Abstract
The Borna disease virus (BDV) p24 phosphoprotein is an abundant protein in BDV-infected cultured cells and animal brains. Therefore, there is a possibility that binding of the p24 protein to cellular factor(s) induces functional alterations of infected neural cells in the brain. To identify a cellular protein(s) that interacts with BDV p24 protein, we performed far-Western blotting with extracts from various cell lines. Using recombinant p24 protein as a probe, we detected a 30-kDa protein in all cell lines examined. Binding between the 30-kDa and BDV p24 proteins was also demonstrated using BDV p24 affinity and ion-exchange chromatography columns. Microsequence analysis of the purified 30-kDa protein revealed that its N terminus showed complete homology with rat amphoterin protein, which is a neurite outgrowth factor abundant in the brain during development. Mammalian two-hybrid and immunoprecipitation analyses also confirmed that amphoterin is a specific target for the p24 protein in vivo. Furthermore, we showed that infection by BDV, as well as purified p24 protein in the medium, significantly decreased cell process outgrowth of cells grown on laminin, indicating the functional inhibition of amphoterin by interaction with the p24 protein. Immunohistochemical analysis revealed decreased levels of amphoterin protein at the leading edges of BDV-infected cells. Moreover, the expression of the receptor for advanced glycation end products, of which the extracellular moiety is a receptor for amphoterin, was not significantly activated in BDV-infected cells during the process of extension, suggesting that the secretion of amphoterin from the cell surface is inhibited by the binding of the p24 protein. These results suggested that BDV infection may cause direct damage in the developing brain by inhibiting the function of amphoterin due to binding by the p24 phosphoprotein.
Borna disease virus (BDV) is the prototype of a new family, Bornaviridae, within the nonsegmented negative-strand RNA viruses, the Mononegavirales (12, 45), which is characterized by low productivity, neurotropism, and nuclear localization for transcription and replication (8). Although BDV was originally described as an agent of nonpurulent encephalomyelitis in horses in Germany (40), BDV infection has now been found in a wide range of vertebrate species, including sheep, cattle, cats, and ostriches (6, 28, 40). Recent epidemiological studies have suggested that BDV infection also occurs in humans and that it may be related to certain psychiatric diseases (7, 13, 22, 27, 43). Human BDV was isolated from the peripheral blood granulocyte cell fraction of a psychiatric patient (35). Furthermore, we have also demonstrated BDV infection in the brain of a schizophrenic patient with a very recent onset of disease (32).
BDV shows noncytolytic replication in cultured cells. However, neonatal rats infected with BDV develop persistent infection and show developmental disturbances affecting specific areas of the brain (4, 9, 14, 19, 42). Neonatal BDV infection also results in a variety of behavioral abnormalities and neuroanatomical disturbances without generalized meningitis or encephalitis (14, 19, 33, 41). Recent studies demonstrated that neonatal BDV infection directly alters concentrations of neurotransmitters, including norepinephrine and serotonin, in the brain (36). Furthermore, BDV infection displayed a progressive decrease in synaptic density and plasticity, especially in the cortex and hippocampus, which preceded a significant dropout of cortical neurons in infected rats (16). These observations indicated that BDV infection shows direct effects on the microenvironment of neural cells in the infected brain in absence of immunopathologically related brain damage.
The present study was performed to identify cellular binding protein(s) of the BDV p24 phosphoprotein. The BDV p24 protein is a nucleus-associated phosphoprotein and is assumed to be a cofactor of the polymerase protein of BDV in replication and transcription (26, 48). Since BDV p24, as well as the BDV p40 nucleoprotein, is abundant in infected cultured cells and animal brains, it is possible that binding of the p24 protein to cellular factor(s) induces functional alterations in the infected neural cell environment. Here we report that BDV p24 specifically binds to amphoterin, which is a neurite outgrowth factor of 30 kDa abundant in the developing brain. The interaction between amphoterin and p24 proteins in vitro and in vivo was confirmed by several different techniques, including far-Western blotting, p24 protein affinity chromatography, and mammalian two-hybrid and immunoprecipitation analyses. We also demonstrated that infection with BDV, as well as purified p24 protein in the medium, significantly inhibited cell process outgrowth of cells grown on laminin. Furthermore, migration activity of the cells to laminin was also decreased by BDV infection. Our results suggest that BDV infection causes a functional disturbance of amphoterin in cells by the interaction of the p24 protein. This, in turn, may result in neurodevelopmental damage in the early brain, as reported in neonatal rats infected with BDV.
MATERIALS AND METHODS
Cell lines and viruses.
MDCK (canine kidney), C6 (rat glioma), SK-N-SH (human neuroblastoma), and COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal calf serum (FCS). The OL cell line, derived from human oligodendroglioma, was grown in high-DMEM glucose (4.5%) supplemented with 10% FCS. Three BDV-infected cell lines, OL/BDV (32), C6BV (10), and SK-N/BDV, obtained by establishing a BDV-strain He80-1 infection in SK-N-SH cells, were maintained under the same conditions as the parental cell lines. These cells produced infectious BDV.
Preparation of whole-cell, cytoplasmic, and nuclear extracts.
To isolate whole-cell extract, cells on plates 100 mm in diameter were resuspended in buffer C (50 mM HEPES-KOH [pH 7.8], 420 mM KCl, 0.1 mM EDTA [pH 8.0], 5 mM MgCl2, 20% glycerol, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg of aprotinin/ml, 2 μg of pepstatin/ml, and 2 μg of leupeptin/ml) by passing through a 25-gauge needle five to ten times. The resulting suspension was centrifuged at 4°C for 15 min at 18,000 × g, and the supernatant was used as the whole-cell lysate. The cytoplasmic and nuclear extracts were prepared as follows. The cells were harvested in phosphate-buffered saline (PBS) and centrifuged at 4°C for 1 min at 2,000 × g. The cell pellets were washed twice with PBS and resuspended in 400 μl of buffer A (10 mM HEPES-KOH [pH 7.8], 10 mM KCl, 0.1 mM EDTA [pH 8.0], and 0.1% NP-40). The suspension was vortexed and centrifuged at 4°C for 1 min at 2,000 × g. The resulting supernatant was used as the cytoplasmic extract. The pellet was resuspended in 100 μl of buffer C and rotated for 30 min at 4°C; then the lysate was centrifuged at 4°C for 15 min at 18,000 × g, and the supernatant was used as the nuclear extract.
