canellas et al. 2015; Marschner 2011). There is evidence to suggest that a diverse array of biostimulants has the capacity to enhance the tolerance of plants to adverse conditions such as drought, salinity, and cold stress (Van Osten et al. 2017). Recent studies have suggested that the volatile compounds found in EOs may offer advantages to plants thriving in challenging or unfavorable environments (Hara et al. 2013; Yamauchi et al. 2015). A distinct category of well-defined biostimulants comprises EOs, which are intricate natural products typically extracted from aromatic plants. These oils exhibit a broad spectrum of biological activities, including resistance against diseases and pests, and they contribute to enhancing plant stress tolerance in the face of various abiotic stress factors (Kahramanoglu and Usanmaz 2021; Kesraoui et al. 2022). In natural settings, EOs influence the growth of neighboring plants, particularly those in proximity to essential oil-producing plants. Certain EOs serve as allelochemicals, exerting inhibitory effects on seed germination (Koul et al. 2008). Their facile metabolic processes have opened avenues for their application in agricultural settings, supplanting the use of synthetic chemicals (Belasli et al. 2020). Utilizing EOs in conjunction with seed coating and priming technologies holds significant promise as a natural biostimulant, contributing substantially to the improvement of both biotic and abiotic stress resistance (Tavares et al. 2002; Lutts et al. 2016). Numerous studies have predominantly concentrated on exploring the antifungal, antibacterial, and insecticidal properties of plant EO (Ben Hsouna et al. 2013, 2019, 2022; Belasli et al. 2020; Moumni et al. 2021; Ben Akacha et al. 2022; Kesraoui et al. 2022). Additionally, there is a growing interest in elucidating the role of EOs in conferring abiotic stress tolerance during the critical stages of germination and seedling growth (Bi̇ngöl and Battal 2017; Souri and Bakhtiarizade 2019; Ben-Jabeur et al. 2019). The application of essential oils or plant extracts as a pre-treatment for seeds has been shown to induce a biostimulant effect, enhancing tolerance to drought stress (Farooq et al. 2018; Ben-Jabeur et al. 2022). The methods employed in seed preparation exert a notable influence on the metabolic, biochemical, and enzymatic activities of the seed (Nile et al. 2013). By employing seed priming, early germination and robust seedling development can be achieved, particularly under arid conditions (Raj and Raj 2019; Zulfiqar 2021).
Rosemary (Rosmarinus officinalis L.), a perennial herb belonging to the Lamiaceae family, originates from the Mediterranean region. Renowned for its resilience, it has become prevalent in numerous countries, attributed to its essential oils, extracts, use as a spice, and diverse biological effects on plants (Jordán et al. 2013). The EOs derived from this plant exhibit a multitude of pharmacological properties (Lemos et al. 2015). The leaves of this plant are abundant in aromatic compounds, as well as iron, calcium, and vitamin B6, imparting a broad spectrum of medicinal and health-related benefits in human health programs (Jalali-Heravi et al. 2011). Rosemary EO showcases a diverse array of properties, including antioxidant, antimicrobial, anti-inflammatory, and insecticidal activities (Abo Ghanima et al. 2020; Da Silva Bomfim et al. 2020; Hashemi Gahruie et al. 2017).
The primary objective of this study was to investigate the biostimulatory properties of RoEO concerning germination, growth, salt tolerance, and antioxidant systems (SOD, CAT, and GPX) in durum wheat seedlings under salt stress conditions. Furthermore, our goal was to elucidate how RoEO regulates the enhancement of salt stress resistance in durum wheat, with a focus on osmotic regulation, redox homeostasis, and molecular responsiveness during the seedling stage.
Materials and methods
Plant materials
The EOs extracted from the needles of Rosmarinus officinalis L., cultivated in Tuscany, Italy, and obtained through steam distillation, were supplied directly by “èssenziale” Azienda Agricola, San Donato in Poggio (FI), Italy. The plants used for extraction were collected in June 2022.
GC–MS analyses of RoEO
The chemical composition of the rosemary essential oil (RoEO) was characterized using a Perkin Elmer Clarus 500 (Waltham, MA, USA) model gas chromatograph coupled to a mass spectrometer equipped with a flame ionization detector (FID) (Ben Akacha et al. 2023). Chromatographic separation of components was achieved using a Stabil wax fused-silica capillary column (Restek, Bellefonte, PA, USA) with dimensions of 60 m × 0.25 mm and a 0.5-mm film thickness. The oven temperature program started at 60 °C and increased to 220 °C for 20 min at a rate of 6 °C/min. Helium served as the carrier gas with a constant flow rate of 1.0 mL/min. Mass spectra were obtained in electron impact (EI) mode at 70 eV in full-scan mode within the range of 35–550 m/z. Compound identification was performed by matching mass spectra with the Wiley 2.2 (Wiley, NY, USA) and NIST 11 (Gaithersburg, MD, USA) databases. Linear retention indices (LRIs) were calculated using a mixture of C8–C25 n-alkanes and compared with literature values (Linstrom and Mallard 2014). Relative average percentages were determined by normalizing peak areas without an internal standard and factor correction. The analysis was conducted in triplicate.
Seed treatment, growth conditions, and measurement of plant growth traits
The experiment utilized seeds from the “Mahmoudi” variety of durum wheat (Triticum turgidum L. var. durum), a widely cultivated Tunisian variety, which were supplied by the Centre d’Appui Chebika-CRDA Kairouan in Tunisia. The seeds underwent surface sterilization following the method described by Bouteraa et al. (2022). To avoid disruption of the normal physiological processes of plants, stunted growth, wilting of leaves, and even plant death, the RoEO was used in moderation and at appropriate concentrations of 1, 2.5, and 5 ppm with the addition of 0.5% dimethyl sulfoxide (DMSO) as a solubilizing agent to ensure homogenous application of the EO. Subsequently, the seeds were categorized into four groups. The initial group was soaked in distilled water, while the remaining three groups were soaked in 1, 2.5, and 5 ppm RoEO for 12 h. Following the soaking process, the seeds underwent three rinses with distilled water and were subsequently air-dried for 48 h at room temperature. Thereafter, 20 seeds were germinated in triplicate using distinct seed batches. The germination process took place on Petri dishes containing sterile filter paper and a half-strength MS medium supplemented with varying concentrations of RoEO (0, 1, 2.5, and 5 ppm). The Petri dishes were then positioned in a growth chamber at 22 °C ± 2.0 with a 12-h photoperiod and 65% relative humidity. The assessment of germination energy (GE) was conducted on the 3rd day post-sowing and was calculated as follows: GE = (number of germinated seeds on day 3/total number of seeds) × 100.
After 8 days of growth, the seedlings were photographed, and seedling length and fresh weight (FW) were determined. The flag leaf area (cm2) was determined using UTHSCA image tool program (http://compdent.uthscsa.edu/dig/itdesc.html). The assays were conducted in triplicate using independent seed lots.
RoEO effect on salt-stressed wheat seedlings
For the salt stress tolerance experiment, wheat seedlings aged 2 days were transplanted into Petri dishes containing half-strength liquid MS medium. This medium was supplemented with 150 mM NaCl and either 0 ppm or 5 ppm of RoEO. The control medium was NaCl-free. The Petri dishes were arranged in accordance with a completely randomized design, with each dish accommodating 20 seeds and each treatment replicated three times. The seedlings were maintained in a constant environment chamber under a 16-h light/8-h dark regime. Nutrient solutions were refreshed every 2 days. After 2 weeks, the seedlings were harvested for subsequent analyses.
Leaf chlorophyll fluorescence
Chlorophyll fluorescence (Fv/Fm) levels were measured on three visually healthy leaves per treatment after a 30-min period of dark adaptation. The quantification was carried out using a portable photosynthesis system, specifically the Handy-Plant Efficiency Analyzer from Hansatech Instruments, following the protocol outlined by Ben Saad et al. (2012).
