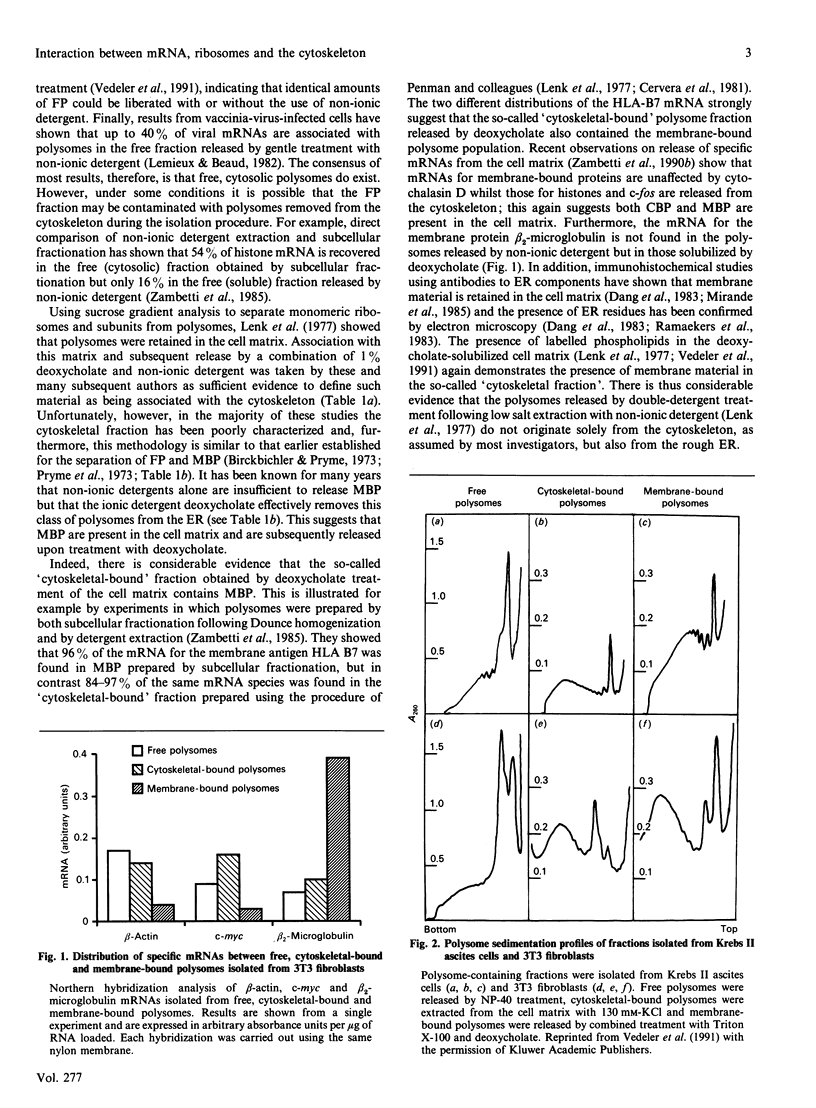
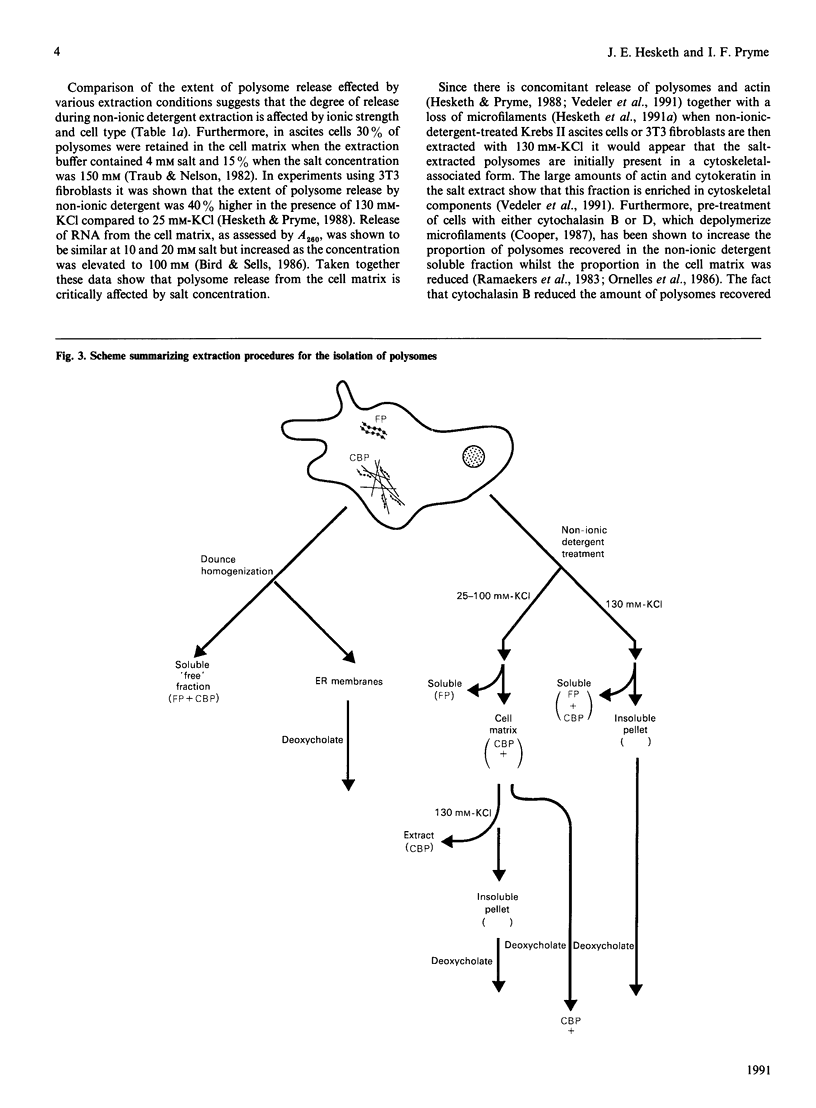
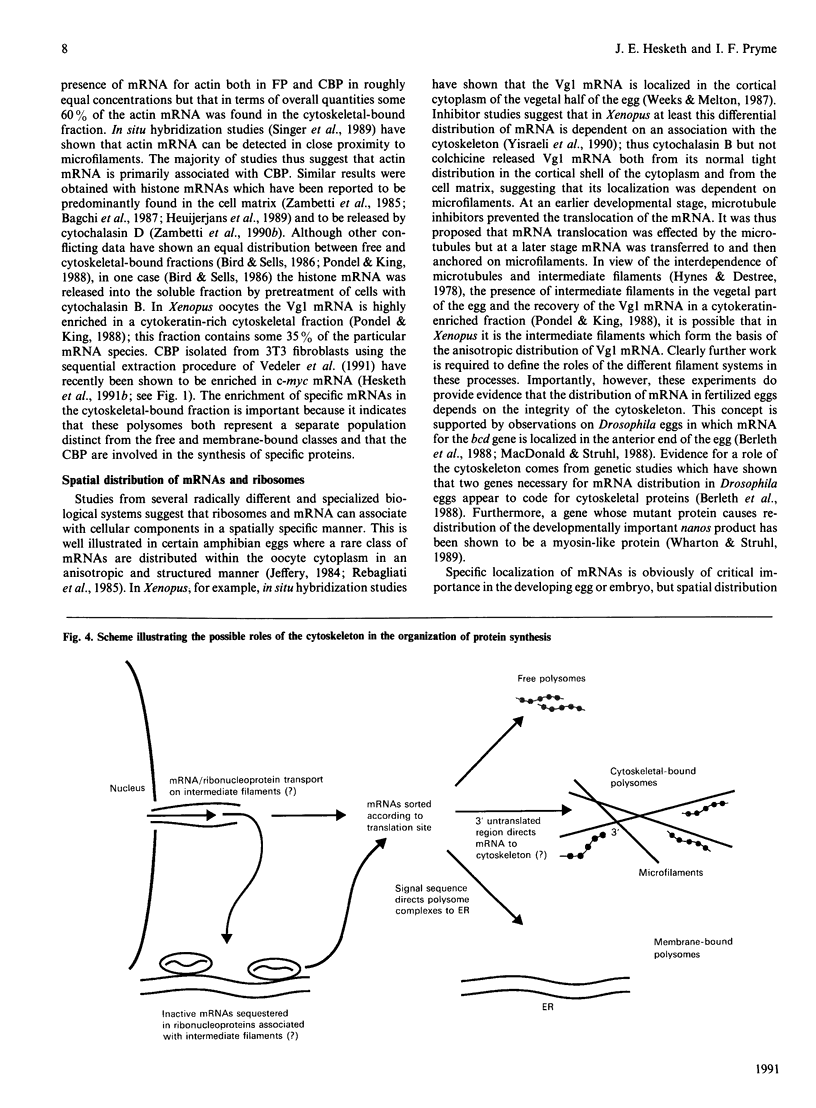
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Fey E. G., Pike S. F., Taylorson C. J., White H. A., Rabin B. R. Preparation and properties of a complex from rat liver of polyribosomes with components of the cytoskeleton. Biochem J. 1983 Oct 15;216(1):215–226. doi: 10.1042/bj2160215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik M., Maschio F. Segregation of specific classes of messenger RNA into free and membrane-bound polysomes. Eur J Biochem. 1981 Feb;114(2):271–284. doi: 10.1111/j.1432-1033.1981.tb05146.x. [DOI] [PubMed] [Google Scholar]
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Bag J., Pramanik S. Attachment of mRNA to the cytoskeletal framework and translational control of gene expression in rat L6 muscle cells. Biochem Cell Biol. 1987 Jun;65(6):565–575. doi: 10.1139/o87-073. [DOI] [PubMed] [Google Scholar]
- Bagchi T., Larson D. E., Sells B. H. Cytoskeletal association of muscle-specific mRNAs in differentiating L6 rat myoblasts. Exp Cell Res. 1987 Jan;168(1):160–172. doi: 10.1016/0014-4827(87)90425-3. [DOI] [PubMed] [Google Scholar]
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birckbichler P. J., Pryme I. F. Fractionation of membrane-bound polysomes, free polysomes, and nuclei from tissue-cultured cells. Eur J Biochem. 1973 Mar 1;33(2):368–373. doi: 10.1111/j.1432-1033.1973.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Bird R. C., Sells B. H. Cytoskeleton involvement in the distribution of mRNP complexes and small cytoplasmic RNAs. Biochim Biophys Acta. 1986 Dec 18;868(4):215–225. doi: 10.1016/0167-4781(86)90057-6. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Relation of ribonuclease and ribonuclease inhibitor to the isolation of polysomes from rat liver. Proc Natl Acad Sci U S A. 1966 May;55(5):1283–1288. doi: 10.1073/pnas.55.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. II. Interaction of ribosomes and membranes. J Mol Biol. 1967 Jun 14;26(2):293–301. doi: 10.1016/0022-2836(67)90298-7. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Darveau A., Sonenberg N. Effect of viral infection on host protein synthesis and mRNA association with the cytoplasmic cytoskeletal structure. J Cell Biol. 1985 Apr;100(4):1209–1218. doi: 10.1083/jcb.100.