Far-Western assay.
The cell extracts were denatured by boiling in sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis using 10% gels. After electrophoresis, proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes, which were then blocked with 5% skimmed milk in PBS–0.05% Tween 20 (PBS-T) overnight at 4°C. As probes, 6 μg of glutathione S-transferase (GST)–p24, GST, or purified p24 protein produced from Escherichia coli per ml was allowed to bind to blotted proteins in PBS-T containing 5% (wt/vol) skimmed milk overnight at 4°C. The expression and purification of the recombinant GST-p24 and removal of GST from GST-p24 were described elsewhere (51). The blots were washed three times with PBS-T for 30 min and were then reacted with anti-GST mouse monoclonal antibody (MAb) or anti-p24 rabbit polyclonal antibody (PAb) in PBS-T containing 5% skimmed milk for 1 h at room temperature. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) or goat anti-rabbit IgG for 1 h at 4°C. Reacted proteins on the membranes were then visualized by the enhanced-chemiluminescence system (Amersham Pharmacia Biotech, Uppsala, Sweden).
BDV p24 affinity column and ion-exchange chromatography.
The BDV p24 affinity column was constructed by binding purified BDV p24 to NHS-activated Sepharose 4FF (Amersham Pharmacia Biotech) as follows. GST was cleaved from the GST-fused BDV p24 with PreScission protease, and purified p24 protein was used in excess to ensure saturation of the N-hydroxysuccinimide-activated Sepharose with ligand-coupling buffer (0.2 M NaHCO3 and 0.5 M NaCl [pH 8.3]). Unbound protein was removed from the column with washing buffer (cold 1 mM HCl), and the ligand-coupling buffer was rapidly applied to the column. The column was then stored overnight at 4°C. For blocking, the column was treated with high pH buffer (0.5 M ethanolamine and 0.5 M NaCl [pH 8.3]) and was washed six times with 3 bed volumes of high pH buffer followed by low pH buffer (0.1 M CH3COOH and 0.5 M NaCl [pH 4.0]). The column was equilibrated with 3 bed volumes of PBS, the cell extract from MDCK cells was loaded onto the column, and unbound proteins were allowed to flow through the column. The column was then washed at 4°C with 5 bed volumes of washing buffer (50 mM Tris-HCl [pH 8.0] and 0.5 M NaCl) and was eluted with buffer B (100 mM glycine-HCl [pH 2.7] and 0.5 M NaCl) until eluting proteins could not be detected by SDS-PAGE analysis with silver staining.
After p24 affinity column chromatography, the samples were subjected to ion-exchange chromatography using HiTrap Q (Amersham Pharmacia Biotech). We examined elution buffers with several pH values (20 mM Tris-HCl [pH 7.0, 7.5, 8.0, or 8.5]), and the most efficient pH for protein elution (7.5) was selected. The fractions containing proteins from the column were collected and subjected to SDS-PAGE and far-Western analysis using the p24 probe as described above.
Microsequence analysis.
Proteins were resolved by SDS-PAGE and transblotted onto PVDF membranes. The membranes were stained for protein with Ponceau S (Sigma Chemical Co., St. Louis, Mo.), and the 30- and 26-kDa bands were excised. The N-terminal sequence of the protein in the excised membrane was analyzed by Biologica Co., Tokyo, Japan. A basic local alignment search tool search (1) of the nonredundant protein database at the National Center for Biotechnology Information was used to identify sequences homologous to those obtained by amino acid sequencing.
Plasmid construction.
The BDV cDNA expression plasmids used for mammalian two-hybrid analysis were generated as follows. BDV cDNAs corresponding to BDV p24 open reading frames were amplified with sense (5′-TGC GGA TCC GTA TGG CAA CGC GAC CAT CGA GTC-3) and antisense (5′-TTG ACG CGT TTA TGG TAT GAT GTC CCA TTC AT-3′) primers using sample RNA from MDCK cells persistently infected with BDV (MDCK/BDV) (17) by reverse transcriptase PCR (RT-PCR). The PCR products were digested with BamHI and MluI and were cloned into vectors for the two-hybrid assay, pBIND and pACT (Promega, Madison, Wis.), and were designated GAL4-p24 and VP16-p24, respectively. To generate GAL4-p40 and VP16-p40, the entire BDV p40 cDNA sequence was digested from pcDL-N. Wild (23) and was cloned into the BamHI and KpnI sites of pBIND and pACT. Rat amphoterin cDNA was amplified with sense (5′-TAC GGA TCC GTA TGG GCA AAG GAG ATC CTA AG-3′) and antisense (5′-TCG ACG CGT CAT GCG TAG AAC CAA CTT ATT CA-3′) primers using RNA from C6 cells by RT-PCR. The resulting cDNA was cloned into the two-hybrid vectors digested with BamHI and KpnI and was designated GAL4-APT and VP16-APT.
Mammalian two-hybrid assay.
COS-7 cells were transfected with luciferase reporter plasmid, pG5luc (Promega), and test plasmids (1.0 μg) using TransFast transfection reagent (Promega) in six-well culture plates. Forty-eight hours after transfection, the cells were lysed in 500 μl of lysis buffer for 15 min at room temperature. After centrifugation at 18,000 × g for 30 s, the cell extracts were assayed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's recommendations.
Immunoprecipitation assay.