Determination of the stress parameters
Electrolyte leakage (El) and the membrane stability index (MSI) were determined following a previously published method by Ben Romdhane et al. (2017). The contents of malondialdehyde (MDA) and H2O2 were measured as previously described by Bouteraa et al. (2022). Furthermore, fresh leaves from the durum wheat plants were gathered to investigate the accumulation of superoxide radicals (O2−) via the nitro blue tetrazolium (NBT) staining following the methodology outlined by Ben Saad et al. (2018).
Determination of osmolyte content
Leaf soluble carbohydrates
The quantification of leaf soluble carbohydrates was conducted through the anthrone method. In this process, 0.2 g of fresh leaf tissue was extracted in 2.5 mL of ice-cold 80% ethanol and heated at 95 °C for 60 min (Ben Hsouna et al. 2020). Following extraction, the solution was filtered, and the alcohol was eliminated through evaporation in a hot water bath. Anthrone reagent was employed to prepare the samples, and their absorbance was measured at 625 nm utilizing a spectrophotometer. The carbohydrate content was determined by referencing a standard glucose curve expressed in µg/g FW (fresh weight).
Proline content
Free proline concentration in fresh leaves was determined spectrophotometrically at 520 nm according to the method described by Ben Saad et al. (2018, 2024).
Activity of antioxidant enzymes
The activities of antioxidant enzymes, namely superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), were quantified spectrophotometrically using methods described in the literature by Ben Saad et al. (2022).
RNA extraction and quantitative real-time PCR assay
Frozen leaves were utilized for the extraction of total RNA using the RNeasy Plant Mini Kit (Qiagen), following the manufacturer’s instructions. For RT-qPCR, 5 µg of total RNA per sample was employed to synthesize first-strand cDNA using SuperScriptTM III reverse transcriptase (Invitrogen), oligo (dT18), and random hexamer primers, following the manufacturer’s protocols to examine the transcript accumulation of seven stress-related genes, including genes associated with membrane transporters (TdNHX1, TdSOS1), antioxidant enzymes (TdSOD, TdCAT), gibberellin oxidase (TdGA20-ox1), and genes involved in nitrogen transport and metabolism (TdNRT2.1 and TdGS); total cDNA from durum wheat was employed in RT-qPCR reactions, following the methods outlined by Ben Romdhane et al. (2022, 2024). Relative expression was determined by the comparative threshold cycle (2−ΔΔCT) method (Livak and Schmittgen 2001), using the cell division control protein (AAA-superfamily of ATPases) (CDC gene) as the housekeeping gene. Three biological replicates were used to calculate relative expression. Primer sequences are listed in supplementary Table S1.
Statistical analyses
The XLSTAT statistical software (https://www.xlstat.com/en/ (accessed on 10 November 2023)) was used to perform statistical analyses, including one-way analysis of variance and principal component analysis. According to the Bonferroni post hoc test, the means marked with different letters in the graphs differ significantly at p < 0.05.
Results
Characterization of the chemical volatile composition in RoEO by GC–MS analysis
Twenty compounds in total were detected and identified by GC/MS in RoEO (Table 1). The most abundant compounds, among other substances detected with almost overlapping relative abundances, were camphor (14.4%), 1,8-cineole (13.0%), levoverbenone (11.4%), and α-pinene (10.1%). Also significant was the content of the sesquiterpene β-caryophyllene (8.6%), as well as that of the three monoterpenes: bornyl acetate (7.9%), limonene (6.4%), and β-myrcene (5.2%).
Table 1.
Chemical volatile composition (percentage mean values ± SD) of R. officinalis essential oil (RoEO)
| N° | Component1 | LRI2 | LRI3 | RoEO4 (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 1020 | 1024 | 10.1 ± 0.02 |
| 2 | β-Thujene | 1115 | 1117 | 2.5 ± 0.02 |
| 3 | β-Myrcene | 1160 | 1166 | 5.2 ± 0.00 |
| 4 | Limonene | 1200 | 1204 | 6.4 ± 0.00 |
| 5 | 1,8-Cineole | 1208 | 1206 | 13.0 ± 0.02 |
| 6 | 3-Heptanone, 6-methyl- | 1266 | 1263 | 6.6 ± 0.00 |
| 7 | p-Cymene | 1285 | 1282 | 1.0 ± 0.02 |
| 8 | 3-Octanol | 1411 | 1406 | 0.2 ± 0.07 |
| 9 | Filifolone | 1426 | 1423 | 3.9 ± 0.05 |
| 10 | 1-Octen-3-ol | 1457 | 1453 | 0.4 ± 0.02 |
| 11 | Linalool | 1508 | 1500 | 3.5 ± 0.02 |
| 12 | Camphor | 1533 | 1528 | 14.4 ± 0.05 |
| 13 | Isocamphopinone | 1535 | 1530 | 1.9 ± 0.08 |
| 14 | Bornyl acetate | 1585 | 1580 | 7.9 ± 0.02 |
| 15 | β-Caryophyllene | 1597 | 1594 | 8.6 ± 0.02 |
| 16 | L-Pinocarveol | 1648 | 1651 | 0.2 ± 0.14 |
| 17 | α-Terpineol | 1705 | 1700 | 1.6 ± 0.02 |
| 18 | Borneol | 1708 | 1705 | 0.9 ± 0.02 |
| 19 | Levoverbenone | 1227 | 1723 | 11.4 ± 0.07 |
| 20 | Myrtenol | 1808 | 1804 | 0.3 ± 0.03 |
| SUM | 100.0 | |||
| Monoterpenes | 28.7 | |||
| Oxygenated monoterpenes | 55.5 | |||
| Sesquiterpenes | 8.6 | |||
| Others | 7.2 |
1The components are reported according to their elution order on the polar column
2Linear Retention Indices calculated using the polar column
3Linear Retention Indices from literature
4Percentage mean values of R. officinalis EO components
The effects of RoEO on seed germination and growth development of wheat seedlings
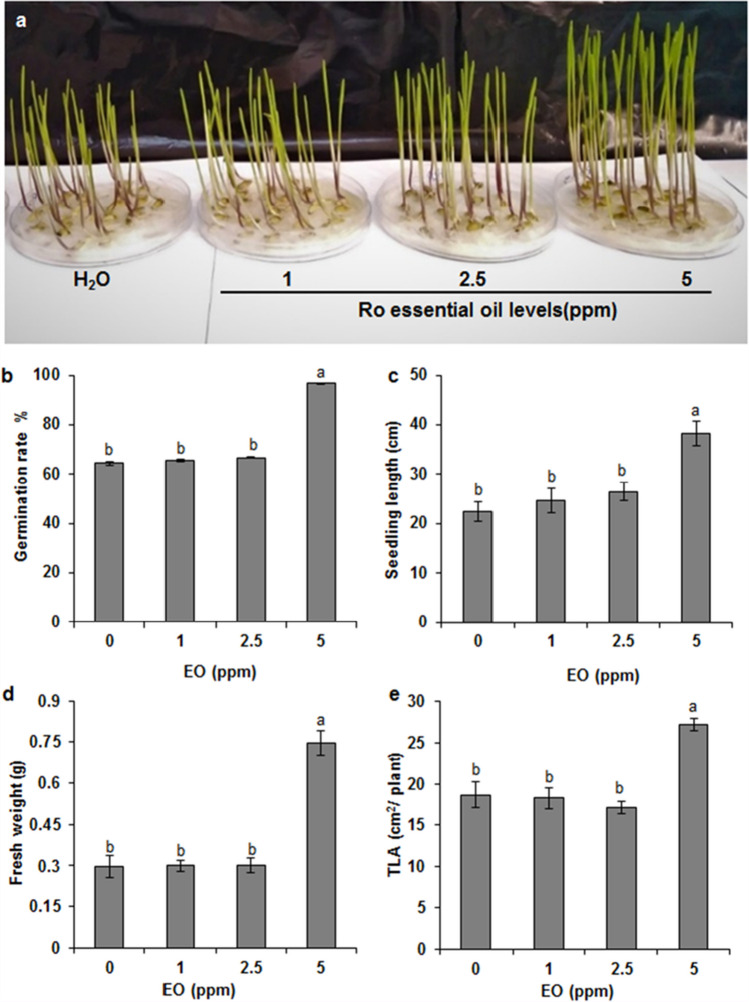
Three RoEO concentrations (1, 2.5, and 5 ppm) were applied to determine the effect of the EO on durum wheat germination (Fig. 1). The results showed that seed coating with 5 ppm RoEO significantly increased the germination rate (100%) compared to the control seeds (without RoEO) (64%) (Fig. 1b). At concentrations of 1 and 2.5 ppm of RoEO, seed wheat germinated 2 days earlier than the control seeds; however, the germination rate was unaffected (Fig. 1b). At 5 ppm, RoEO caused seedling elongation (~ 1.7-fold) (Fig. 1c) and increased its fresh weight (~ 2.55-fold) (Fig. 1d), as well as total leaf area (~ 1.5-fold) (Fig. 1e). At this RoEO concentration, the development of the second leaf was more pronounced, while the stem and roots grew longer (Fig. 1) than those of the control and those of the seeds treated with 1 and 2.5 ppm RoEO. As no phytotoxicity symptoms occurred, we considered RoEO at 5 ppm an optimal concentration for wheat seed germination and applied it in subsequent experiments under saline conditions.