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnieu A., Piechaczyk M., Marty L., Cuny M., Blanchard J. M., Fort P., Jeanteur P. Sequence determinants of c-myc mRNA turn-over: influence of 3' and 5' non-coding regions. Oncogene Res. 1988 Sep;3(2):155–166. [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancker P., Löw I., Hasselbach W., Wieland T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975 Aug 19;400(2):407–414. doi: 10.1016/0005-2795(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Yang D. C., Pollard T. D. Association of methionyl-tRNA synthetase with detergent-insoluble components of the rough endoplasmic reticulum. J Cell Biol. 1983 Apr;96(4):1138–1147. doi: 10.1083/jcb.96.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L., Banker G. A., Steward O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature. 1987 Dec 3;330(6147):477–479. doi: 10.1038/330477a0. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Martins de Sa C., Harper F., Olink-Coux M., Huesca M., Scherrer K. The association of prosomes with some of the intermediate filament networks of the animal cell. J Cell Biol. 1988 Oct;107(4):1517–1530. doi: 10.1083/jcb.107.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardesty B., Kudlicki W., Chen S. C., Fullilove S., Kramer G. Involvement of the membrane skeleton in the regulation of the cAMP-independent protein kinase and a protein phosphatase that control protein synthesis. Haematol Blood Transfus. 1987;31:268–273. doi: 10.1007/978-3-642-72624-8_57. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Campbell G. P., Reeds P. J. Rapid response of protein synthesis to insulin in 3T3 cells: effects of protein kinase C depletion and differences from the response to serum repletion. Biosci Rep. 1986 Sep;6(9):797–804. doi: 10.1007/BF01117102. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Campbell G. P., Whitelaw P. F. c-myc mRNA in cytoskeletal-bound polysomes in fibroblasts. Biochem J. 1991 Mar 1;274(Pt 2):607–609. doi: 10.1042/bj2740607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J. E., Horne Z., Campbell G. P. Immunohistochemical evidence for an association of ribosomes with microfilaments in 3T3 fibroblasts. Cell Biol Int Rep. 1991 Feb;15(2):141–150. doi: 10.1016/0309-1651(91)90105-r. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Pryme I. F. Evidence that insulin increases the proportion of polysomes that are bound to the cytoskeleton in 3T3 fibroblasts. FEBS Lett. 1988 Apr 11;231(1):62–66. doi: 10.1016/0014-5793(88)80703-8. [DOI] [PubMed] [Google Scholar]