OL cells were cotransfected with FLAG-tagged amphoterin (pcD-APT/FLAG) and p24 expression (pP-wild) plasmids. The construction of pP-wild is described elsewhere (46). At 48 h posttransfection, transfected cells were labeled for 6 h in methionine-free Eagle's medium with 150 μCi of [35S]methionine per ml. After labeling, the cells were lysed by freeze-thaw cycling in a buffer containing 10 mM Tris (pH 7.6), 150 mM NaCl, 0.5% Nonidet P-40, and 1.0 mM phenylmethylsulfonyl fluoride. After centrifugation, the soluble fraction was immunoprecipitated with anti-FLAG MAb for 2 h at 4°C, and the precipitates were then recovered by incubation with protein G-agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) for 2 h at 4°C. After thorough washing, proteins bound to the agarose beads were separated by SDS-PAGE as described previously (23, 46).
Neurite outgrowth and transfilter migration assays.
The culture plates were coated with the indicated amounts of laminin (Sigma Chemical Co.) at 4°C overnight. The wells were then washed twice with PBS and blocked with 1% bovine serum albumin (BSA). After FCS starvation for 2 h, the BDV-infected or uninfected cells were cultured on plates in serum-free DMEM at 37°C for 3 to 4 h to induce outgrowth or extension of the cells. The cells were then fixed with 4% paraformaldehyde and stained with 0.05% toluidine blue or hematoxylin, and proportions of neurite-bearing cells (processes longer than 1 diameter of the cell soma) in at least four independent fields were counted under a light microscope (Nikon Co., Tokyo, Japan). To determine the viability of cells during the assay, trypan blue or Hoechst staining was also performed in the C6 cells cultured with purified p24 protein and antiamphoterin antibody.
The migration assay was carried out in Transwell chambers (filter pore size, 12 μm; Corning Costar) essentially as described previously (15). Briefly, the lower surface of the filter was coated with the indicated amounts of laminin at 4°C overnight. The filter was washed twice with PBS and blocked with BSA. Cells were placed in the upper chamber at 2 × 105 cells/ml and were cultured for 6 h or overnight. After fixing with cold methanol, the cells were stained with 0.05% toluidine blue and removed from the upper surface of the filter. The cells that had migrated to the lower surface were then examined under a light microscope. Photomicrographs were taken with a Fujix digital camera HC-300Zi (Nikon Co.), and five independent fields per filter were measured for the proportion of migrated cells in the observation fields using MacScope software (MITANI Co., Fukui, Japan).
Rat amphoterin expression and antibody production.
A baculovirus expression system encoding for rat amphoterin protein was prepared as follows. A cDNA fragment encoding rat amphoterin was amplified with sense (APT-1; 5′-TCG GAA TTC CAA CTA AAC ATG GGC AAA GGA GA-3′) and antisense (APT-2; 5′-TCG GGT ACC CAT GCG TAG AAC CAA CTT ATT CA-3) primers by RT-PCR, and the amplified fragment was inserted into the EcoRI and KpnI sites of the pcDL-His eukaryotic expression plasmid (23, 46) to create pcDL-His-AMP. To generate pFastBac-AMP donor plasmid for the baculovirus expression system, a rat amphoterin cDNA fragment was digested from pcDL-His-AMP and was cloned into the PstI and KpnI sites of the pFastBac baculovirus donor plasmid (GIBCO/BRL, Grand Island, N.Y.). Recombinant amphoterin-expressing baculovirus (Bac-AMP) was constructed using the BAC-to-BAC Baculovirus Expression System (GIBCO/BRL) according to the manufacturer's recommendations. Purification of histidine-tagged amphoterin produced in the baculovirus expression system was performed using a His-Trap Kit (Amersham Pharmacia Biotech) according to the manufacturer's recommendations from Bac-AMP-infected High Five cells (Invitrogen, Carlsbad, Calif.). Antiamphoterin immune sera were prepared by immunization of rabbits. Briefly, 4-week-old rabbits were immunized intramuscularly with recombinant rat amphoterin in Freund's complete adjuvant, and booster injections were continued until production of antibodies to amphoterin was observed on immunoblotting.
Northern blot analysis.
Total RNAs were extracted from BDV-infected or uninfected C6 cells grown on laminin-coated plates using an RNA isolation kit (Nippon Gene, Toyama, Japan). Aliquots of 5 μg of total RNA were electrophoresed through a 1% agarose gel containing 2.2% formaldehyde and transferred onto nylon membranes (GeneScreen Plus; NEN-Dupont, Boston, Mass.). The membranes were then analyzed by Northern (RNA) blot hybridization with [32P]rUTP-labeled antisense riboprobes. To generate the riboprobes, amphoterin cDNA was amplified with APT-1 and APT-2 by RT-PCR and was inserted into the polycloning site of pBluescript II SK(−). The plasmid coding β-actin cDNA (pB-actin) was kindly provided by J. Katahira (Osaka University). The plasmid DNAs were linearized with EcoRI (for amphoterin probe) and Eco81I (for β-actin probe), and 32P-labeled riboprobes were synthesized with in vitro transcription using T7 or T3 RNA polymerase. The blotted membranes were hybridized with the probes (106 cpm/ml) in ULTRAhyb buffer (Ambion, Inc., Austin, Tex.) at 68°C overnight. After washing, specific reaction was detected by exposure to X-ray film at −70°C.
Semiquantitative RT-PCR analysis of expression of receptor for advanced glycation end products (RAGE).
Total RNAs were extracted from BDV-infected or uninfected C6 cells (2 × 105 cells) grown with or without laminin coat. First-strand cDNAs were synthesized from aliquots of 1 μg of total RNAs by the Thermoscript RT-PCR System (GIBCO/BRL). The resulting cDNAs were used as templates for PCR amplification with the following primers: 5′-GAG CCA CTT ATG CTG AGC TG-3′ and 5′-CTG TGA GCT CTG ACC GAA GC-3′. PCR was performed in a total volume of 25 μl containing 2 μl of cDNA and 1.25 U of Taq polymerase (Amplitaq Gold; Perkin-Elmer). The reaction mixture was preincubated at 94°C for 5 min followed by 32 cycles of PCR at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. As a control for RNA input, levels of β-actin (primers, 5′-ATG GTG GGA ATG GGT CAG AAG-3′ and 5′-TAT CCT GAC CCT GAA GTA CCC CAT-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (GAPDH detection primer set; TOYOBO Inc., Tokyo, Japan) were assayed. The amplification products were resolved on 1.5% agarose gels. The images of agarose gels were captured electronically, and the pixels were inverted. The intensity of each band was quantified using NIH Image.
