Abstract
Producing hydrogen efficiently through water electrolysis could greatly reduce fossil fuel consumption. As well as this renewable energy source will also help combat global warming and boost economic investment opportunities. This paper studied some factors affecting the performance of oxy-hydrogen/hydroxy (HHO) gas generator, such as applied voltage (from 10.5 to 13.0 V) and electrolyte solution concentration (from 0.05 to 0.20 M), using a dry fuel cell based on the electrolyzing technique of water. The results revealed that the HHO gas production rate, power consumption, and temperature change of electrolyte solution increased significantly with increasing the tested applied voltage and electrolyte concentration. This study concluded that the optimum conditions for producing HHO gas ranged from 11.5 to 12.0 V for applied voltage and from 0.05 to 0.10 M for KOH concentration according to the lowest specific energy and highest HHO gas generator efficiency. Under the previous optimum conditions, the highest productivity, specific energy, and efficiency of the HHO gas generator were 343.9 cm3 min−1, 3.43 kW h m−3, and 53.79%, respectively, using 12.0 V for applied voltage and 0.10 M for electrolyte solution concentration. These findings provide an unambiguous direction for adjusting the operational factors (applied voltage and electrolyte concentration) for efficient HHO gas production and use in different applications. Furthermore, the required energy to operate the HHO gas generator can be obtained from renewable sources.
Keywords: Renewable energy, Hydrogen fuel, Oxy-hydrogen, HHO generator, Water electrolysis
Subject terms: Energy harvesting, Engineering
Introduction
Fossil fuels still supplies about 82% of all energy consumed globally1,2. However, fossil fuels generate greenhouse gas emissions such as CO, CO2, SOx, NOx, and unburned hydrocarbons (HC), contributing to climate change (global warming) and negatively impacting human health and the environment. Furthermore, fossil fuels become expensive due to increasing demand and the depletion of their sources3,4. In order to reduce the negative effects of the combustion of fossil fuels, it has become necessary to utilize renewable and ecologically friendly energy sources. Oxy-hydrogen or hydroxy (HHO) gas is a promising alternative fuel that has several advantages over fossil fuels5. The advantages of HHO gas include its high flammability, more oxygen, fast burning rate, and zero carbon compared to fossil fuels6. HHO gas is an effective fuel that can be used in different applications such as internal combustion engines, cooking, heating, desalinating water, welding, and metal cutting, etc. So oxy-hydrogen gas can be employed as an alternative energy source for solving the shortage problem of petroleum fuel and reducing environmental pollution7.
Holladay et al.8 and Nnabuife et al.9 classified water-splitting methods into three types: electrolysis, thermolysis, and photoelectrolysis10–12. Renewable and non-renewable resources are used to produce hydrogen gas9. Electrolysers can also use renewable energy surplus to produce green hydrogen, enhancing the electrical grid’s stability. In addition, medium- to long-term energy storage can be achieved with hydrogen13. The main features of the water-splitting methods are shown in Table 1.
Table 1.
A few characteristics of solar energy to hydrogen conversion methods according to Wang et al.14.
| Methods | Thermochemical | Electrolysis | Photoelectrolysis | Photochemical |
|---|---|---|---|---|
| Reaction mechanism | Thermal spitting | Electric potential | Electric potential | Photon-activated electron |
| Form of energy input | Thermal | Electricity | Electricity | Photon |
| Solar energy-capturing device | No | No | Yes | Yes |
| External or internal energy supply | External | External | Internal | Internal |
| Basic components for engineering apparatus | More than 3 thermal reactors | 2 electrodes and electrolyte | 2 electrodes, electrolyte, and sunlight window | At least 1 sensitizer, at least 1 catalyst, and sunlight window |
| H2 and O2 produced separately or in mixture | Separately | Separately | Mix | Mix |
| Overall production efficiency | 45% | 30% | 16% | 10% |
| Suitable for large scale production or fueling stations | Large scale | Medium scale and fueling station | Fueling station | Fueling station |
| Additional hydrogen distribution network | Needed | Depends on production scale | Not necessarily | Not necessarily |
Wang et al.14 found that the technology of water electrolysis powered by solar electricity is more mature than other techniques. Additionally, for small-scale hydrogen production in distributed facilities, certain methods, particularly water electrolysis, may be more cost-effective8.
The electrolysis technique refers to an electrolysis cell that can separate hydrogen (H2) and oxygen (O2) from water molecules using DC electricity15. Water electrolysis can produce HHO gas from dry or wet cells16. When comparing two types of HHO gas production cells (dry and wet cells), the dry cell is vastly superior to the wet cell, producing significantly more HHO gas under identical input conditions. For cell operation and maintenance, the dry cell is more dependable and suitable than its wet cell; its safety features are far more reassuring than those of the wet cell17. The dry cell is favorable because of its simplicity, easy manufacturing, and assembly. HHO dry cell is economical, made from locally available materials, and can be used with internal combustion engines supplying its required energy from the battery2. Furthermore, HHO gas can be applied as an additional fuel in a diesel or gasoline engine without any modifications or the need for a storage tank; the addition of HHO gas to a diesel engine led to increased torque output by 19.1% whereas the average values of specific fuel consumption, CO emission, HC emission was decreased by 14, 13.5, and 5% respectively18. The generator of HHO gas was designed, constructed, and consisted of 3 anodes (A), 3 cathodes (C), and 20 neutral (N) plates from SS grade of 316 L and investigated the effect of voltage, time, and electrolyte solution concentration; the authors reported that; increasing voltage, time and electrolyte solution concentration lead to increase the yield of hydroxy gas4.
Many types of electrolytes with different concentrations can be used for the production of HHO gas, such as potassium hydroxide (KOH), sodium chloride (NaCl), sodium hydroxide (NaOH), sulphuric acid (H2SO4), etc., are added to pure water for increasing the electrical conductivity19. KOH produces more HHO gas than NaOH because it has a higher stability and compatibility with metallic components20. Also, potassium hydroxide is the perfect electrolyte to increase the generation of the brown gas; furthermore, vertical orientation is ideal for plates at a gap of 2–3 mm between each plate21. El-Kassaby et al.22 reported that the highest production of HHO gas was 18 L h−1 using 2 neutral plates, 1 mm distance, and 6 g L−1 of KOH. Also, the thermal efficiency of gasoline engines was increased by 10%; consequently, fuel consumption decreased by 34%. As well as the CO, HC, and NOx values for exhaust gas emissions decreased to approximately 18, 14, and 15%, respectively. Alam and Pandey15 investigated the effect of DC current and voltage, electrolyte solution concentration, reaction temperature, and time on the HHO gas production rate. Their results indicated that the optimum production of HHO was obtained at 1 A current, 5 V potential, and 1 mol electrolytic concentration. Also, the increase in voltage, cell temperature, and electrolytic concentration improved hydroxy gas production by about 30–40% with a reduction in energy consumption by about 35%. Mustaqim & Maulana23 produced HHO gas by using different concentrations from NaOH (1, 2, 3, 4, and 5 M) as electrolyte and LaCoO3 (0, 0.025, 0.05, 0.33 and 0.67 wt%) as catalyst. They reported that; the optimum production of HHO occurs using 3 M of Sodium hydroxide, whereas; the addition of LaCoO3 as a catalyst led to a drop HHO gas production rate for all tested concentrations of catalyst. Whereas Almassri et al.24 studied the effects of the consumed power, and reaction time on the production of HHO by using a dry HHO cell and a solution of ammonium hydroxide (NH4OH) as an electrolyte; they found that the productivity of HHO increases with increasing the consumed power and reaction time, for producing of one kilogram of hydrogen the energy consumption was about 70.5600 MJ. Also, they reported that the ammonium hydroxide induced significant corrosion of metals, which is considered a drawback of ammonia hydroxide.