- Heuijerjans J. H., Pieper F. R., Ramaekers F. C., Timmermans L. J., Kuijpers H., Bloemendal H., Van Venrooij W. J. Association of mRNA and eIF-2 alpha with the cytoskeleton in cells lacking vimentin. Exp Cell Res. 1989 Apr;181(2):317–330. doi: 10.1016/0014-4827(89)90091-8. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne Z., Hesketh J. Immunological localization of ribosomes in striated rat muscle. Evidence for myofibrillar association and ontological changes in the subsarcolemmal:myofibrillar distribution. Biochem J. 1990 May 15;268(1):231–236. doi: 10.1042/bj2680231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne Z., Hesketh J. Increased association of ribosomes with myofibrils during the skeletal-muscle hypertrophy induced either by the beta-adrenoceptor agonist clenbuterol or by tenotomy. Biochem J. 1990 Dec 15;272(3):831–833. doi: 10.1042/bj2720831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Fulton A. B. Cotranslational assembly of myosin heavy chain in developing cultured skeletal muscle. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6174–6178. doi: 10.1073/pnas.84.17.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R. Spatial distribution of messenger RNA in the cytoskeletal framework of ascidian eggs. Dev Biol. 1984 Jun;103(2):482–492. doi: 10.1016/0012-1606(84)90335-x. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Lara J., Wambach M. Nontranslated cellular mRNAs are associated with the cytoskeletal framework in influenza virus or adenovirus infected cells. Virology. 1989 Apr;169(2):312–322. doi: 10.1016/0042-6822(89)90156-6. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Rome L. H. Vaults: large cytoplasmic RNP's that associate with cytoskeletal elements. Mol Biol Rep. 1990;14(2-3):121–122. doi: 10.1007/BF00360441. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lemieux R., Beaud G. Expression of vaccinia virus early mRNA in Ehrlich ascites tumor cells. 2. Part of the polysomes at an early stage of virus infection are not bound to the cytoskeleton. Eur J Biochem. 1982 Dec 15;129(2):273–279. doi: 10.1111/j.1432-1033.1982.tb07049.x. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Lequang H., Gauthier D. Isolation of hamster brain polyribosomes-cytoskeleton complexes. Neurochem Res. 1989 Mar;14(3):239–243. doi: 10.1007/BF00971317. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Meadus W. J., Pramanik S., Bag J. Cytoskeleton-bound mRNA for a 40-kDa polypeptide in rat L6 cells is not always translated. Exp Cell Res. 1990 Mar;187(1):25–32. doi: 10.1016/0014-4827(90)90111-m. [DOI] [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Louvard D., Reggio H., Pailliez J. P., Waller J. P. Association of an aminoacyl-tRNA synthetase complex and of phenylalanyl-tRNA synthetase with the cytoskeletal framework fraction from mammalian cells. Exp Cell Res. 1985 Jan;156(1):91–102. doi: 10.1016/0014-4827(85)90264-2. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Nicosia R. F., Olsen C., Hille M. B., Jeffery W. R. The cytoskeletal framework of sea urchin eggs and embryos: developmental changes in the association of messenger RNA. Dev Biol. 1983 Feb;95(2):447–458. doi: 10.1016/0012-1606(83)90046-5. [DOI] [PubMed] [Google Scholar]
- Nielsen P., Goelz S., Trachsel H. The role of the cytoskeleton in eukaryotic protein synthesis. (A minireview). Cell Biol Int Rep. 1983 Apr;7(4):245–254. doi: 10.1016/0309-1651(83)90057-7. [DOI] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Pondel M. D., King M. L. Localized maternal mRNA related to transforming growth factor beta mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7612–7616. doi: 10.1073/pnas.85.20.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryme I. F. Domains of rough endoplasmic reticulum (a review). Mol Cell Biochem. 1989 Jun 1;87(2):93–103. doi: 10.1007/BF00219253. [DOI] [PubMed] [Google Scholar]
- Pryme I. F., Garatun-Tjeldstö O., Birckbichler P. J., Weltman J. K., Dowben R. M. Synthesis of immunoglobulins by membrane-bound polysomes and free polysomes from plasmacytoma cells. Eur J Biochem. 1973 Mar 1;33(2):374–378. doi: 10.1111/j.1432-1033.1973.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Pryme I. F. The nuclear-associated endoplasmic reticulum. Int J Biochem. 1989;21(2):119–125. doi: 10.1016/0020-711x(89)90099-2. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Benedetti E. L., Dunia I., Vorstenbosch P., Bloemendal H. Polyribosomes associated with microfilaments in cultured lens cells. Biochim Biophys Acta. 1983 Sep 9;740(4):441–448. doi: 10.1016/0167-4781(83)90093-3. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Savitz A. J., Meyer D. I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature. 1990 Aug 9;346(6284):540–544. doi: 10.1038/346540a0. [DOI] [PubMed] [Google Scholar]