IFA.
Intracellular localization of amphoterin and BDV p24 proteins was analyzed by indirect immunofluorescence assay (IFA). The cells were grown on laminin for 3 to 4 h at 37°C and were fixed with 4% paraformaldehyde prior to treatment with 0.4% Triton X-100. After reaction with the anti-p24 MAb (49) and antiamphoterin PAb as the first antibodies, the cells were stained with fluorescein isothiocynate-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-mouse IgG antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Immunofluorescence was detected using an epifluorescence microscope (Nikon Co.).
RESULTS
BDV p24 phosphoprotein binds a 30-kDa protein.
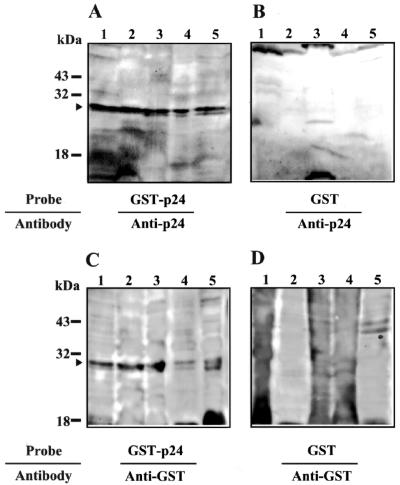
Previous studies have demonstrated that BDV p24 protein is a major product among the viral proteins, and it is always detected in BDV-infected cultured cells and rat brain cells (2, 3). These observations suggest a possibility that BDV p24 may directly alter the host cell environments by binding to host cellular factor(s). Therefore, we attempted to identify the cellular factor(s) that binds to BDV p24 phosphoprotein. We first performed far-Western blotting using extracts of several cell lines from various species, including human (OL and SK-N-SH), monkey (COS-7), rat (C6), and dog (MDCK). Details of the isolation of cell extracts and far-Western analysis are described in Materials and Methods. As a probe, recombinant BDV p24 protein expressed in E. coli was purified as a GST-tagged fusion protein and used. The purified GST protein was also used as a negative control probe. After hybridization of these probes, p24-binding proteins were detected with anti-p24 PAb or anti-GST MAb. As shown in Fig. 1, an intense band was found at around 30 kDa in all cell lines examined using GST-p24 protein as the probe (Fig. 1A and C, arrowhead). No unambiguous reactive band was detected in the GST-probed membranes (Fig. 1B and D). Furthermore, we detected the 30-kDa bands in both the nuclear and cytoplasmic fractions of the cell lines (data not shown).
FIG. 1.
Far-Western blot analysis of Borna disease virus phosphoprotein. Whole-cell extracts from various cell lines were loaded onto a 10% polyacrylamide gels. After blotting onto PVDF membranes, far-Western blotting was performed with recombinant GST-p24 (A and C) or GST (B and D) protein purified from E. coli as probes. The reacted proteins were then detected with anti-p24 PAb (A and B) or anti-GST MAb (C and D). Lanes: 1, MDCK (dog); 2, OL (human); 3, C6 (rat); 4, SK-N-SH (human); and 5, COS-7 (monkey). The appropriate sizes of molecular markers are shown.
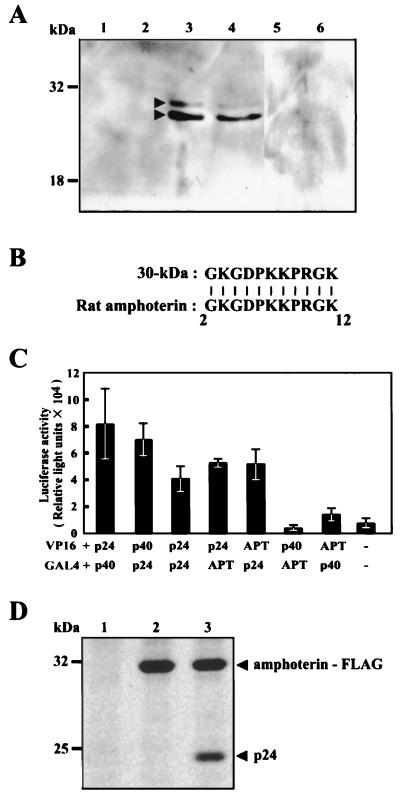
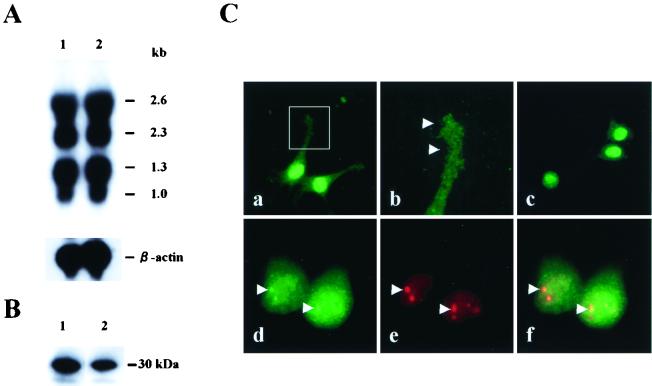
To verify the binding of BDV p24 to the 30-kDa protein, we next constructed a BDV p24 affinity column using recombinant p24 protein from E. coli (see Materials and Methods). The column was loaded with cellular extract from MDCK cells and was washed extensively to remove nonspecifically bound proteins. The specifically bound proteins were than eluted with buffer B. For further purification of the p24-binding proteins, the eluted fractions from the p24 affinity column were subjected to ion-exchange chromatography with elution buffers of different pH. Our results indicated that elution buffer of pH 7.5 effectively eluted proteins from the column (data not shown), so this pH was therefore selected for further analysis. Several fractions from the ion-exchange column were then subjected to SDS-PAGE and far-Western blotting with the BDV p24 probe. As shown in Fig. 2A, we clearly detected two bands at 30 and 26 kDa in some fractions from the column, confirming that BDV p24 protein can specifically bind to cellular factors of around 30 kDa.