Fig. 1.
Effect of RoEO at different concentrations (1, 2.5, and 5 ppm) on the seed germination and durum wheat seedlings phenotype. a This photo was taken eight days after sowing seeds treated with different RoEO essential oil concentrations. Effect of increasing RoEO concentrations on b germination rate, c seedling length, d fresh weight, and e total leaf area (TLA) of durum wheat seeds. Values are the means ± SE of three biological replicates. Means sharing the same letter do not significantly differ at p < 0.05. Twenty seeds were assessed for each treatment replicate
Effects of RoEO on wheat seedling growth, chlorophyll fluorescence, and osmolyte accumulation under salinity
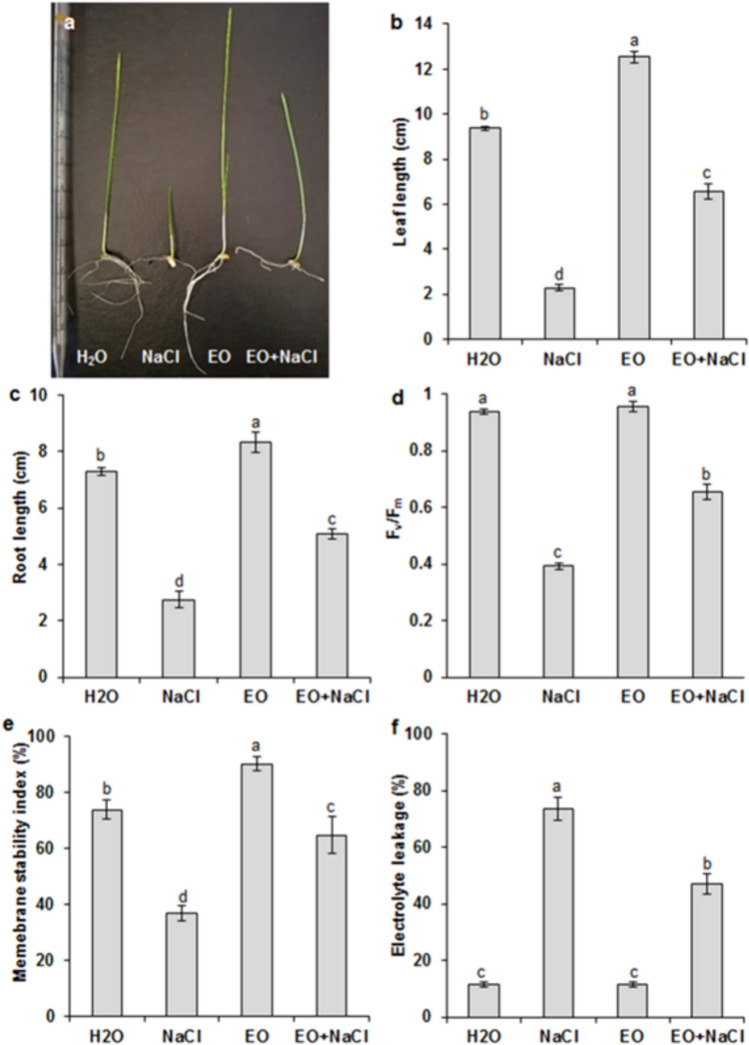
Under salinity (150 mM NaCl), both shoots and roots grew shorter than those of untreated control wheat plants (Fig. 2a). The application of 5 ppm RoEO had a stimulatory effect on wheat seedling growth. When added to salt-treated seedlings, RoEO intensified plant elongation and biomass accretion (Fig. 2a, c). Similarly, total chlorophyll fluorescence (Fv/Fm) in the leaves of durum wheat seedlings exposed to salt stress increased by half after the application of 5 ppm RoEO (Fig. 2d). Additionally, RoEO treatment had no effect on the Fv/Fm ratio in non-stressed plants. This could be due to the biostimulatory effect of RoEO on the photosynthetic capacity of photosystem II following exposure to salt stress.
Fig. 2.
Effect of RoEO at 5 ppm on plant phenotype (a), leaf length (b), root length (c), leaf chlorophyll fluorescence (Fv/Fm) (d), leaf membrane stability index (MSI) (e), and electrolyte leakage (f) grown under saline (150 mM NaCl) and non-saline conditions (H2O). Values are the means ± SE (n = 3). Means denoted by the same letter did not differ significantly at p < 0.05
RoEO effect on stress indicators under salinity
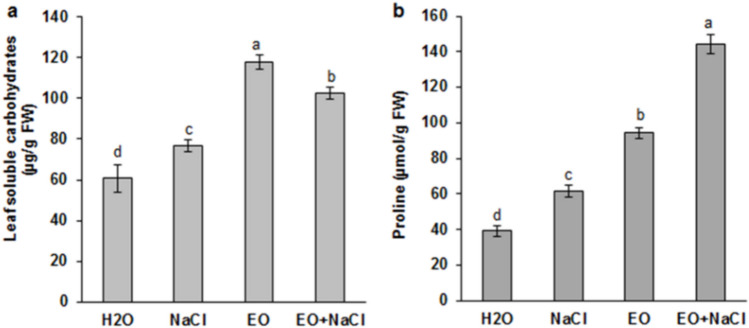
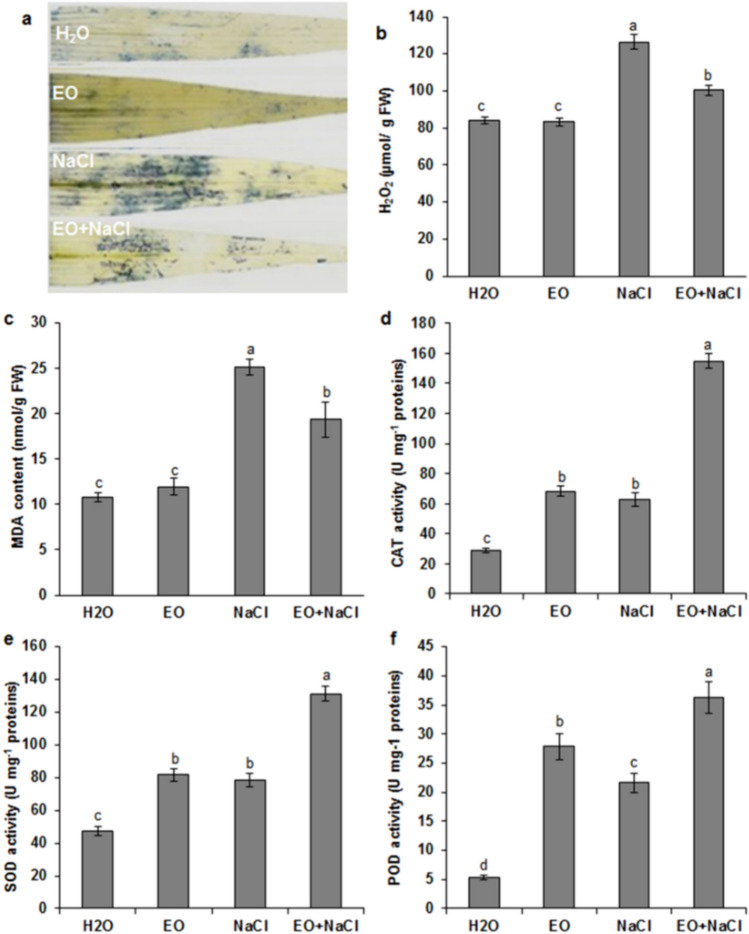
RoEO treatment considerably alleviated salinity stress-induced growth reduction, increased MSI, and maintained low EL compared to the plants grown under salt stress conditions (Fig. 2e, f). The influence of RoEO on salt-treated wheat seedlings was monitored via assessment of osmolyte accumulation. The salt-stressed wheat seedlings had significantly higher leaf soluble carbohydrates and proline contents than unstressed seedlings (Fig. 3a, b). RoEO application further increased osmolyte accumulation by ~ 1.5-fold and 1.33-fold, in the case of proline and carbohydrates, respectively (Fig. 3a, b). For the non-stressed plants, RoEO stimulated the accumulation of soluble carbohydrate and proline, by 40% and 71%, respectively, compared to the control conditions. Additionally, the effect of RoEO on oxidative status of wheat seedlings was examined by measuring the changes in the accumulation of the reactive oxygen species H2O2 and O2−, as well as in the content of MDA, a marker of lipid peroxidation. As shown in Fig. 4a and c, salt exposure caused a significant increase in foliar H2O2 and MDA levels (~ 1.56-fold and 2.27-fold, respectively) compared to the control plants. Remarkably, MDA, O2−, and H2O2 accumulation was considerably reduced in seedlings treated with RoEO under salt stress conditions (Fig. 4a, c). In the absence of stress, RoEO did not affect H2O2 and MDA contents.
Fig. 3.
Influence of RoEO application on the leaf soluble carbohydrate content (a) and proline content (b) of durum wheat seedlings subjected or not to 150 mM NaCl. Values are the means ± SE (n = 3). Different letters indicate significant differences at p < 0.05