Despite the availability of studies related to testing the performance of dry cell HHO gas generators using water electrolysis, there is still a gap in the literature when examining the performance indicators. Furthermore, little research has been conducted on the applied voltage effect on the performance of the hydroxy gas generator within the voltage range of rechargeable batteries (12 V) in addition to photovoltaic cell systems that produce power at the same voltage which may be used to run the hydroxy gas generator to produce the green hydrogen fuel. Also, few researchers have focused on developing hydroxy gas generators on a small scale for sustainable energy purposes. This highlights the need for more research to determine the optimal operating conditions to achieve the highest efficiency level.
Therefore, the main target of this research was to develop and manufacture an oxy-hydrogen gas generator from a dry cell type with high energy-conversion efficiency from locally available materials, characterized by simplicity in construction, ease of maintenance, and operation. Additionally, study some factors affecting the performance of HHO gas generator. Specifically, the impact of applied voltage and electrolyte solution concentration on productivity, power consumption, change in electrolyte temperature, specific energy requirements, and efficiency of HHO gas generator were studied using water electrolyzing under a laboratory scale.
Materials and methods
HHO gas generator
The developed HHO gas generator (Fig. 1) consists of five main parts used for oxy-hydrogen (HHO) gas production: power supply, control and monitoring panel, electrolyte temperature gauge, electrolyzer, and measuring tool of HHO volume.
Fig. 1.
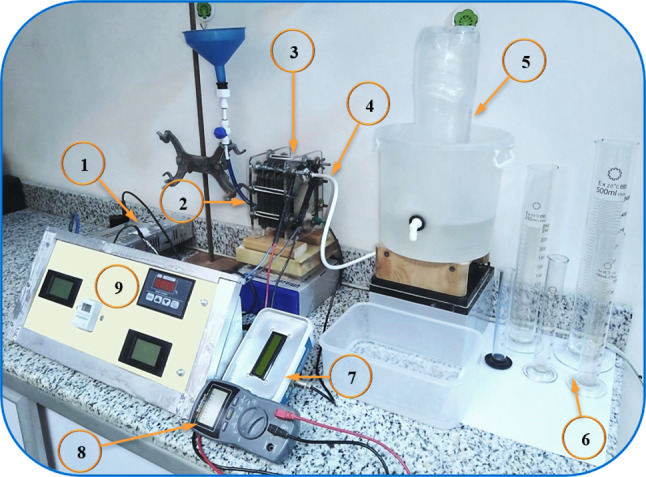
Final experimental system of HHO gas generator. (1) Power supply “DC”, (2) Electrolyte inlet, (3) Dry electrolyzer, (4) HHO outlet, (5) Bubbler, (6) Graduated cylinders, (7) Digital temperature gauge, (8) Digital AVO-meter and (9) Control and monitoring panel.
Power supply
The power supply was used to convert AC (alternating current) into DC (direct current). The main specifications of the power supply are as follows: model number: S-360-12, AC input: 110/220 V ± 15%, DC output: 12 V 30 A, and contain adjustable range for DC voltage from 10.44 to 15.24 V.
Control and monitoring panel
A simple panel was carried out for control and monitoring. This panel consists of (1) AC digital multi-function meter; the product type is PZEM 061. This device can simultaneously measure and show electrical parameters (voltage, current, power, and energy) while operating the system. AC Voltage measurement range is 80 to 260 V with accuracy of 1 V. Current measurement range is 0 to 100 A with accuracy of 0.01 A. Power measurement range is from 0 to 22 kW with accuracy of 0.1 W in range (0.0 to 999.9 W), 1 W in range (1000 to 9999W) and 0.1 kW in range (10.0 to 22.0 kW). (2) A programmable switch timer from type TM-615 was used to set the operating time of the experiment; this timer was manufactured by Sinotimer and can be automatically switched on and switched off according to experiment time. (3) DC digital multi-function meter with a shunt (50A/75 mV); the type of product is PZEM 051. Also, this device can be measured and show electrical parameters (voltage, current, power, and energy) simultaneously while operating the system. DC Voltage measurement range is 6.5 to 100 V with an accuracy of 0.01 V. Current measurement range is 0 to 100 A with accuracy of 0.01 A. Power measurement range is from 0 to 10 kW with accuracy of 0.1 W in range (0.0 to 999.9 W), 1 W in range (1000 to 9999W). (4) Adjustable PWM (pulse width modulation) was used to restrict the amount of electric current flowing into the HHO generator. PWM is one of the techniques for controlling current and voltage via regulating the ratio of pulse width to the period of a square signal in the form of an applied periodic voltage to the electric load as a power source25. The main features of the PWM module used are as follows: Model: Q8-42S, input voltage: 6–60 VDC, output current: 0–30 A, and regulation range 0–100%.
Digital electrolyte temperature gauge
The temperature gauge is an electronic circuit that consists of Arduino UNO R3 with microcontroller ATmega328 chip, waterproof temperature sensor, and Blue Screen LCD (1602) Display Module with IIC Interface for Arduino, as shown in Fig. 2. The temperature sensor was connected to the HHO cell to sense the temperature of the electrolyte solution.
Fig. 2.
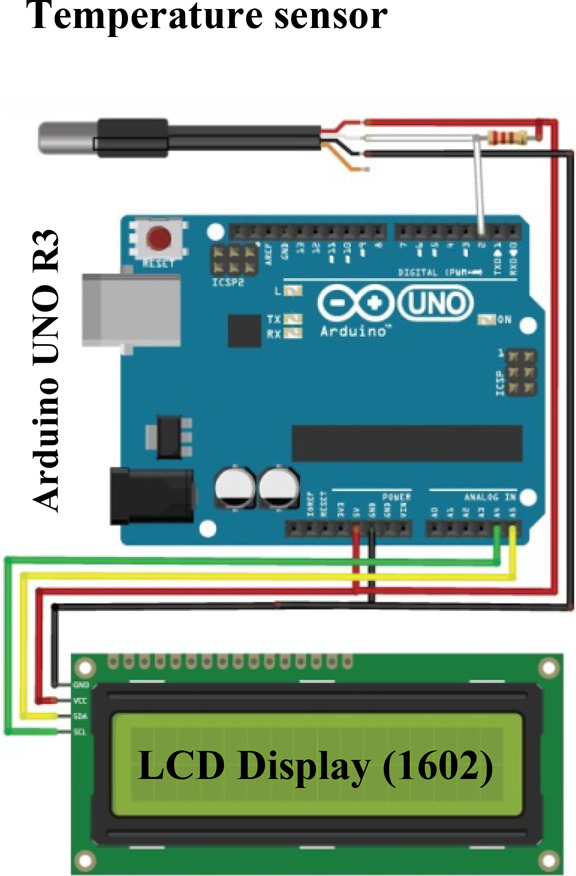
Electronic circuit diagram of electrolyte temperature measuring device.