FIG. 2.
The Borna disease virus phosphoprotein binds a 30-kDa protein. (A) Extract from MDCK cells was loaded onto a BDV p24 affinity column. After elution of proteins, the fractions were subsequently subjected to ion-exchange chromatography. Each fraction was then subjected to SDS–10% PAGE and far Western blot analysis using recombinant p24 protein and anti-p24 PAb. Lanes indicate fraction numbers. The sizes of molecular markers are also shown. (B) The amino acid sequences of purified 30-kDa proteins. The N-terminal sequence of 30-kDa protein was determined by microsequence analysis. Alignment of the 30-kDa protein was performed by a BLAST search of the GenBank database. (C) The Borna disease virus phosphoprotein specifically binds rat amphoterin in vivo. COS-7 cells were cotransfected with the indicated constructs and a luciferase reporter plasmid. After 48 h of incubation, luciferase activity was measured in cell extracts. APT, amphoterin; p24, BDV p24 phosphoprotein; p40, BDV p40 nucleoprotein; −, control. Bars and vertical lines represent the means and standard deviations of four independent experiments, respectively. (D) Immunoprecipitation of BDV p24 and amphoterin proteins. 35S-labeled OL cells were cotransfected with p24- and FLAG-tagged amphoterin expression plasmids, and interaction between the proteins was analyzed by immunoprecipitation using anti-FLAG antibody. Lane 1, control plasmid transfected; lane 2, pcD-APT/FLAG alone; lane 3, pcD-APT/FLAG and pP-wild.
To determine the origins of the proteins purified from the p24 affinity and ion-exchange chromatography columns, we transferred the proteins onto PVDF membranes followed by microsequencing analysis by Biologica Co. as described in Materials and Methods. The amino acid sequences of the N termini of proteins were obtained. The sequence of the lower band exactly matched that of BDV p24 protein, indicating that the band was p24 eluted from the BDV p24 affinity column. The N-terminal amino acid sequence of the upper band was GKGDPKKPRGK (Fig. 2B). As shown by a basic local alignment search tool search (1) of the nonredundant protein database at the National Center for Biotechnology Information, these sequences were identical to those present in rat amphoterin–HMG-1. Amphoterin–HMG-1 is a developmentally regulated nonhistone heparin-binding protein of 30 kDa that is abundantly expressed in the embryonic brain and in transformed cells from various species (34, 37, 38). The sequence of amphoterin–HMG-1 is well conserved across different species (34). These results suggested that the p24-binding protein identified in the MDCK cells was a dog homologue of the rat protein, amphoterin.
Amphoterin specifically binds to BDV p24 protein in vivo.
To confirm the binding of amphoterin to BDV p24 protein in vivo, we next used a GAL4/VP16-based mammalian two-hybrid system in COS-7 cells. The rat amphoterin cDNA (30) was reverse transcribed and amplified from total RNA extracted from a rat glioma cell line, C6. The cDNAs corresponding to amphoterin and BDV p24 and p40 ORFs were fused to the VP16 transactivating domain (VP16-APT, VP16-p24, and VP16-p40) or the GAL4 DNA-binding domain (GAL4-APT, GAL4-p24, and GAL4-p40). Each combination of the individual constructs and a luciferase reporter plasmid were cotransfected into COS-7 cells. Forty-eight hours after transfection, luciferase activity was measured in cell extracts as an index of protein-protein interactions (Fig. 2C). As previously reported, interaction between BDV p40 and p24 proteins was clearly demonstrated in cells transfected with VP16-p40 and GAL4-p24 or with VP16-p24 and GAL4-p40 (Fig. 2C). Furthermore, oligomerization of the p24 protein was also observed in cells cotransfected with VP16-p24 and GAL4-p24 (Fig. 2C). Transfection with VP16-APT and GAL4-p24 or VP16-p24 and GAL4-APT showed significant luciferase activities compared with the negative control plasmids (Fig. 2C). In contrast, no interaction was detected between VP16-APT and GAL4-p40-transfected cells. We repeated this experiment at least six times and obtained similar results in each experiment. For further verification of the binding between the p24 protein and amphoterin in vivo, immunoprecipitation analysis was performed in 35S-labeled OL cells that are cotransfected with p24- and FLAG-tagged amphoterin-expression plasmids. The transfected cells were harvested and immunoprecipitated with anti-FLAG antibody. As shown in Fig. 2D, the p24 protein was clearly coimmunoprecipitated with amphoterin (Fig. 2D, lane 3). Furthermore, amphoterin protein was also observed to be immunoprecipitated by using anti-p24 MAb in the cells (data not shown), strongly confirming that amphoterin specifically interacts with BDV p24 protein in vivo.
BDV p24 protein inhibits neurite outgrowth of cells.