Fig. 4.
Influence of Ro essential oil application on the contents of H2O2 (a), MDA (b), and the activities of SOD (c), CAT (d), and POD (e) in durum wheat seedlings subjected or not to 150 mM NaCl. Values are means ± SEM (n = 3). Means sharing the same letter do not significantly differ at p < 0.05
The activities of the antioxidant enzymes SOD, CAT, and POD
In comparison with control plants, a marked increase in CAT, SOD, and POD activities was recorded in leaves treated with RoEO alone (by 2-, 1.66-, and 5.2-fold, respectively) and those subjected to salt stress only (Fig. 4d–f). The activities of CAT and SOD were comparable in seedlings treated either with RoEO or salt. The combination of NaCl stress and RoEO treatment tended to enhance CAT, SOD, and POD activities to the greatest extent (~ 5-fold, ~ 2.8-fold, and ~ 7-fold, respectively, in relation to the control) (Fig. 4d, f).
Molecular responses of wheat plants treated with RoEO and salinity
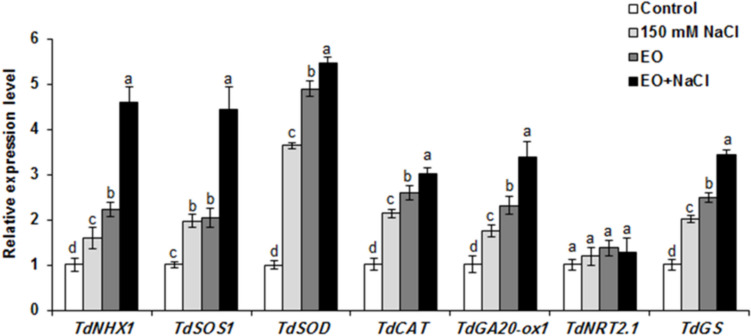
RT-qPCR was conducted to analyze the expression levels of seven stress-related genes (TdNHX1, TdSOS1, TdSOD, TdCAT, TdGA20-ox1, TdNRT2.1, and TdGS) in the leaves of durum wheat. Generally, the application of RoEO was capable of modulating gene expression in wheat seedlings. Salt-stressed wheat seedlings treated with RoEO had significantly higher transcript levels of six stress-related genes (TdNHX1, TdSOS1, TdSOD, TdCAT, TdGA20-ox1, and TdGS) than under both control and saline conditions (Fig. 5). Only in the case of the TdNRT2 gene (related to nitrate transport) were the expression levels stable, regardless of the treatment (Fig. 5).
Fig. 5.
Transcriptional profiles of seven stress-related genes (TdNHX1, TdSOS1, TdSOD, TdCAT, TdGA20-ox1, TdNRT2.1, and TdGS) in the leaves of durum wheat seedlings subjected or not to salt stress and 5 ppm RoEO application for 15 days. The data represent means of three independent experiments. Values are the means ± SE (n = 3). Means sharing the same letter do not significantly differ at p < 0.05
Principal component analysis for stress-related parameters
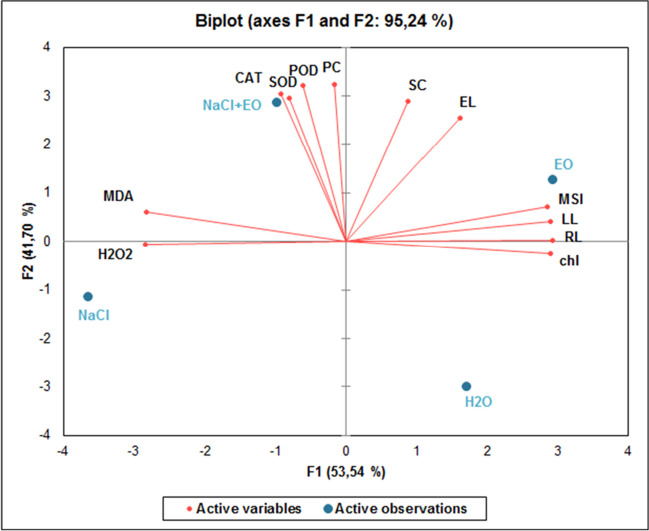
To identify possible correlations between treatments and the different evaluated parameters, principal component analysis (PCA) was carried out (Fig. 6, Table 2). Both principal factorial planes 1 and 2 have eigenvalues greater than 5 and contributed to 53.54% and 41.70% of the total variance, respectively (Table 2). The most important characters contributing to F1 were root length, leaf length, chlorophyll content, membrane stability index, malondialdehyde content, and hydrogen peroxide content (score > 9.4). All other parameters were responsible for the variance in F2. Seedlings treated with RoEO were clustered with growth-related parameters on the positive side of F1. Seedlings subjected to salt stress were clustered with malondialdehyde and hydrogen peroxide content traits on the negative side of F1, while RoEO-treated seedlings under salt stress conditions are clustered with salinity tolerance linked parameters, including SOD, CAT, and POD antioxidant enzyme activities and proline content almost at the center F1 and the positive side of F2. These results suggest that RoEO treatments may play a role in promoting growth in wheat seedlings and enhancing their ability to deal with salinity.
Fig. 6.