Electrolyser
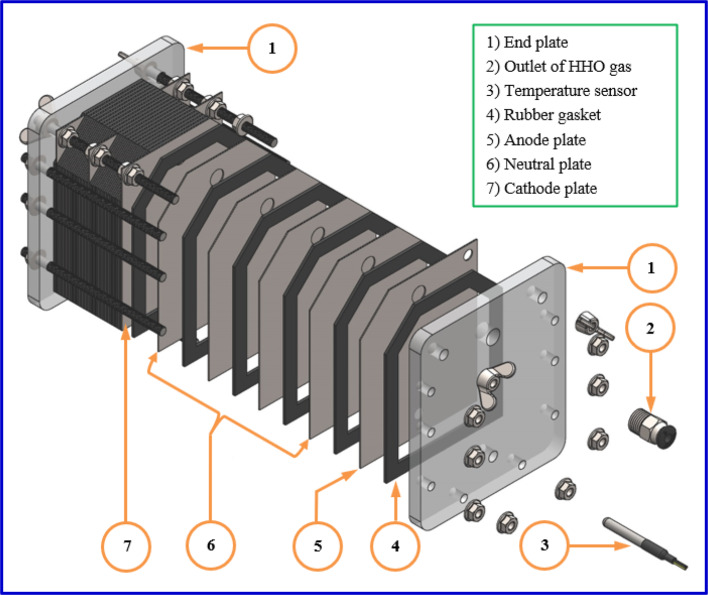
The developed electrolyzer is a type of dry fuel cell; this electrolyzer was designed and manufactured to produce HHO gas using a water electrolysis method. In this method, hydrogen and oxygen are separated by electrolysis of electrolyte solution. The mixture of produced gases (HHO) contains 66.6% hydrogen and 33.3% oxygen26. Distilled water is not electrically conductive, so potassium hydroxide (KOH) was added to distilled water to boost its electrical conductivity. The KOH was in the shape of scales, the minimum assay was 85%, the molecular mass was 56.11 g mol−1, and the KOH was purchased from EL-Nasr Pharmaceutical Chemicals Co. The main body of the electrolyzer (Fig. 3) comprises 26 electrodes, 27 rubber gaskets, and two plates of acrylic (Plexiglass). In addition, small installation components such as; fully threaded rods, screw nuts, hoses, pneumatic connectors, and electrical connection wires. The electrodes were made of 316-L grade stainless steel (SS). The SS material was chosen because it has high resistance to corrosion and rusting against alkaline solutions. The electrodes were arranged as follows: 1A 4N 1C and so on, where (A) represents the anode plate, (N) represents the neutral plate, and (C) represents the cathode plate. This arrangement was repeated to obtain the final electrode configuration of 3A 3C 20N. For a single stack, we used the following arrangement (1A 4N 1C = 6 plates = 5 cells). The voltage of each cell is computed by dividing the source voltage by the number of cells, resulting in 2.1 to 2.6 V. According to the applied voltage range (10.5 to 13 V) in our investigation, this agrees with Bob Boyce, “the approximate value of 2–3 V per cell allows the generator to operate at appropriate conditions”7,27. The main dimensions of the anode/cathode and neutral plates were 120 mm × 100 mm × 0.8 mm. The plates of the anode/cathode are cut from one side at the top, whereas the neutral plates are cut from the upper left and right sides. The goal of cutting both sides in the neutral plates is to avoid a direct connection between anode/cathode plates and neutral plates28.
Fig. 3.
3D Assembly drawing of main body of the developed electrolyzer.
Two holes were drilled in all electrodes (SS plates), the upper hole with a diameter of 11.5 mm to ensure exit of the HHO gas produced by the electrolysis process and the lower hole with a diameter of 7.5 mm to ensure evenly distributed electrolyte solution throughout all cell gaps. In addition, another hole was drilled in the anode/cathode plates at the top corner with a diameter of 7 mm to connect the electrical charge by using the two screw rods. A hollow rubber gasket with main dimensions of 120 × 100 × 2 mm was used to prevent leakage of the electrolyte solution and produced HHO gas, in addition to insulating the electrodes from each other. Two plates from transparent acrylic (Plexiglass) were used on the two sides of the electrolyzer as cover plates; this material is flexible and resistant to corrosion against corrosive materials. A hole was drilled in the bottom of the left acrylic plate for an electrolyte solution inlet. For the HHO gas outlet, a hole was drilled in the top of the right acrylic plate, as shown in Fig. 3. The general specifications of the developed HHO gas generator are shown in Table 2.
Table 2.
Specifications of the developed Electrolyser.
| Items | Details |
|---|---|
| HHO generator type | Dry cell |
| Plate material | Stainless steel (316 L) |
| The main dimensions of the plate, mm | 120 × 120 × 0.8 |
| No. of plates | 26 plates |
| No. of rubber gaskets | 27 gaskets |
| Thickness of gaskets, mm | 2 |
| Plate configuration | 3A 3C 20N |
| Power input | 12 V–DC |
| Catalyst electrolyte | KOH |
| Type of water | Distilled water |
Measuring tool of HHO volume
The produced volume of HHO gas was measured by displacement water under atmospheric pressure. The displacement water compressed by HHO gas produced from the generator was collected and then poured into the graduated cylinder to determine its volume.
The experimental procedure
The production of oxy-hydrogen/HHO gas was carried out using the electrolysis method of water according to the following steps: the electrolysis cell was washed with distilled water several times and then dried. For preparing the electrolyte solution with the required concentration, the required amount of potassium hydroxide was weighed by a digital electrical balance with an accuracy of 0.1 g and then dissolved manually in distilled water for about five minutes using a conical flask to obtain a homogeneous solvent. Then, the determined amount of electrolyte solution (315 cm3) was measured using a measuring cylinder and poured into the developed electrolysis cell. The electrolyte solution level is about 13 mm under the gas vent hole. The timer was set for the determined experiment time to 1 min for all experiments; before the electrolysis process began, the starting temperature of the electrolyte solution was constant at 32 ± 0.5 °C for all experiments. During the test, the power consumption in the electrolysis process was measured and recorded at the middle and finish of the experiment, and then the average value was taken. After the experiment time elapsed, the finish temperature of the electrolyte solution was measured and recorded. Then, the produced volume of HHO was measured using the displacement water method using graduated cylinders. After finishing and confirming that the HHO gas production process will run smoothly and without any problems, the following variables: applied voltage (10.5, 11.0, 11.5, 12.0, 12.5, and 13 V) and electrolyte concentration (0.05, 0.10, 0.15, and 0.20 M) were studied. All experiments were repeated five times, and the mean value was taken.
Performance evaluation of the HHO gas generator
Productivity of HHO gas generator
The productivity [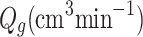 ] of HHO gas generator was calculated by using the following equation:
] of HHO gas generator was calculated by using the following equation:
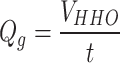 |
1 |
where 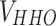 is the volume of HHO gas (
is the volume of HHO gas (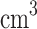 ) and
) and 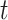 is the time of operating (
is the time of operating (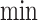 ).
).
Power consumption
The DC power consumption [P (W)] was directly measured by using the digital multi-function meter Model No; (PZEM-051); the measured value (DC power) while operating the HHO generator found that agreement with the value of calculated power via the equation shown below:
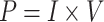 |
2 |
where 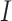 is the DC current consumed (Amperes) and
is the DC current consumed (Amperes) and 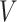 is the DC voltage difference (Volts).
is the DC voltage difference (Volts).
Change in temperature
The change in electrolyte solution temperature [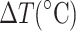 ] was calculated by using the following equation:
] was calculated by using the following equation:
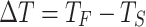 |
3 |
where 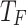 is the final temperature (
is the final temperature (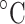 ) and
) and 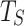 is the starting temperature (
is the starting temperature (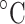 ).