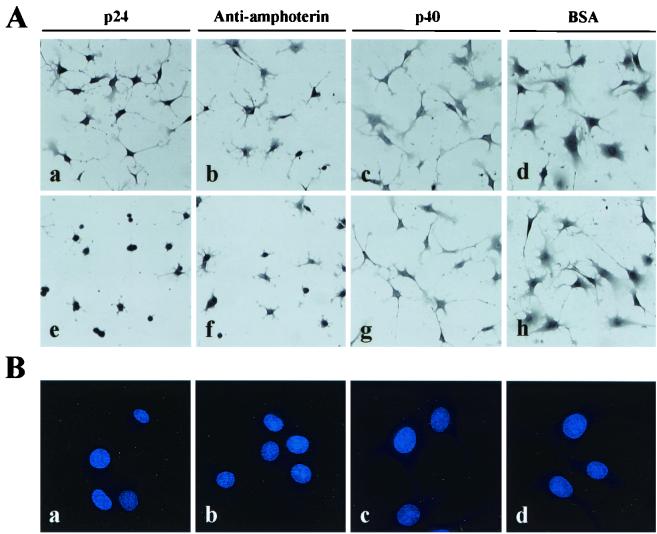
Previous studies have demonstrated an extracellular role in neurite outgrowth for amphoterin (37, 39). Amphoterin was found to be the endogenously occurring ligand that binds to the extracellular moiety of RAGE (18). Amphoterin-RAGE interaction enhances neurite outgrowth when extension of cytoplasmic processes is stimulated by matrix proteins such as laminin (15, 30, 34, 39). Therefore, we next examined whether the p24 protein can directly inhibit cell process outgrowth of neural cells. To observe the process efficiently, C6 glial cells were cultured on laminin-coated plates in medium containing purified p24 protein or antiamphoterin PAb. As a negative control, the cells were also cultured with purified p40 protein or BSA. As shown in Fig. 3A, the p24 protein in the medium, as well as antiamphoterin PAb, efficiently inhibited extension of the cells in a dose-dependent manner (Fig. 3A, panels a, b, e, and f), while the p40 protein and BSA did not show any effect on the cell process of C6 cells (Fig. 3A, panels c, d, g, and h). Figure 3B shows that cell process inhibition in p24 and antiamphoterin administration is not simply apoptosis or death of the cells. This observation suggested that binding between p24 and amphoterin proteins inhibits the cell process outgrowth of neural cells by interfering with amphoterin-RAGE interaction on the cell surface.
FIG. 3.
BDV p24 protein inhibits neurite outgrowth. (A) C6 cells were grown on laminin (2.5 μg/ml)-coated plates for 3 to 4 h with purified p24 protein (panels a and e), antiamphoterin antibody (panels b and f), purified p40 protein (panels c and g), or BSA (panels d and h). Ten (panels a to d) and 40 (panels e to h) μg of each protein/ml were added to the culture medium. The cells were fixed and briefly stained with hematoxylin. (B) Hoechst dye staining of C6 cells. C6 cells were cultured for 4 h on laminin with 40 μg of p24 (a), antiamphoterin antibody (b), p40 protein (c), or BSA (d) per ml and were stained with Hoechst 33342.
BDV infection inhibits neurite outgrowth and migration of neural cells.
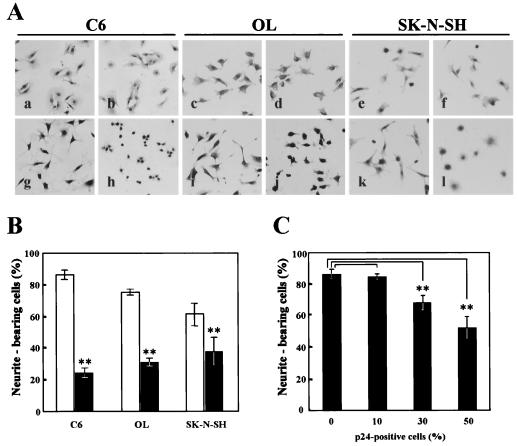
The results described suggest a possibility that BDV infection can influence the functions of amphoterin by expression of the p24 protein. To examine this possibility, we investigated the functional abilities of amphoterin in several BDV-infected neural cell types, C6BV, OL/BDV, and SK-N/BDV. The infected cells were cultured on laminin-coated plates, and the proportions of cell process outgrowth of the cells were determined after 3 to 4 h in serum-free culture. As shown in Fig. 4, the number of neurite-bearing cells was significantly reduced in the BDV-infected cells (Fig. 4A, panels g to l), although no apparent differences were observed between the infected and uninfected cells when cultured in normal medium (Fig. 4A, panels a to f). The cell process outgrowths of the BDV-infected cells were reduced to 30 to 60% of those in uninfected cells (Fig. 4B). To demonstrate whether BDV infection causes the decrease of the cell process outgrowth in these cells, we infected BDV to C6 cells and the cell process was observed at different times postinfection. The cells were collected at different percentages of the infection and were replated onto a laminin-coated plate. As shown in Fig. 4C, the number of the outgrowth-possessing cells was gradually decreased in association with increases in the percentage of p24-positive cells, indicating that BDV directly inhibits outgrowth processes of infected cells.
FIG. 4.
BDV infection inhibits neurite outgrowth and migration of neural cells. (A) BDV-infected (panels b, d, f, h, j, and l) and uninfected (panels a, c, e, g, i, and k) C6, OL, and SK-N-SH cells were grown under normal conditions (panels a to f) or on plates coated with laminin (2.5 μg/ml) (panels g to l). The cells were cultured for 3 to 4 h and were then fixed and briefly stained with hematoxylin. The proportions of neurite-bearing cells in at least four independent fields were counted in persistently (B) and acutely infected (C) cells under a light microscope. Bars and vertical lines represent the means and standard deviations of independent experiments, respectively. Black and white bars indicate BDV-infected and uninfected cells, respectively. Double asterisks indicate the level of statistical significance (P < 0.005) determined by Student's t test using the Statcel software (Oms publishing Inc., Tokyo, Japan).