Principal component analysis (PCA). Red circles represent the parameters analyzed. Blue circles represent different growth conditions, H2O (control), NaCl (salt treatment at 150 mM), EO (seeds primed with RoEO), and NaCl + EO (seeds primed with RoEO under 150 mM NaCl). F1-F2 principal factorial plane explains 95.24% of the variation between all studied parameters and different conditions. RL, root length; LL, leaf length; chl, chlorophyll; MSI, membrane stability index; EL, electrolyte leakage; SC, sugar content; PC, proline content; H2O2, hydrogen peroxide content; MDA, malondialdehyde content; SOD, superoxide dismutase activity; CAT, catalase activity; POD, peroxidase activity
Table 2.
PCA results for the different parameters studied
| F1 | F2 | F3 | |
|---|---|---|---|
| RL | 1.000 | 0.000 | 0.000 |
| LL | 0.981 | 0.015 | 0.004 |
| chl | 0.980 | 0.006 | 0.014 |
| MSI | 0.953 | 0.046 | 0.000 |
| EL | 0.304 | 0.595 | 0.101 |
| SC | 0.091 | 0.765 | 0.144 |
| PC | 0.003 | 0.952 | 0.044 |
| MDA | 0.942 | 0.033 | 0.024 |
| H2O2 | 0.950 | 0.000 | 0.050 |
| CAT | 0.077 | 0.799 | 0.124 |
| SOD | 0.099 | 0.850 | 0.051 |
| POD | 0.044 | 0.942 | 0.014 |
Values ≥ 0.94 are presented in bold and indicate important traits for PCA. RL root length, LL leaf length, chl chlorophyll, MSI membrane stability index, EL electrolyte leakage, SC sugar content, PC proline content, H2O2 hydrogen peroxide content, MDA malondialdehyde content, SOD superoxide dismutase activity, CAT catalase activity, POD peroxidase activity
Discussion
The impact of abiotic stress on agricultural productivity is manifested through diminished crop growth, which contributes to global food crises. Presently, environmentalists are focused on securing food resources by enhancing crop production in challenging environments. Augmenting the germination potential of seeds holds paramount importance as a criterion for enhancing crop competitiveness through efficient water and nutrient utilization. However, under stress conditions such as salinity, germination is inhibited by Na+ and Cl− toxicity, which is reflected in the osmotic potential and ROS production (Yan et al. 2013; Astaneh et al. 2018). Lutts et al. (2016) suggested that subjecting seeds to transient stress agents during germination within embryos can result in stress memory, fostering more efficient adaptation to subsequent stress episodes. The use of plant extracts and aromatic oils in agriculture has been proposed as an environmentally friendly and cost-effective approach (Farooq et al. 2018; Ben-Jabeur et al. 2022). Day (2016) findings revealed that the EO extracted from safflower plants’ stem and root tissues had varying effects on the germination rates of wheat, barley, sunflower, and chickpea. In this current study, our objective was to investigate the impact of seed treatment with RoEO on the germination performance, growth establishment, and responses to stress, along with the mechanisms involved in stress amelioration under salinity conditions. It appears that the applied EO influences plant growth characteristics akin to other organic compounds, serving as a biostimulant (Marschner 2011; Souri and Römheld 2009). Numerous reports highlight the biostimulant effects of phenolic compounds and plant extracts on seed germination, rooting, and shoot development. These effects are observed when treating seeds, leaves, or soil with EOs (Kisiriko et al. 2021). In our current study, we propose that the application of 5 ppm of RoEO is adequate to enhance salt stress tolerance in durum wheat plants. Nevertheless, understanding its precise mode of action is a complex matter that necessitates further investigation. Binbir et al. (2019) demonstrated that the germination rate decreased, and the dry weight of the seedlings increased as a consequence of treating corn seeds with lavender EO. Ben-Jabeur et al. (2022) illustrated that employing the seed coating technique with thyme EO resulted in enhanced vegetative growth and grain yield. This improvement was associated with the regulation of ABA (abscisic acid) in plants experiencing drought stress. Hara (2020) reported that biostimulants containing EO heat tolerance enhancers (HTLEs) such as isothiocyanates and monoterpenes may be useful in reducing yield and crop quality losses due to various stresses, especially heat. Increased yields were obtained with thyme EO. Basu et al. (2016) demonstrated that elevated chlorophyll levels resulting from EO treatments support the hypothesis that certain mechanisms are triggered to mitigate damage caused by drought stress. Consistent with these findings, our results indicate that RoEO alleviated the adverse effects of salt stress on photosynthetic efficiency, as evidenced by an increase in the chlorophyll fluorescence (Fv/Fm) value. The substantial increases in soluble sugar contents observed in RoEO-treated wheat seedlings under salt stress may be attributed to the potential role of RoEO in maintaining photosynthetic efficiency. The application of thyme EO was found to minimize chlorophyll damage and limit the accumulation of anthocyanins at the onset of severe stress. These observations suggest that thyme EO plays a protective role in preserving photosynthetic capacity, preventing photo-oxidation damage, and impeding stress-induced leaf senescence (Hsiao et al. 1984), which is related to the potential of thyme EO to enhance the peroxidase-mediated antioxidative mechanisms (Ben-Jabeur et al. 2015). Soluble sugars act as osmoregulators that tend to increase in plants under salt stress and are able to reduce membrane permeability and affect osmotic pressure (Chaves 1991; Baki et al. 2000). Proline has been reported to accumulate in many plant species under a wide range of environmental stress conditions (Ashraf and Harris 2004; Claussen 2005; Xiong et al. 2014). Thus, proline is known to play several roles in plants, especially in osmotic regulation and ROS removal (Szabados and Savouré 2010). In the present study, at 150 mM NaCl, RoEO treatment increased proline and leaf soluble carbohydrate contents compared to the untreated control plants. Consequently, the results indicated that RoEO could accumulate osmoregulatory substances to regulate the cell osmotic balance.
Salt stress often leads to excessive accumulation of ROS and causes oxidative damage to proteins, lipids, and nucleic acids. Excessive amounts of ROS in plant cells lead to lipid peroxidation and simultaneously increase the amounts of H2O2 and MDA. In this study, H2O2 and MDA accumulation increased significantly in the leaves of durum wheat under salt stress conditions but the exogenous application of RoEO reduced this increasing trend. This suggests that RoEO probably acts to protect the integrity of the cell membrane by minimizing oxidative stress, leading to lipid peroxidation. According to Bailly (2019), low levels of reactive oxygen species (ROS) positively impact germination, whereas high ROS levels can lead to oxidative damage, inhibiting seed germination. The heightened germination observed with seed priming using EO is attributed to the mitigation of ROS accumulation, maintaining a low level conducive to germination (Hussain et al. 2019). Antioxidant defenses, including the activation of SOD, CAT, and POD, often play an important role in preventing damage to plant cell membrane systems due to ROS accumulation (Jaleel et al. 2009; Khan et al. 2019; Li et al. 2020). In the present study, we reported that the activities of SOD, POD, and CAT increased in the leaves of durum wheat plants in response to salinity. These enzymes were able to scavenge free radicals and ROS, which led to effective protection of the plasma membrane and eventually reduced accumulation of lipid peroxidation products in the membrane. The activation of these enzymes was further enhanced when RoEO was applied. Therefore, these results demonstrated that RoEO could reduce the accumulation of ROS and prevent the deterioration of membrane structure and function in durum wheat cells in response to salt stress. A similar effect of EO application was revealed in avocado fruit. Exposure to thyme EO increased the activities of antioxidant enzymes (SOD, POD, and CAT) in comparison with untreated control fruit (Sellamuthu et al. 2013). Furthermore, Ben-Jabeur et al. (2015) validated the ability of thyme EO to safeguard tomato seedlings through the accumulation of peroxidases, which serve as the first line of defense against ROS. Additionally, EOs from Allium sativum and R. officinalis were identified as preservatives for quality parameters in treated strawberry fruits against Colletotrichum nymphaeae. This preservation was attributed to the stimulation of peroxidase activity and the synthesis of phenolic compounds (Hosseini et al. 2020).