).
Specific energy
The specific energy requirement [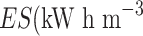 )] was calculated by using the following equation:
)] was calculated by using the following equation:
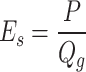 |
4 |
where 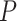 is the consumed power (kW) and
is the consumed power (kW) and 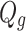 is the productivity of HHO gas
is the productivity of HHO gas 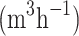 .
.
Efficiency of HHO gas generator
The efficiency [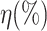 ] of the HHO gas generator is the ratio of energy gained and energy consumed22. The HHO gas is composed of two moles of hydrogen and one mole of oxygen, therefore the HHO gas composition of 1/3 volume percent oxygen and 2/3 percent hydrogen, so the volume of hydrogen is 0.66% of the produced volume of HHO gas (
] of the HHO gas generator is the ratio of energy gained and energy consumed22. The HHO gas is composed of two moles of hydrogen and one mole of oxygen, therefore the HHO gas composition of 1/3 volume percent oxygen and 2/3 percent hydrogen, so the volume of hydrogen is 0.66% of the produced volume of HHO gas (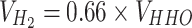 )29. The efficiency of the HHO gas generator was calculated as illustrated in the following formula30:
)29. The efficiency of the HHO gas generator was calculated as illustrated in the following formula30:
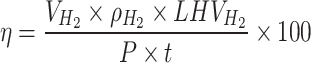 |
5 |
where 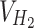 is the volume of hydrogen (
is the volume of hydrogen (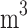 ),
), 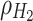 is the density of hydrogen (
is the density of hydrogen (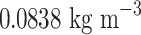 ),
), 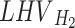 is the lower heating value of hydrogen (
is the lower heating value of hydrogen (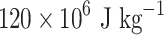 ),
), 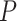 is the DC power consumption (
is the DC power consumption (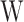 ), and
), and 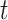 is the time (
is the time ( ).
).
The results obtained from this study were statistically analyzed using the following programs: Microsoft Excel and SPSS “V. 23”.
Results and discussion
Performance of the developed HHO gas generator includes the following items: productivity, power consumption, amount of change in electrolyte solution temperature, specific energy requirement, and efficiency of HHO gas generator.
Productivity
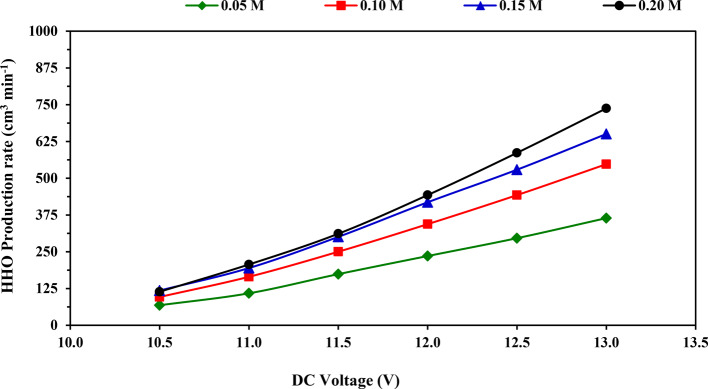
Figure 4 shows the impact of applied voltage on the rate of HHO gas production at tested KOH concentrations. As expected, it was found that the rate of HHO gas production increased gradually by increasing the applied voltage for all tested KOH concentrations, and a similar trend of this result was observed in the literature4,15,16,31. This result could be caused by an increase in the density of uniform charge, ions exchange on the surface of the electrode, and acceleration of the reaction kinetics2. The highest value of the HHO production rate is 737.6 cm3 min−1 at the applied voltage of 13 V using KOH concentration of 0.20 M, while the lowest value of the HHO production rate is 68.17 cm3 min−1 at applied voltage of 10.5 V using KOH concentration of 0.05 M. The rate of HHO gas production increased from 68.17 to 364.4, 96.25 to 547.8, 118.1 to 650.5, and 114.1 to 737.6 cm3 min−1 by increasing the applied voltage from 10.5 to 13.0 V at four tested KOH concentrations 0.05, 0.10, 0.15 and 0.20 M, respectively as displayed in Table 3.
Fig. 4.
Impact of applied voltage on HHO gas production rate at tested KOH concentrations.
Table 3.
Average values of HHO production rate at tested voltages and electrolytic concentrations, [mean value ± (SD)] and Duncan’s test result.
| DC voltage (V) | HHO Gas production rate (cm3 min−1) | Mean | |||
|---|---|---|---|---|---|
| Electrolytic solution concentration (M) | |||||
| 0.05 | 0.10 | 0.15 | 0.20 | ||
| 10.5 | 68.17 (3.06) | 96.25 (2.42) | 118.1 (2.82) | 114.1 (4.82) | 99.17a |
| 11.0 | 108.8 (3.29) | 164.9 (2.40) | 194.7 (4.73) | 206.8 (6.87) | 168.8b |
| 11.5 | 174.0 (8.15) | 250.2 (12.0) | 300.7 (11.7) | 311.3 (4.44) | 259.0c |
| 12.0 | 235.5 (5.72) | 343.9 (6.68) | 418.3 (19.8) | 442.8 (4.09) | 360.1d |
| 12.5 | 296.2 (6.80) | 442.7 (7.82) | 529.0 (11.9) | 586.3 (2.54) | 463.5e |
| 13.0 | 364.4 (4.10) | 547.8 (6.46) | 650.5 (22.1) | 737.6 (18.1) | 575.1f |
| Mean | 207.84a | 307.62b | 368.54c | 399.82d | – |
Different superscript letters (a,b,c, etc.) indicate significant differences among groups using the Duncan Multiple-Range Test (P < 0.05).
When comparing with the previous studies, Budiman et al.28 produced HHO gas by dry cell and used NaCl as an electrolyte (500 cm3 of water + 100 g of NaCl), and they reported that the highest production of HHO gas was 175 cm3 at time of (3.0–3.5 min) using battery source (12 V–10 A). Meanwhile, Essuman et al.4 observed that increasing electrolyte strength and voltage correspondingly improved the yield of HHO gas. Their findings revealed that the optimal yield rate of 136.2 cm3 min−1 of HHO gas was achieved when the generator was run at 13 V and 0.025 M KOH. Whereas, Sudrajat et al.32 discovered that using a 22.4 g L−1 of KOH catalyst resulted in an average HHO gas production of 230.3 cm3 min−1 at 12 V. Also, they found that the volumes of HHO gas produced were 152, 176, and 258 cm3 for HHO gas generator configurations 3A 3C 12N, 3A 3C 16N, and 3A 3C 20N respectively at using 0.02 M KOH for 50 s.
Duncan Multiple Range Test (DMRT) in Table 3 revealed that the mean impact of applied voltage on the HHO production rate, the average values of production rate increased significantly at 5% level (P < 0.05) from 99.17 to 575.1 cm3 min−1 with increased the applied voltage from 10.5 to 13 V.
From Table 3, the results of Duncan’s test for the mean impact of KOH concentration on the rate of HHO gas production rate revealed that the average values of production rate increased significantly from 207.84 to 399.82 cm3 min−1 with increasing the KOH concentration from 0.05 to 0.20 M.