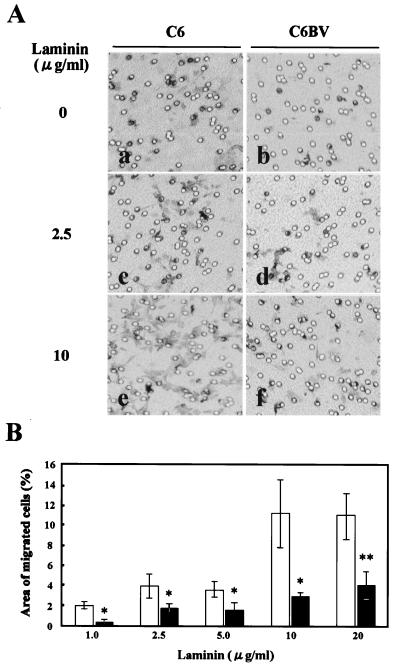
Recent studies have also revealed that amphoterin-RAGE interaction plays an important role in cell motility (15, 47). Therefore, we next used a transfilter migration assay to assess motility of the BDV-infected C6 cells. As shown in Fig. 5, BDV infection clearly affected the migration activity of the infected cells to the laminin-coated surface; the infected cells showed a reduction of the activity to about 30% of that of uninfected C6 cells (Fig. 5B). Furthermore, migration activity of the cells was correlated with the amount of laminin on the lower surface (Fig. 5B). These results suggested that BDV infection affects the functional abilities of amphoterin in infected cells.
FIG. 5.
BDV infection affects cell migration. (A) The lower surface of the Transwell filter was coated with laminin (0 [panels a and b], 2.5 [panels c and d], and 10 [panels e and f] μg/ml), and the BDV-infected (panels b, d, and f) or uninfected (panels a, c, and e) C6 cells were placed in the upper chamber. The cells that migrated from the upper chamber to the lower were studied under a microscope after 6 h. (B) The migrated cells were stained with toluidine blue, and the proportion of migrated cells was measured in five randomly different fields of each filter in at least four independent experiments. Bars and vertical lines represent the means and standard deviations of the proportion of migrated cells in each field, respectively. Black and white bars indicate BDV-infected and uninfected cells, respectively. Single (P < 0.05) and double (P < 0.005) asterisks indicate the level of statistical significance determined by Student's t test.
Expression and intracellular localization of amphoterin in BDV-infected cells.
To examine whether the p24 protein in BDV-infected cells affects the levels of amphoterin mRNA and protein expression, we next performed Northern and Western blot analyses using total RNAs and proteins from BDV-infected and uninfected C6 cells after 3 to 4 h of culture on laminin, respectively. As shown in Fig. 6A and B, both amphoterin transcript and protein were detected at similar levels in the infected and uninfected cells, indicating that the production of amphoterin was not affected by BDV infection.
FIG. 6.
Expression and intracellular localization of amphoterin. Expression of amphoterin mRNA (A) and protein (B) in BDV-infected and uninfected C6 cells. Total RNAs and proteins were isolated from the cells grown on laminin (2.5 μg/ml) and reacted with amphoterin-specific [32P]recombinant UTP-labeled riboprobe (A) and antiamphoterin PAb (B), respectively. As a control for RNA blotting, an β-actin probe was also used. Sizes of the bands are shown. (C) Localization of amphoterin in cells. The BDV-infected (panels c to f) and uninfected (panels a and b) C6 cells were cultured on laminin (2.5 μg/ml) for 3 to 4 h and were fixed. IFA was performed with antiamphoterin PAb (fluorescein isothiocyanate, green) and anti-p24 MAb (Cy3, red). Arrowheads indicate dot staining of amphoterin protein at the leading edge of uninfected C6 cells (panel b) and BDV-specific foci in the nuclei of infected cells (panels d to f).
Previous studies have demonstrated that amphoterin becomes localized to growth cones or leading edges in cells during the process of extension and is secreted from the cell surface to promote neurite outgrowth by the interaction with RAGE (15, 30, 34, 38). Thus, we next investigated intracellular localization of amphoterin in BDV-infected cells. After culture on laminin for 3 h, the infected and uninfected C6 cells were fixed and stained with anti-p24 MAb and antiamphoterin PAb. As previously demonstrated (30, 34), amphoterin was diffusely found in both the nucleus and cytoplasm in the cells in both infected (Fig. 6C, panel c) and uninfected cells (Fig. 6C, panel a), while the protein was also clearly localized at the growth cone of the uninfected cells (Fig. 6C, panels a and b, arrowhead). On the other hand, the localization of amphoterin at the growth cone or leading edge was found to be reduced in the BDV-infected cells (Fig. 6C, panel c). Furthermore, the p24 MAb revealed that the BDV p24 protein was colocalized with amphoterin protein in the infected cells. Colocalization of the proteins was also detected at the BDV-specific foci in the nuclei of infected cells (Fig. 6C, panels d to f, arrowheads). These observations suggested the possibility that the binding of p24 protein may influence intracellular movement of amphoterin and inhibits the leading-edge localization of the protein in the cells.
Level of RAGE transcription in BDV-infected cells.
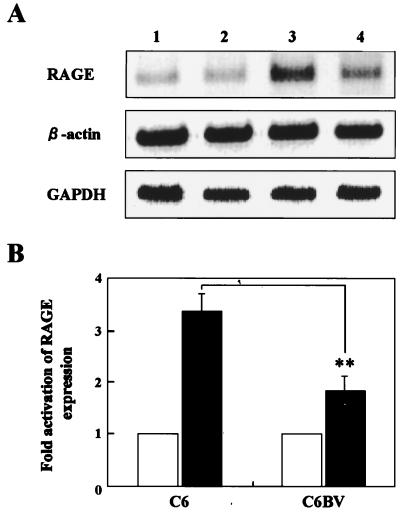
Previous studies have also demonstrated that interaction of amphoterin with RAGE up-regulates the level of RAGE transcription through the Spl-binding sites within the promoter of RAGE (25, 39). Thus, to examine the hypothesis that the p24 protein blocks amphoterin-RAGE interaction by inhibiting secretion of amphoterin from the cell surface and affects the extracellular roles of amphoterin, such as neurite outgrowth and cell motility, we analyzed expression level of RAGE mRNA in BDV-infected cells. The BDV-infected and uninfected C6 cells were cultured with or without laminin coating for 3 to 4 h, and total cellular RNAs were extracted from the cells. To determine the expression level of RAGE in the cells, we used semiquantitative RT-PCR as described in Materials and Methods. As shown in Fig. 7A, expression of RAGE mRNA was clearly activated in uninfected C6 cells grown on laminin (Fig. 7A, lane 3, and B). On the other hand, the BDV-infected C6 cells showed a reduced activation level of RAGE mRNA to almost 55% of that of uninfected cells even in culture on laminin-coated plates, although expression of the RAGE mRNA was equally detected in the BDV-infected and uninfected cells when the cells were cultured without laminin (Fig. 7A, lanes 1, 2, and 4, and B). We repeated this experiment at least three times and always obtained similar results in each experiment. This result demonstrated that secretion of amphoterin protein from the cell surface was reduced in the BDV-infected neural cells.