As genes from NHX family encode tonoplast Na+/H+ antiporters responsible for Na+ sequestration in vacuoles, the enhanced expression of TdNHX1 gene detected in our study may indicate that similar mechanisms of salinity tolerance were probably activated by RoEO treatment. This assumption is supported by the elevated accumulation of osmolytes (proline and soluble sugars) to align osmolarity between vacuole and the cytoplasm. On the other hand, the SOS1 protein, localized in the plasma membrane, which partially mediates Na+ efflux from the cell expression of the TdSOS1 gene encoding this antiporter, was also elevated in RoEO-treated wheat, suggesting that removal of excess Na+ was facilitated by RoEO application. RoEO clearly promotes both mechanisms of Na+ neutralization in durum wheat. The biostimulatory activity of RoEOs was also manifested in boosted antioxidant activity. A greater accumulation of TdSOD and TdCAT transcripts occurred in parallel with the enhanced activity of the enzymes. Our results are in line with recent findings highlighting the crucial role of the genes encoding antioxidant enzymes in plant resilience in challenging environments. Another gene upregulated by RoEO treatment was TdGA20-ox1, encoding the enzyme of gibberellin (GA) biosynthetic pathway, which is responsible for the synthesis of active GA forms (Li et al. 2020). Increased expression of the gene was likely associated with higher accumulation of active GA, which facilitated the growth of the seedlings. A similar tolerance mechanism was reported for other monocotyledon species, such as rice (Li et al. 2020), grown under saline or saline-alkaline conditions.
The expression pattern of the TdNRT2.1 gene encoding nitrate transporter was not affected by either salinity or RoEO application. This may indicate that applied conditions did not interfere with nitrogen assimilation in durum wheat, unlike in salinity-stressed or maize (Ertani et al. 2013). On the other hand, we observed enhanced expression of the TdGS gene involved in nitrogen metabolism, particularly amino acid biosynthesis, including proline. Higher expression of this gene may be interrelated with intensified synthesis of free amino acids as osmoprotectants under RoEO application.
Conclusions
In conclusion, our study demonstrated the positive effects of RoEO during the early stages of seedling development as well as on salinity tolerance in the durum wheat cultivar “Mahmoudi.” At an optimal dose (5 ppm), RoEO acted as a biostimulant, increasing the germination rate and biomass accretion and ameliorating physiological performance under saline conditions. Our comprehensive biochemical and molecular analyses revealed that RoEO boosted the main defense mechanisms towards salinity stress: enhancement of photosynthetic efficiency, synthesis of osmoprotectants for osmotic adjustment, transcriptional modulation of salt stress-related genes, and ROS scavenging. Overall, the application of RoEO represents a promising environmentally friendly alternative approach for enhancing wheat tolerance and preserving plant yield potential under salt stress conditions, making it a preferred choice for sustainable agricultural practices. Considering the chemical composition of RoEO, future studies will be carried out to better understand the mechanism by which this EO exerts its biostimulatory effect through a targeted study on the main compounds.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to “èssenziale” Claudio Gori Agricultural Company, San Donato in Poggio (FI), Italy, for providing Rosmarinus officinalis L. essential oil. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large research group projects to Narjes Baazaoui (Project under grant number (RGP. 2/88/45)).
Author contribution
Rania Ben Saad: conceptualization, data curation, investigation, methodology, project administration, resources, software, validation, writing—original draft. Walid Ben Romdhane: conceptualization, formal analysis, funding acquisition, project administration, supervision, writing—review and editing. Alina Wiszniewska: visualization, writing—review and editing. Narjes Baazaoui: data curation, formal analysis. Mohamed Taieb Bouteraa: data curation, formal analysis. Yosra Chouaibi: data curation. Mohammad Y. Alfaifi: data curation. Miroslava Kačániová: visualization, writing—review and editing. Natália Čmiková: data curation, formal analysis, writing—review and editing. Anis Ben Hsouna: data curation, formal analysis, resources. Stefania Garzoli: funding acquisition, validation, methodology, project administration, resources, visualization, writing—original draft, supervision.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This research was partially funded by the Tunisian Ministry of Higher Education and Scientific Research (Program contract 2023–2026) and by the King Khalid University (Project under grant number (RGP. 2/88/45)).
Data availability
All generated data are included in this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abo Ghanima MM, Elsadek MF, Taha AE, Abd El-Hack ME, Alagawany M, Ahmed BM, Elshafie MM, El-Sabrout K (2020) Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals 10(2):245. 10.3390/ani10020245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharabsheh HM, Seleiman MF, Hewedy OA, Battaglia ML, Jalal RS, Alhammad BA, Schillaci C, Ali N, Al-Doss A (2021) Field crop responses and management strategies to mitigate soil salinity in modern agriculture: a review. Agronomy 11(11):2299. 10.3390/agronomy11112299 [Google Scholar]
- Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166(1):3–16. 10.1016/j.plantsci.2003.10.024 [Google Scholar]
- Astaneh RK, Bolandnazar S, Nahandi FZ, Oustan S (2018) The effects of selenium on some physiological traits and K, Na concentration of garlic (Alliumsativum L.) under NaCl stress. Inf Process Agric 5(1):156–161. 10.1016/j.inpa.2017.09.003 [Google Scholar]
- Bailly C (2019) The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J 476(20):3019–3032. 10.1042/BCJ20190159 [DOI] [PubMed] [Google Scholar]
- Baki GKA, Siefritz F, Man H-M, Weiner H, Kaldenhoff R, Kaiser WM (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23(5):515–521. 10.1046/j.1365-3040.2000.00568.x [Google Scholar]
- Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000Research 5:1554. 10.12688/f1000research.7678.1 [DOI] [PMC free article] [PubMed]
- Belasli A, Ben Miri Y, Aboudaou M, Aït Ouahioune L, Montañes L, Ariño A, Djenane D (2020) Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurusnobilis L.): potential use as wheat preservative. Food Sci Nutr 8(9):4717–4729. 10.1002/fsn3.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Akacha B, Švarc-Gajić J, Elhadef K, Ben Saad R, Brini F, Mnif W, Smaoui S, Ben Hsouna A (2022) The essential oil of Tunisian halophyte Lobulariamaritima: a natural food preservative agent of ground beef meat. Life 12(10):1571. 10.3390/life12101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Akacha B, Ben Hsouna A, Generalić Mekinić I, Ben Belgacem A, Ben Saad R, Mnif W, Kačániová M, Garzoli S (2023) Salviaofficinalis L. and Salviasclarea essential oils: chemical composition, biological activities and preservative effects against Listeria monocytogenes inoculated into minced beef meat. Plants 12:3385. 10.3390/plants12193385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hsouna A, Ben Saad R, Zouari N, Ben Romdhane W, Brini F, Ben Salah R (2019) Stress associated protein from Lobulariamaritima: heterologous expression, antioxidant and antimicrobial activities with its preservative effect against Listeriamonocytogenes inoculated in beef meat. Int J Biol Macromol 132:888–896. 10.1016/j.ijbiomac.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Ben Hsouna A, Dhibi S, Dhifi W, Ben Saad R, Brini F, Hfaidh N, Almeida JRGDS, Mnif W (2022) Lobulariamaritima leave extract, a nutraceutical agent with antioxidant activity, protects against CCl4-induced liver injury in mice. Drug Chem Toxicol 45(2):604–616. 10.1080/01480545.2020.1742730 [DOI] [PubMed] [Google Scholar]