- Scherrer K. Prosomes, subcomplexes of untranslated mRNP. Mol Biol Rep. 1990 Feb;14(1):1–9. doi: 10.1007/BF00422709. [DOI] [PubMed] [Google Scholar]
- Schröder H. C., Diehl-Seifert B., Rottmann M., Messer R., Bryson B. A., Agutter P. S., Müller W. E. Functional dissection of nuclear envelope mRNA translocation system: effects of phorbol ester and a monoclonal antibody recognizing cytoskeletal structures. Arch Biochem Biophys. 1988 Mar;261(2):394–404. doi: 10.1016/0003-9861(88)90355-4. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Pitot H. C. Functional studies of polysome-membrane interactions in vitro. Adv Enzyme Regul. 1973;11:255–272. doi: 10.1016/0065-2571(73)90019-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Langevin G. L., Lawrence J. B. Ultrastructural visualization of cytoskeletal mRNAs and their associated proteins using double-label in situ hybridization. J Cell Biol. 1989 Jun;108(6):2343–2353. doi: 10.1083/jcb.108.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Steward O., Levy W. B. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982 Mar;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Actin mRNA localizes in the absence of protein synthesis. J Cell Biol. 1990 Dec;111(6 Pt 1):2397–2403. doi: 10.1083/jcb.111.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svardal A. M., Pryme I. F. Aspects of the role of the endoplasmic reticulum in protein synthesis. Subcell Biochem. 1980;7:117–170. doi: 10.1007/978-1-4615-7948-9_3. [DOI] [PubMed] [Google Scholar]
- Svardal A. M., Pryme I. F., Dalen H. Ultrastructure and polysome content of the microsomal subfractions of mouse plasmacytoma cells. Mol Cell Biochem. 1981 Feb 11;34(3):165–175. doi: 10.1007/BF02359621. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Lolait S. J., Mathy J. P., Baum R. Association of mitochondria with intermediate filaments and of polyribosomes with cytoplasmic actin. Cell Tissue Res. 1980;211(1):163–169. doi: 10.1007/BF00233731. [DOI] [PubMed] [Google Scholar]
- Traub P., Nelson W. J. Polyribosomes are not associated with vimentin-type intermediate filaments in Ehrlich ascites tumor cells. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1177–1185. doi: 10.1515/bchm2.1982.363.2.1177. [DOI] [PubMed] [Google Scholar]