Power consumption
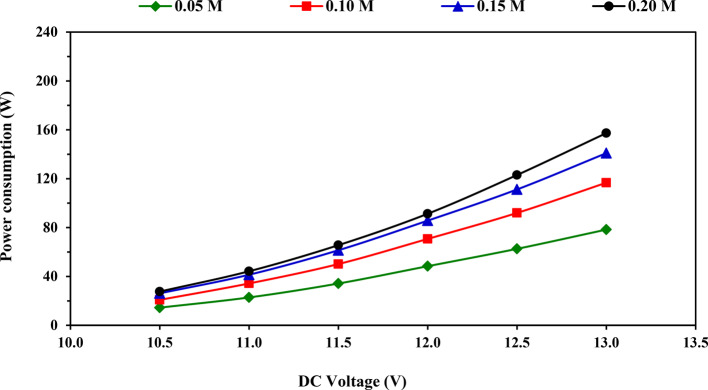
Figure 5 illustrates the impact of applied voltage on the consumed power at tested KOH concentrations. It was found that the consumed power increased gradually by increasing the applied voltage for all tested KOH concentrations. This result may be because of the increase in the consumed current with increasing the applied voltage, which followed an increase in power consumption. In addition, increasing the KOH concentration of the electrolyte solution leads to increased electrical conductivity33.
Fig. 5.
Impact of applied voltage on power consumption at tested KOH concentrations.
The results indicated that the power consumption increased from 14.46 to 78.34, 20.81 to 116.7, 26.16 to 140.9, and 27.62 to 157.3 W with increasing the applied voltage from 10.5 to 13.0 V at four tested KOH concentrations 0.05, 0.10, 0.15 and 0.20 M, respectively as displayed in Table 4. Duncan’s test in Table 4 revealed that the mean impact of applied voltage on the power consumption, the average values of power consumption increased significantly (P < 0.05) from 22.26 to 123.3 W with increased the applied voltage from 10.5 to 13 V. Also, the result of Duncan’s test for the mean effect of KOH concentration on power consumption showed that the average values of power consumption increased significantly from 43.47 to 84.85 W with increasing the KOH concentration from 0.05 to 0.20 M.
Table 4.
Average values of DC power consumption at tested voltages and electrolytic concentrations, [mean value ± (SD)] and Duncan’s test result.
| DC voltage (V) | DC Power consumption (W) | Mean | |||
|---|---|---|---|---|---|
| Electrolytic solution concentration (M) | |||||
| 0.05 | 0.10 | 0.15 | 0.20 | ||
| 10.5 | 14.46 (0.59) | 20.81 (0.47) | 26.16 (0.89) | 27.62 (1.18) | 22.26a |
| 11.0 | 22.81 (0.29) | 34.26 (0.34) | 41.41 (0.23) | 44.24 (0.92) | 35.68b |
| 11.5 | 34.21 (0.21) | 50.14 (1.31) | 61.44 (2.04) | 65.60 (0.53) | 52.85c |
| 12.0 | 48.38 (0.32) | 70.72 (0.63) | 85.68 (3.16) | 91.31 (1.07) | 74.02d |
| 12.5 | 62.59 (0.15) | 91.97 (0.92) | 111.2 (3.39) | 123.0 (3.68) | 97.19e |
| 13.0 | 78.34 (0.32) | 116.7 (0.86) | 140.9 (4.54) | 157.3 (5.10) | 123.3f |
| Mean | 43.47a | 64.10b | 77.80c | 84.85d | – |
Different superscript letters (a,b,c, etc.) indicate significant differences among groups using the Duncan Multiple-Range Test (P < 0.05).
Change in temperature
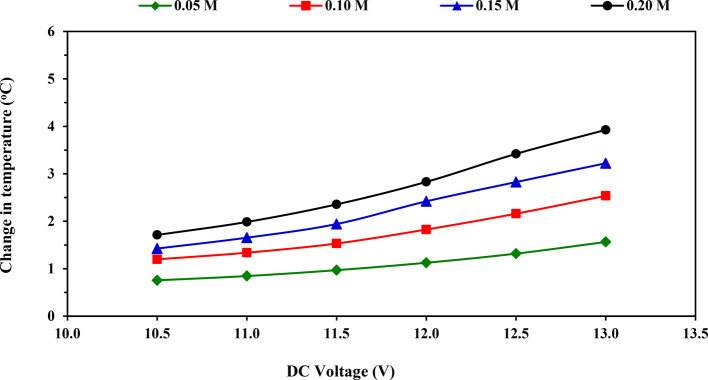
Figure 6 shows the impact of applied voltage on change in electrolyte solution temperature when the experiment is over at tested KOH concentrations. It’s noted that the temperature change increased gradually with increasing the applied voltage for all tested KOH concentrations. Increasing the applied voltage led to an increase in the consumed current and, therefore, followed a rise in power consumption, which increased the HHO production rate. However, there is a negative side effect: an increase in the temperature of electrolyte solution during the electrolysis process34. This may occur due to an increase in electron mobility and an increase in heat transfer rate on the plates16.
Fig. 6.
Impact of applied voltage on change in temperature at tested KOH concentrations.
Also, the obtained results revealed that the amount of change in temperature increased from 0.76 to 1.56, 1.20 to 2.54, 1.43 to 3.22, and 1.71 to 3.93 °C with increasing the voltage from 10.5 to 13.0 V at four tested KOH concentrations 0.05, 0.10, 0.15 and 0.20 M, respectively as displayed in Table 5. Duncan’s test result in Table 5 indicates the average impact of applied voltage on the change in electrolyte solution temperature; the average values of change in temperature increased significantly from 1.27 to 2.81 °C with increasing the applied voltage from 10.5 to 13 V. Table 5 also illustrates Duncan’s test result for the average impact of KOH concentration on temperature change. The average temperature change values increased significantly from 1.10 to 2.71 °C with increasing the KOH concentration from 0.05 to 0.20 M.
Table 5.
Average values of change in temperature at tested voltages and electrolytic concentrations, [mean value ± (SD)] and Duncan’s test result.
| DC voltage (V) | Change in temperature (oC) | Mean | |||
|---|---|---|---|---|---|
| Electrolytic solution concentration (M) | |||||
| 0.05 | 0.10 | 0.15 | 0.20 | ||
| 10.5 | 0.76 (0.05) | 1.20 (0.12) | 1.43 (0.03) | 1.71 (0.05) | 1.27a |
| 11.0 | 0.85 (0.03) | 1.34 (0.03) | 1.65 (0.03) | 1.99 (0.03) | 1.46b |
| 11.5 | 0.97 (0.03) | 1.53 (0.03) | 1.94 (0.07) | 2.36 (0.03) | 1.70c |
| 12.0 | 1.13 (0.01) | 1.83 (0.05) | 2.42 (0.16) | 2.83 (0.06) | 2.05d |
| 12.5 | 1.32 (0.04) | 2.16 (0.06) | 2.83 (0.15) | 3.42 (0.11) | 2.43e |
| 13.0 | 1.56 (0.09) | 2.54 (0.07) | 3.22 (0.29) | 3.93 (0.19) | 2.81f |
| Mean | 1.10a | 1.77b | 2.25c | 2.71d | – |
Different superscript letters (a,b,c, etc.) indicate significant differences among groups using the Duncan Multiple-Range Test (P < 0.05).