- Ben Hsouna A, Ghneim-Herrera T, Ben Romdhane W, Dabbous A, Ben Saad R, Brini F, Abdelly C, Ben Hamed K (2020) Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct Plant Biol 47(10):912. 10.1071/FP19303 [DOI] [PubMed] [Google Scholar]
- Ben Hsouna A, Hamdi N, Ben Halima N, Abdelkafi S (2013) Characterization of essential oil from Citrusaurantium L. flowers: antimicrobial and antioxidant activities. J Oleo Sci 62(10):763–772. 10.5650/jos.62.763 [DOI] [PubMed] [Google Scholar]
- Ben-Jabeur M, Vicente R, López-Cristoffanini C, Alesami N, Djébali N, Gracia-Romero A, Serret MD, López-Carbonell M, Araus JL, Hamada W (2019) A novel aspect of essential oils: coating seeds with thyme essential oil induces drought resistance in wheat. Plants 8(10):371. 10.3390/plants8100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jabeur M, Chamekh Z, Jallouli S, Ayadi S, Serret MD, Araus JL, Trifa Y, Hamada W (2022) Comparative effect of seed treatment with thyme essential oil and Paraburkholderiaphytofirmans on growth, photosynthetic capacity, grain yield, δ 15 N and δ 13 C of durum wheat under drought and heat stress. Ann Appl Biol 181(1):58–69. 10.1111/aab.12754 [Google Scholar]
- Ben-Jabeur M, Ghabri E, Myriam M, Hamada W (2015) Thyme essential oil as a defense inducer of tomato against gray mold and fusarium wilt. Plant Physiol Biochem 94:35–40. 10.1016/j.plaphy.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Ben Romdhane W, Ben-Saad R, Meynard D, Verdeil J-L, Azaza J, Zouari N, Fki L, Guiderdoni E, Al-Doss A, Hassairi A (2017) Ectopic expression of Aeluropuslittoralis plasma membrane protein gene AlTMP1 confers abiotic stress tolerance in transgenic tobacco by improving water status and cation homeostasis. Int J Mol Sci 18(4):692. 10.3390/ijms18040692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Romdhane W, Al-Ashkar I, Ibrahim A, Sallam M, Al-Doss A, Hassairi A (2024) Aeluropuslittoralis stress-associated protein promotes water deficit resilience in engineered durum wheat. Heliyon 10(10):e30933. 10.1016/j.heliyon.2024.e30933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Romdhane W, Ben Saad R, Meynard D, Zouari N, Tarroum M, Ali A, Droc G, Périn C, Morel J-B, Fki L, Al-Doss A, Guiderdoni E, Hassairi A (2022) Expression of an A20/AN1 stress-associated protein from Aeluropus littoralis in rice deregulates stress-related genes. J Plant Growth Regul 41(2):848–862. 10.1007/s00344-021-10344-z [Google Scholar]
- Ben Saad R, Ben Halima N, Ghorbel M, Zouari N, Ben Romdhane W, Guiderdoni E, Al-Doss A, Hassairi A (2018) AlSRG1, a novel gene encoding an RRM-type RNA-binding protein (RBP) from Aeluropus littoralis, confers salt and drought tolerance in transgenic tobacco. Environ Exp Bot 150:25–36. 10.1016/j.envexpbot.2018.03.002 [Google Scholar]
- Ben Saad R, Ben Romdhane W, Bouteraa MT, Jemli S, Ben Hsouna A, Hassairi A (2024) Development of a marker-free engineered durum wheat overexpressing Lobulariamaritima GASA1 with improved drought tolerance. Plant Physiol Biochem 212:108775. 10.1016/j.plaphy.2024.108775 [DOI] [PubMed] [Google Scholar]
- Ben Saad R, Ben Romdhane W, Bouteraa MT, Jrad O, Ben Hsouna A (2022) LobulariaMaritima thioredoxin-H2 gene mitigates salt and osmotic stress damage in tobacco by modeling plant antioxidant system. Plant Growth Regul 97(1):101–115. 10.1007/s10725-022-00805-0 [Google Scholar]
- Ben Saad R, Fabre D, Mieulet D, Meynard D, Dingkuhn M, Al-Doss A, Guiderdoni E, Hassairi A (2012) Expression of the Aeluropuslittoralis AlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant Cell Environ 35(3):626–643. 10.1111/j.1365-3040.2011.02441.x [DOI] [PubMed] [Google Scholar]
- Binbir U, Coşkun Y, Türkmen C, Cikili Y, Taş İ (2019) Alleloopathic effects of lavender (Lavandula x intermedia) essential oil on the seed germination and seedling development of dent corn (Zea mays indentata Sturt.). III. International ISCMP Joint Science of Materials and Polymers Piristina Kosovo
- Bi̇ngöl Ö, Battal P (2017) Verbascum cheiranthifolium Boiss. var. asperulum (Boiss.) Murb. ve Salvia limbata C. A. Meyer Ekstraktlarının Zea mays L. ve Portulaca oleraceae L. Tohumlarının Çimlenmesi Üzerine Allelopatik Etkisinin Araştırılması. Türkiye Tarımsal Araştırmalar Derg 4(2). 10.19159/tutad.299228
- Bouteraa MT, Mishra A, Romdhane WB, Hsouna AB, Siddique KHM, Saad RB (2022) Bio-stimulating effect of natural polysaccharides from Lobulariamaritima on durum wheat seedlings: improved plant growth, salt stress tolerance by modulating biochemical responses and ion homeostasis. Plants 11(15):1991. 10.3390/plants11151991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgari R, Franzoni G, Ferrante A (2019) Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9(6):306. 10.3390/agronomy9060306 [Google Scholar]
- Canellas LP, Olivares FL, Aguiar NO, Jones DL, Nebbioso A, Mazzei P, Piccolo A (2015) Humic and fulvic acids as biostimulants in horticulture. Sci Hortic 196:15–27. 10.1016/j.scienta.2015.09.013 [Google Scholar]
- Chaves MM (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42(1):1–16. 10.1093/jxb/42.1.1 [Google Scholar]
- Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168(1):241–248. 10.1016/j.plantsci.2004.07.039 [Google Scholar]
- Da Silva Bomfim N, Kohiyama CY, Nakasugi LP, Nerilo SB, Mossini SAG, Romoli JCZ, Graton Mikcha JM, Abreu Filho BAD, Machinski M Jr (2020) Antifungal and antiaflatoxigenic activity of rosemary essential oil (RosmarinusOfficinalis L.) against Aspergillus flavus. Food Addit Contam Part A 37(1):153–161. 10.1080/19440049.2019.1678771 [DOI] [PubMed] [Google Scholar]
- Day S (2016) Impact of essential oils obtained from safflower stem and roots on germination and seedling growth of wheat barley sunflower and chickpea. Turkish Journal Of Agriculture - Food Science And Technology 4(8):706–711
- Ertani A, Schiavon M, Muscolo A, Nardi S (2013) Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zeamays L. plants. Plant Soil 364:145–158. 10.1007/s11104-012-1335-z [Google Scholar]
- Farooq M, Nadeem F, Arfat MY, Nabeel M, Musadaq S, Cheema SA, Nawaz A (2018) Exogenous application of allelopathic water extracts helps improving tolerance against terminal heat and drought stresses in bread wheat (Triticumaestivum L. Em. Thell.). J Agron Crop Sci 204(3):298–312. 10.1111/jac.12261 [Google Scholar]
- Hara M (2020) Potential use of essential oils to enhance heat tolerance in plants. Z Für Naturforschung C 75(7–8):225–231. 10.1515/znc-2019-0233 [DOI] [PubMed] [Google Scholar]
- Hara M, Harazaki A, Tabata K (2013) Administration of isothiocyanates enhances heat tolerance in Arabidopsisthaliana. Plant Growth Regul 69(1):71–77. 10.1007/s10725-012-9748-5 [Google Scholar]