- Vedeler A., Pryme I. F., Hesketh J. E. Insulin and step-up conditions cause a redistribution of polysomes among free, cytoskeletal-bound and membrane-bound fractions in Krebs II ascites cells. Cell Biol Int Rep. 1990 Mar;14(3):211–218. doi: 10.1016/s0309-1651(05)80003-7. [DOI] [PubMed] [Google Scholar]
- Vedeler A., Pryme I. F., Hesketh J. E. The characterization of free, cytoskeletal and membrane-bound polysomes in Krebs II ascites and 3T3 cells. Mol Cell Biochem. 1991 Feb 2;100(2):183–193. doi: 10.1007/BF00234167. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Struhl G. Structure of the Drosophila BicaudalD protein and its role in localizing the the posterior determinant nanos. Cell. 1989 Dec 1;59(5):881–892. doi: 10.1016/0092-8674(89)90611-9. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Stereo high-voltage electron microscopy of whole cells of the human diploid line, WI-38. Am J Anat. 1976 Nov;147(3):303–323. doi: 10.1002/aja.1001470305. [DOI] [PubMed] [Google Scholar]
- Yang F., Demma M., Warren V., Dharmawardhane S., Condeelis J. Identification of an actin-binding protein from Dictyostelium as elongation factor 1a. Nature. 1990 Oct 4;347(6292):494–496. doi: 10.1038/347494a0. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Melton D. A. The material mRNA Vg1 is correctly localized following injection into Xenopus oocytes. Nature. 1988 Dec 8;336(6199):592–595. doi: 10.1038/336592a0. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Schmidt W., Stein G., Stein J. Subcellular localization of histone messenger RNAs on cytoskeleton-associated free polysomes in HeLa S3 cells. J Cell Physiol. 1985 Nov;125(2):345–353. doi: 10.1002/jcp.1041250225. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Stein J., Stein G. Role of messenger RNA subcellular localization in the posttranscriptional regulation of human histone gene expression. J Cell Physiol. 1990 Jul;144(1):175–182. doi: 10.1002/jcp.1041440123. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Wilming L., Fey E. G., Penman S., Stein J., Stein G. Differential association of membrane-bound and non-membrane-bound polysomes with the cytoskeleton. Exp Cell Res. 1990 Dec;191(2):246–255. doi: 10.1016/0014-4827(90)90011-x. [DOI] [PubMed] [Google Scholar]
- Zumbé A., Stähli C., Trachsel H. Association of a Mr 50,000 cap-binding protein with the cytoskeleton in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2927–2931. doi: 10.1073/pnas.79.9.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]