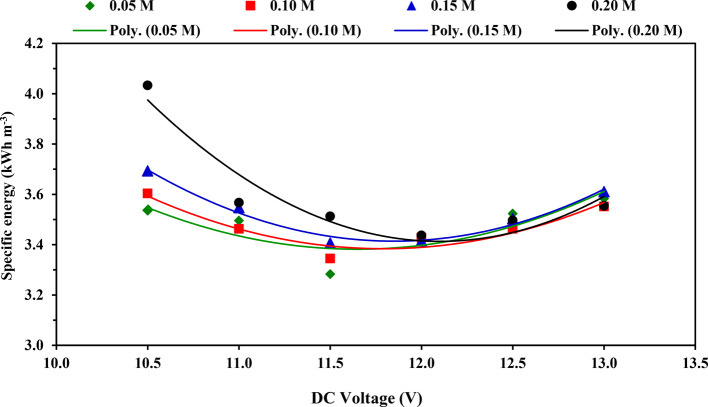
Specific energy requirement
Figure 7 shows the impact of applied voltage on the specific energy requirement at tested KOH concentrations. Generally, we discovered that increasing the electrolytic solution concentration from 0.05 to 0.2 M increased the specific energy required for all applied voltages from 10.5 to 13 V. This could be attributed to an increase in the temperature of the electrolytic solution during operation, which is likely to increase current consumption hence increasing the specific energy required for the production of HHO gas according to Nabil27 and Nabil and Dawood7.
Fig. 7.
Impact of applied voltage on specific energy at tested KOH concentrations.
For tested KOH concentrations 0.05, 0.10, and 0.15 M, the results indicated that specific energy values decreased gradually with increasing the applied voltage from 10.5 to 11.5 V. In contrast, as the applied voltage increased from 11.5 to 13 V, the specific energy increased. In comparison, using the KOH concentration of 0.20 M, the specific energy decreased gradually with the increase in the applied voltage from 10.5 to 12.0 V, which increased with the increase in the applied voltage to 13.0 V, as shown in Fig. 7 and Table 6. So, the production of HHO gas is more efficient when the specific energy value drops and less efficient when the specific energy value rises. This could be due to a gradual increase in the electrolyte solution’s temperature resulting in an increase in electric current drawn, which increases power consumption faster than the rate of increased HHO gas production because a part of the electric power is consumed in electrolyte solution heating with agree with Wu et al.35 and Hassan et al.34. According to the literature, Rusdianasari et al.36 employed an HHO gas generator. They found that the specific energy consumption was 29.18 kWh m−3. Meanwhile, Patil et al.37 found that producing 1 m3 of HHO gas needs 15 kWh. Whereas Wu et al.35 found that the required specific energy was 3.31 kWh for every 1 m3 HHO gas.
Table 6.
Average values of specific energy at applied voltages and electrolytic concentrations, [mean value ± (SD)] and Duncan’s test result.
| DC voltage (V) | Specific energy (kWh m−3) | Mean | |||
|---|---|---|---|---|---|
| Electrolytic solution concentration (M) | |||||
| 0.05 | 0.10 | 0.15 | 0.20 | ||
| 10.5 | 3.54 (0.16) | 3.60 (0.04) | 3.69 (0.14) | 4.03 (0.05) | 3.72d |
| 11.0 | 3.50 (0.08) | 3.46 (0.06) | 3.55 (0.07) | 3.57 (0.07) | 3.52b |
| 11.5 | 3.28 (0.16) | 3.34 (0.15) | 3.41 (0.06) | 3.51 (0.06) | 3.39a |
| 12.0 | 3.43 (0.08) | 3.43 (0.04) | 3.42 (0.04) | 3.44 (0.04) | 3.43a |
| 12.5 | 3.52 (0.08) | 3.46 (0.03) | 3.50 (0.05) | 3.50 (0.10) | 3.50b |
| 13.0 | 3.58 (0.04) | 3.55 (0.05) | 3.61 (0.01) | 3.55 (0.03) | 3.57c |
| Mean | 3.47a | 3.48a | 3.53b | 3.60c | – |
Different superscript letters (a,b,c, etc.) indicate significant differences among groups using the Duncan Multiple-Range Test (P < 0.05).
Duncan’s test in Table 6 revealed that the mean effect of applied voltage on the specific energy requirement, the average values of specific energy decreased significantly (P < 0.05) from 3.72 to 3.39 kWh m−3 with increasing the voltage from 10.5 to 11.5 V while, the average values of specific energy are not significantly at using voltage from 11.5 to 12.0 V whereas, the average values of specific energy increased significantly from 3.43 to 3.57 kWh m−3 with increasing the voltage from 12.0 to 13.0 V.
Also, Table 6 illustrates the result of Duncan’s test for the average impact of KOH concentration on the specific energy requirement; the average values of specific energy increased significantly from 3.48 to 3.60 kWh m−3 with increasing the KOH concentration from 0.01 to 0.20 M whereas, the average values of specific energy are not significantly at using KOH concentrations of 0.05 and 0.01 M.
The outcomes also showed that the relationship between specific energy and applied voltage was a second-degree polynomial equation, which can be expressed using Eqs. (6–9) for the tested electrolyte concentrations.
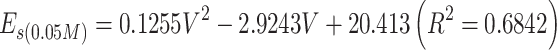 |
6 |
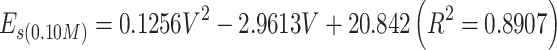 |
7 |
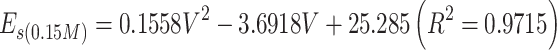 |
8 |
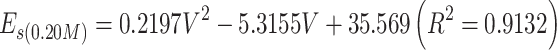 |
9 |
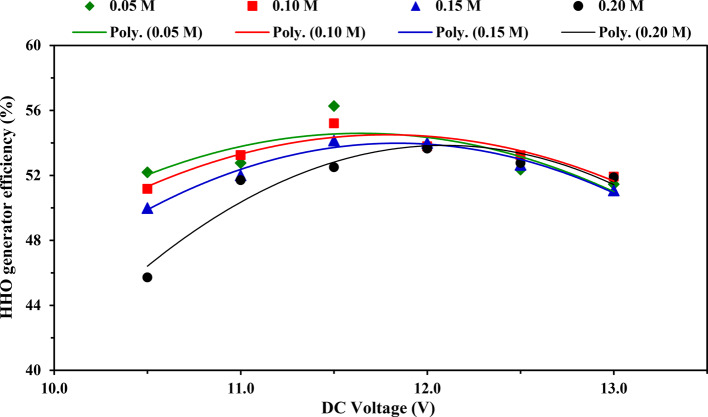
Efficiency
Figure 8 illustrates the impact of applied voltage on the efficiency of the HHO gas generator at tested KOH concentrations. For tested KOH concentrations 0.05, 0.10, and 0.15 M, the results indicated that the values of HHO gas generator efficiency increased gradually with increasing the applied voltage from 10.5 to 11.5 V, after which it decreased with increasing the applied voltage up to 13.0 V. In comparison, when using the KOH concentration of 0.20 M, the generator efficiency increased gradually with increasing the applied voltage from 10.5 to 12.0 V, after which it decreased with increasing the applied voltage up to 13.0 V, as shown in Fig. 8 and Table 7, a similar trend of this outcome was reported El Kady et al.2. The decline in HHO gas generator efficiency could be attributed to loss some of the consumption electrical power in raising the temperature of the electrolyte solution which causes decreasing of HHO production rate7,16.
Fig. 8.
Impact of applied voltage on generator efficiency at tested KOH concentrations.
Table 7.