- Hashemi Gahruie H, Hosseini SMH, Taghavifard MH, Eskandari MH, Golmakani M-T, Shad E (2017) Lipid oxidation, color changes, and microbiological quality of frozen beef burgers incorporated with shirazi thyme, cinnamon, and rosemary extracts. J Food Qual 2017:1–9. 10.1155/2017/6350156 [Google Scholar]
- Hsiao AI, Vidaver W, Quick WA (1984) Acidification, growth promoter, and red light effects on germination of skotodormant lettuce seeds (Lactucasativa). Can J Bot 62(6):1108–1115. 10.1139/b84-154 [Google Scholar]
- Hosseini S, Amini J, Saba MK, Karimi K, Pertot I (2020) Preharvest and postharvest application of garlic and rosemary essential oils for controlling anthracnose and quality assessment of strawberry fruit during cold storage. Front Microbiol 11:1855. 10.3389/fmicb.2020.01855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Hussain S, Khaliq A, Ali S, Khan I (2019) Physiological, biochemical, and molecular aspects of seed priming. In Priming and pretreatment of seeds and seedlings: implication in plant stress tolerance and enhancing productivity in crop plants; Hasanuzzaman M, Fotopoulos V (eds). Springer: Singapore, pp 43–62. 10.1007/978-981-13-8625-1_3
- Jalali-Heravi M, Moazeni RS, Sereshti H (2011) Analysis of Iranian rosemary essential oil: application of gas chromatography–mass spectrometry combined with chemometrics. J Chromatogr A 1218(18):2569–2576. 10.1016/j.chroma.2011.02.048 [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31(3):427–436. 10.1007/s11738-009-0275-6 [Google Scholar]
- Jordán MJ, Lax V, Rota MC, Lorán S, Sotomayor JA (2013) Effect of the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L essential oil and its polyphenolic extract. Ind Crops Prod 48:144–152. 10.1016/j.indcrop.2013.04.031 [Google Scholar]
- Kahramanoglu I, Usanmaz S (2021) Roles of citrus secondary metabolites in tree and fruit defence against pests and pathogens. Nat Resour Hum Health 1:51–62. 10.53365/nrfhh/141637 [Google Scholar]
- Kesraoui S, Andrés MF, Berrocal-Lobo M, Soudani S, Gonzalez-Coloma A (2022) Direct and indirect effects of essential oils for sustainable crop protection. Plants 11(16):2144. 10.3390/plants11162144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MN, Zhang J, Luo T, Liu J, Rizwan M, Fahad S, Xu Z, Hu L (2019) Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind Crops Prod 140:111597. 10.1016/j.indcrop.2019.111597 [Google Scholar]
- Kisiriko M, Anastasiadi M, Terry LA, Yasri A, Beale MH, Ward JL (2021) Phenolics from medicinal and aromatic plants: characterisation and potential as biostimulants and bioprotectants. Molecules 26(21):6343. 10.3390/molecules26216343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul O, Walia S, Dhaliwal GS (2008) Essential oils as green pesticides potential and constraints. Biopestic Int 4(1):63–84
- Laraus J (2004) The problems of sustainable water use in the Mediterranean and research requirements for agriculture. Ann Appl Biol 144(3):259–272. 10.1111/j.1744-7348.2004.tb00342.x [Google Scholar]
- Lemos MF, Lemos MF, Pacheco HP, Endringer DC, Scherer R (2015) Seasonality modifies rosemary’s composition and biological activity. Ind Crops Prod 70:41–47. 10.1016/j.indcrop.2015.02.062 [Google Scholar]
- Li Z, Cheng B, Peng Y, Zhang Y (2020) Adaptability to abiotic stress regulated by γ-aminobutyric acid in relation to alterations of endogenous polyamines and organic metabolites in creeping bentgrass. Plant Physiol Biochem 157:185–194 [DOI] [PubMed] [Google Scholar]
- Linstrom P, Mallard W (2014) NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899. Web address: 10.18434/T4D303. (Retrieved in December 2023)
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lutts S, Benincasa P, Wojtyla L, Kubala S, Pace R, Lechowska K, Quinet M, Garnczarska M (2016) Seed priming new comprehensive approaches for an old empirical technique. In: Araujo S, Balestrazzi A (eds) A book new challenges in seed biology basic and translational research driving seed technology chapter
- Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic Press [Google Scholar]
- Moumni M, Romanazzi G, Najar B, Pistelli L, Ben Amara H, Mezrioui K, Karous O, Chaieb I, Allagui MB (2021) Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of Cucurbits. Antibiotics 10(2):104. 10.3390/antibiotics10020104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile SH, Thiruvengadam M, Wang Y, Samynathan R, Shariati MA, Rebezov M, Nile A (2013) Physiological performance of wheat seeds coated with micronutrients. J Seed Sci 35:28–34 [Google Scholar]
- Raj AB, Raj SK (2019) Seed priming: an approach towards agricultural sustainability. J Appl Nat Sci 11(1):227–234. 10.31018/jans.v11i1.2010 [Google Scholar]
- Sellamuthu PS, Sivakumar D, Soundy P, Korsten L (2013) Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol Technol 81:66–72. 10.1016/j.postharvbio.2013.02.007 [Google Scholar]
- Singh N, Joshi E, Sasode D, Dangi RS, Chouhan N (2020) Soil fertility, macro and micro nutrient uptake and their use efficiencies under integrated nutrient management in groundnut (Arachis hypogaea L.). Int J Chem Stud 8(1):1983–1987. 10.22271/chemi.2020.v8.i1ac.8556 [Google Scholar]
- Souri MK (2016) Aminochelate fertilizers: the new approach to theold problem; a review. Open Agric 1(1):118–123. 10.1515/opag-2016-0016 [Google Scholar]
- Souri MK, Bakhtiarizade M (2019) Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci Hortic 243:472–476. 10.1016/j.scienta.2018.08.056 [Google Scholar]
- Souri MK, Römheld V (2009) Split daily applications of ammonium can not ameliorate ammonium toxicity in tomato plants. Hortic Environ Biotechnol 50(5):384–391 [Google Scholar]
- Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Tahami MK, Jahan M, Khalilzadeh H, Mehdizadeh M (2017) Plant growth promoting rhizobacteria in an ecological cropping system: a study on basil (Ocimumbasilicum L.) essential oil production. Ind Crops Prod 107:97–104. 10.1016/j.indcrop.2017.05.020 [Google Scholar]
- Tauler M, Baraza E (2015) Improving the acclimatization and establishment of Arundo donax L. plantlets, a promising energy crop, using a mycorrhiza-based biofertilizer. Ind Crops Prod 66:299–304. 10.1016/j.indcrop.2014.12.039 [Google Scholar]
- Tavares LC, de Rufino CA, Brunes AP, Friedrich FF, Barros ACSA, Villela FAP, Tworkoski T (2002) Herbicide effects of essential oils. Weed Sci 50(4):425–431. 10.1614/0043-1745(2002)050[0425:HEOEO]2.0.CO;2 [Google Scholar]
- Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4(1):5. 10.1186/s40538-017-0089-5 [Google Scholar]
- Yamauchi Y, Kunishima M, Mizutani M, Sugimoto Y (2015) Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci Rep 5(1):8030. 10.1038/srep08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Shao H, Shao C, Chen P, Zhao S, Brestic M, Chen X (2013) Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant 35(10):2867–2878. 10.1007/s11738-013-1325-7 [Google Scholar]
- Xiong H, Li J, Liu P, Duan J, Zhao Y, Guo X, Li Y, Zhang H, Ali J, Li Z (2014) Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS ONE 9(3):e92913. 10.1371/journal.pone.0092913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Cao H, Fang W, Pan J, Chen J, Zhang J, Shen W (2017) Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: a case study on germination. Ecotoxicol Environ Saf 145:303–312. 10.1016/j.ecoenv.2017.07.055 [DOI] [PubMed] [Google Scholar]
- Zulfiqar F (2021) Effect of seed priming on horticultural crops. Sci Hortic 286:110197. 10.1016/j.scienta.2021.110197 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All generated data are included in this article.