Average values of HHO gas generator efficiency at tested voltages and electrolytic concentrations, [mean value ± (SD)] and Duncan’s test result.
| DC voltage (V) | HHO gas Generator efficiency (%) | Mean | |||
|---|---|---|---|---|---|
| Electrolytic solution concentration (M) | |||||
| 0.05 | 0.10 | 0.15 | 0.20 | ||
| 10.5 | 52.18 (2.33) | 51.16 (0.54) | 49.98 (1.85) | 45.72 (0.62) | 49.76d |
| 11.0 | 52.76 (1.16) | 53.24 (0.85) | 52.00 (1.09) | 51.70 (1.04) | 52.42b |
| 11.5 | 56.27 (2.70) | 55.20 (2.38) | 54.13 (0.91) | 52.49 (0.85) | 54.52a |
| 12.0 | 53.84 (1.25) | 53.79 (0.66) | 53.99 (0.65) | 53.65 (0.61) | 53.82a |
| 12.5 | 52.35 (1.24) | 53.24 (0.50) | 52.64 (0.77) | 52.76 (1.47) | 52.75b |
| 13.0 | 51.45 (0.51) | 51.92 (0.70) | 51.06 (0.16) | 51.87 (0.47) | 51.58c |
| Mean | 53.14a | 53.09a | 52.30b | 51.37c | – |
Different superscript letters (a,b,c, etc.) indicate significant differences among groups using the Duncan Multiple-Range Test (P < 0.05).
Duncan’s test in Table 7 indicates the average impact of applied voltage on the efficiency of HHO generator. The average values of efficiency of HHO gas generator increased significantly from 49.76 to 54.52% with increasing the applied voltage from 10.5 to 11.5 V. In contrast, the average HHO generator efficiency values are not significant at applied voltage from 11.5 to 12.0 V. In contrast, the average values of efficiency of HHO generator decreased significantly from 53.82 to 51.58% with increasing the applied voltage from 12.0 to 13.0 V.
Also, Table 7 illustrates the Duncan’s test result for the mean effect of KOH concentration on the efficiency of HHO generator. The average values of generator efficiency decreased significantly from 53.09 to 51.37% with the increase in the KOH concentration from 0.01 to 0.20 M. Meanwhile, the average values of generator efficiency are not significant when using KOH concentrations of 0.05 and 0.01 M.
In comparison with previous studies, Sudarmanta et al.25 stated that the HHO gas generator efficiency was 20.06% using a PWM system with a 40% duty cycle, a specific energy input of 33,121 MJ kg−1, and generator temperature can be maintained below 60 °C. While, Najafi et al.30 demonstrated that the generator efficiency of HHO gas improved with increasing the concentration of NaOH catalyst from 2 to 6 g L−1 and then dropped with increasing the catalyst concentration from 6 to 10 g L−1; also, they found that the maximum efficiency of 39.12% was achieved at 6 g L−1 of NaOH catalyst.
Moreover, the findings revealed that the relationship between HHO generator efficiency and applied voltage was a second-degree polynomial equation, which can be expressed for the tested electrolyte concentrations using Eqs. (10–13).
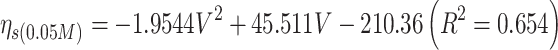 |
10 |
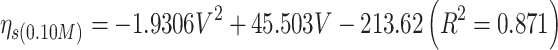 |
11 |
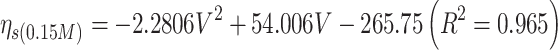 |
12 |
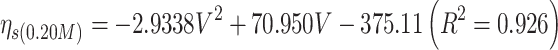 |
13 |
Conclusion
The developed HHO generator exhibits ease of manufacturing, operation, and maintenance, and its design has successfully facilitated the production of HHO gas. Within the range of tested potassium hydroxide (KOH) concentrations (0.05 to 0.20 M) and applied voltages (10.5 to 13.0 V), the results demonstrated a significant increase in HHO gas production rate with higher applied voltages and electrolyte solution concentrations. It was also observed that the power consumption for HHO gas production increased notably with increasing applied voltage and electrolyte concentration. Furthermore, an increase in applied electrolyte concentration and voltage led to a corresponding rise in temperature. From this study, the optimum conditions for producing HHO gas ranged from 11.5 to 12 V for voltage and from 0.05 to 0.10 M for KOH concentration according to the lowest specific energy and highest HHO gas generator efficiency. Under the previous optimum conditions, the highest productivity, specific energy, and efficiency of the HHO gas generator were 343.9 cm3 min−1, 3.43 kW h m−3, and 53.79%, respectively, using 12.0 V for applied voltage and 0.10 M for electrolyte solution concentration. In the future, other variables and new technologies will be studied to enhance generator efficiency, in addition to using renewable sources (such as solar energy, wind energy, etc.) for producing eco-friendly fuel (hydrogen).
Author contributions
“Conceptualization, K. A. M. A. and A. M. M; methodology, H.A. A. S.; software, A. M. M.; validation, W. A. E. M. ; formal analysis, A. M. M.; investigation, H.A. A. S.; resources, N. S. A.; data curation, N. S. A.; writing—original draft preparation, A. M. M.; writing—review and editing, K. A. M. A.; visualization, A. M. M.; supervision, A. M. M.; project administration, W. A. E. M.; funding acquisition, A. M. M. All authors have read, reviewed and agreed to the published version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data and materials are available with the corresponding author upon request. in this email: khaledabdeen@azhar.edu.eg.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaulin, N. & Le Billon, P. Climate change and fossil fuel production cuts: Assessing global supply-side constraints and policy implications. Clim. Policy20, 888–901 (2020). [Google Scholar]
- 2.El Kady, M. A., El Fatih Farrag, A., Gad, M. S., El Soly, A. K. & Abu Hashish, H. M. Parametric study and experimental investigation of hydroxy (HHO) production using dry cell. Fuel282, 118825 (2020). [Google Scholar]
- 3.de Fátima Palhares, D. D. A., Vieira, L. G. M. & Damasceno, J. J. R. Hydrogen production by a low-cost electrolyzer developed through the combination of alkaline water electrolysis and solar energy use. Int. J. Hydrogen Energy43, 4265–4275 (2018). [Google Scholar]
- 4.Essuman, S. P. K., Nyamful, A., Agbodemegbe, V. & Debrah, S. K. Experimental studies of the effect of electrolyte strength, voltage and time on the production of Brown’s (HHO) gas using oxy-hydrogen generator. Open J. Energy Effic.08, 64–80 (2019). [Google Scholar]
- 5.Subramanian, B. & Ismail, S. Production and use of HHO gas in IC engines. Int. J. Hydrogen Energy43, 7140–7154 (2018). [Google Scholar]
- 6.Al-Rousan, A. A. & Musmar, S. A. Effect of anodes-cathodes inter-distances of HHO fuel cell on gasoline engine performance operating by a blend of HHO. Int. J. Hydrogen Energy43, 19213–19221 (2018). [Google Scholar]
- 7.Nabil, T. & Khairat Dawood, M. M. Enabling efficient use of oxy-hydrogen gas (HHO) in selected engineering applications; transportation and sustainable power generation. J. Clean. Prod.237, 117798 (2019). [Google Scholar]
- 8.Holladay, J. D., Hu, J., King, D. L. & Wang, Y. An overview of hydrogen production technologies. Catal. Today139, 244–260 (2009). [Google Scholar]
- 9.Nnabuife, S. G. et al. A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact. Clean Technol.5, 1344–1380 (2023). [Google Scholar]
- 10.Kannan, K. et al. Hydrothermally synthesized mixed metal oxide nanocomposites for electrochemical water splitting and photocatalytic hydrogen production. Int. J. Hydrogen Energy48, 36412–36426 (2023). [Google Scholar]
- 11.Kannan, K., Chanda, D., Meshesha, M. M. & Yang, B. L. Impressive efficiency of zinc oxide-manganese oxide/MAX composite in two-electrode system for photovoltaic-electrolyzer water splitting. Colloids Surfaces A Physicochem. Eng. Asp.689, 133599 (2024). [Google Scholar]
- 12.Kannan, K. et al. Facial synthesis of p-p heterojunction composites: Evaluation of their electrochemical properties with photovoltaics-electrolyzer water splitting using two-electrode system. Int. J. Hydrogen Energy48, 13814–13826 (2023). [Google Scholar]
- 13.Liponi, A., Baccioli, A., Ferrari, L. & Desideri, U. Techno-economic analysis of hydrogen production from PV plants. E3S Web Conf.334, 01001 (2022). [Google Scholar]
- 14.Wang, Z., Roberts, R. R., Naterer, G. F. & Gabriel, K. S. Comparison of thermochemical, electrolytic, photoelectrolytic and photochemical solar-to-hydrogen production technologies. Int. J. Hydrogen Energy37, 16287–16301 (2012). [Google Scholar]
- 15.Alam, N. & Pandey, K. M. Experimental study of hydroxy gas (HHO) production with variation in current, voltage and electrolyte concentration. IOP Conf. Ser. Mater. Sci. Eng.225, 1–9 (2017). [Google Scholar]
- 16.El Soly, A. K., El Kady, M. A., Farrag, A. E. F. & Gad, M. S. Comparative experimental investigation of oxy-hydrogen (HHO) production rate using dry and wet cells. Int. J. Hydrogen Energy46, 12639–12653 (2021). [Google Scholar]
- 17.Shah, S. A. Q., Ali, Z., Larik, J. & Kaimkhani, A. A. Comparative study of dry cell and wet cell for the HHO gas generation as a supplement fuel for I.C. engine. In 2018 International Conference on Computing, Mathematics and Engineering Technologies: Invent, Innovate and Integrate for Socioeconomic Development, iCoMET 2018 - Proceedings vols 2018-Janua 1–8 (2018).
- 18.Yilmaz, A. C., Uludamar, E. & Aydın, K. Effect of hydroxy (HHO) gas addition on performance and exhaust emissions in compression ignition engines. Int. J. Hydrogen Energy35, 11366–11372 (2010). [Google Scholar]
- 19.Manu, P. V., Sunil, A. & Jayaraj, S. Experimental investigation using an on-board dry cell electrolyzer in a CI engine working on dual fuel mode. Energy Procedia90, 209–216 (2016). [Google Scholar]
- 20.Eissa, M. S., Aissa, W. A., Hassan, H. & Abdel-Mohsen, H. S. Improving SI engines performance to obtain lower operating cost and emissions using in-situ produced HHO gas. Appl. Model. Simul.6, 107–114 (2022). [Google Scholar]
- 21.Muthu, V. S. S., Osman, S. A. & Osman, S. A. A review of the effects of plate configurations and electrolyte strength on production of brown gas using dry cell oxy-hydrogen generator. J. Adv. Res. Fluid. Mech. Therm. Sci.99, 1–8 (2022). [Google Scholar]
- 22.El-Kassaby, M. M., Eldrainy, Y. A., Khidr, M. E. & Khidr, K. I. Effect of hydroxy (HHO) gas addition on gasoline engine performance and emiss ions. Alex. Eng. J.55, 243–251 (2016). [Google Scholar]
- 23.Mustaqim, I. Z. & Maulana, D. W. The effect of NaOH and LaCoO3 perovskite on HHO gas production rate. J. Phys. Conf. Ser.2344, 012003 (2011). [Google Scholar]
- 24.Almassri, G. S., Jaballa, K. M. H., Maatug, B. & Alqaed, S. Design of dry HHO cell to produce hydrogen using electrolysis. J. Alasmarya Univ. Basic Appl. Sci.10.59743/jauas.7.1.5 (2022). [Google Scholar]
- 25.Sudarmanta, B., Darsopuspito, S. & Sungkono, D. Application of dry cell hho gas generator with pulse width modulation on sinjai spark ignition engine performance. Int. J. Res. Eng. Technol.05, 105–112 (2016). [Google Scholar]
- 26.Kuracina, M., Fiala, J. & Soldán, M. Study of selected characteristics of 8-cell HHO generator using various concentrations of NaOH solutions. Adv. Mater. Res.1001, 109–113 (2014). [Google Scholar]
- 27.Nabil, T. Efficient use of oxy-hydrogen gas (HHO) in vehicle engines. J. Eur. Des Syst. Autom.52, 87–96 (2019). [Google Scholar]
- 28.Budiman, A., Yerizam, M. & Bow, Y. Design of dry cell HHO generator using NaCl solution for hydrogen production. Indones. J. Fundam. Appl. Chem.7, 8–15 (2022). [Google Scholar]
- 29.Sambandam, P., Murugesan, P., Shajahan, M. I., Sethuraman, B. & Hussein, H. M. A. Sustainability and environmental impact of hydroxy addition on a light-duty generator powered with an ethanol gasoline blend. J. Renew. Energy Environ.9, 82–92 (2022). [Google Scholar]
- 30.Najafi, B. et al. Effects of low-level hydroxy as a gaseous additive on performance and emission characteristics of a dual fuel diesel engine fueled by diesel/biodiesel blends. Eng. Appl. Comput. Fluid Mech.15, 236–250 (2021). [Google Scholar]
- 31.Mohammed, H. J. & Ali, N. A. Fabricating and study effect of the concentrations electrolyte for an alkaline electrolysis cell. AIP Conf. Proc.2144, 1–6 (2019). [Google Scholar]
- 32.Sudrajat, A., Mayfa Handayani, E., Tamaldin, N. & Kamal Mat Yamin, A. Principle of generator HHO hybrid multistack type production technologies to increase HHO gas volume. SHS Web Conf.49, 02016 (2018). [Google Scholar]
- 33.Rusdianasari, Bow, Y., Dewi, T. & Risma, P. Hydrogen gas production using water electrolyzer as hydrogen power. In ICECOS 2019—3rd International Conference on Electrical Engineering and Computer Science, Proceeding 127–131 (2019). 10.1109/ICECOS47637.2019.8984438.
- 34.Hassan, H., Aissa, W., Eissa, M. S. & Abdel-Mohsen, H. The effect of introducing HHO gas into the intake manifold of Spark ignition engine (SIE). Int. J. Appl. Energy Syst.2, 77–81 (2020). [Google Scholar]
- 35.Wu, Y. et al. Experimental Investigation of producing Brown’s gas using a metal-plate electrolyzer for diesel vehicle applications. Energy Technol.5, 244–252 (2017). [Google Scholar]
- 36.Rusdianasari, R., Bow, Y. & Dewi, T. HHO Gas Generation in Hydrogen Generator using Electrolysis. IOP Conf. Ser. Earth Environ. Sci.258, 012007 (2019). [Google Scholar]
- 37.Patil, N. N., Chavan, C. B., More, A. S. & Baskar, P. Generation of oxy-hydrogen gas and its effect on performance of spark ignition engine. IOP Conf. Ser. Mater. Sci. Eng.263, 062036 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available with the corresponding author upon request. in this email: khaledabdeen@azhar.edu.eg.