FIG. 7.
Semiquantitative RT-PCR analysis of RAGE expression in BDV-infected cells. (A) Total RNAs were extracted from the BDV-infected (lanes 2 and 4) and uninfected (lanes 1 and 3) C6 cells, and RT-PCR was performed as described in Materials and Methods. As a control for RNA input, the transcript of β-actin and GAPDH in the cells was also amplified. Lanes: 1 and 2, uncoated; 3 and 4, laminin coated. The amplification products were resolved on 1.5% agarose gels, and the images of agarose gels were captured electronically. (B) The fold activation of RAGE mRNA in each culture was calculated by the intensity of each band quantified using NIH Image software. Black and white bars indicate laminin-coated and uncoated plates, respectively. The level of statistical significance was determined by Student's t test. ∗∗, P = 0.0036.
DISCUSSION
The p24 phosphoprotein of BDV is an abundant protein in infected cultured cells and in infected animal brains (2, 3). Although the precise role of the protein in the viral life cycle has not yet been determined, it is assumed that the protein associates and cooperates with the pol protein to play a pivotal role in viral transcription and replication in the nucleus (26, 48). In this study, we demonstrated that a 30-kDa protein specifically binds to the bacterially and mammalianly expressed BDV p24 protein using far-Western blotting, p24 protein affinity chromatography, and mammalian two-hybrid and immunoprecipitation analyses (Fig. 1 and 2). Microsequencing revealed that the N-terminal sequence of the 30-kDa protein exactly matches that of rat amphoterin–HMG-1 protein (Fig. 2B). Note that the possibility that far-Western blotting using denaturing of cellular lysates fails to detect other specific binding proteins in the cells remains.
Amphoterin is a 30-kDa heparin-binding protein, which was first isolated from the perinatal rat brain as a neurite outgrowth-promoting adhesive factor (37). The level of amphoterin in the rat brain gradually decreases during brain development (18, 30), indicating that this protein is developmentally regulated. The sequence of amphoterin is well conserved across different species; the sequences of human (52), pig (49), cow (21), and rat homologues (30) are all composed of 214 amino acids, and the degree of homology is more than 98%. Indeed, we detected the 30-kDa protein in all cell lines from various species examined by far-Western blotting (Fig. 1). Furthermore, amphoterin has a highly dipolar sequence homologous to the HMG-1 group proteins (30). As HMG-1 protein, amphoterin must have a feature of DNA-binding protein and play roles in transcription, replication, chromatin assembly, and stabilization of chromatin structure in the nucleus (5, 24).
Amphoterin is abundantly expressed in immature and malignant cells. The protein has been shown to be highly enriched at the growth cones and was concentrated at the leading edges of neuronal cells in the extending processes (15, 30, 34). Previous studies suggested that although amphoterin lacks a classic secretion signal, the protein plays essential roles for the cell processes at the cell surface (11, 18, 29, 37, 39). Surface coating of culture plates with amphoterin efficiently induced neurite outgrowth of primary rat brain cells (30, 37). On the other hand, antibody to amphoterin inhibited neurite extension in cultured neuronal cells (34). Furthermore, it has recently been demonstrated that amphoterin is an endogenously occurring ligand that binds to the extracellular moiety of RAGE (18). The amphoterin-RAGE interaction directly mediated neurite outgrowth in neural cell lines and primary rat brain cells (18). Also, the neurite outgrowth reaction of the cells was blocked in the presence of soluble RAGE or anti-RAGE antibody. These observations indicated that amphoterin plays an important function in neurite outgrowth of neural cells as a protein secreted from the cell surface.
In addition to the neurite outgrowth function, amphoterin has been suggested to have several other extracellular roles. Daston and Ratner (11) reported that amphoterin is important for neuron-glial cell interaction, which is correlated with diminished amphoterin levels in glial cells. Furthermore, a function of amphoterin in the early phase of cell differentiation was also suggested (29). Recently, it was reported that amphoterin regulates cell migration of immature and transformed cells (15) and that blockage of RAGE-amphoterin signaling suppresses tumor growth and metastases (47). Moreover, it has been also demonstrated that activation of RAGE by amphoterin promotes cell survival through increased expression of the antiapoptotic protein Bcl-2 (20). Together with these observations, it is also likely that amphoterin is critical in maturation or construction of the central nervous system (CNS), as well as in network formation of neuronal cells in the developing or injured brain.
Previous studies demonstrated that persistent infection with BDV induced functional alterations of the brain in the absence of immunopathology-related brain damage (4, 9, 14, 19, 42). It has been reported that BDV-infected neonatal rats show developmental damage and neuroanatomical disturbances characterized by degeneration of hippocampal neurons, cortical shrinkage, cerebellar hypoplasia, and degeneration of Purkinje cell neurons in the cerebellum (14, 16, 41, 42). Furthermore, chronic astrocytosis and microgliosis, as well as progressive decreases in synaptic density and plasticity, were observed in the brains of neonatally infected rats (16, 19, 44). These observations suggested that BDV infection could directly affect brain development or its function without direct destruction of infected neuronal cells.
The binding between BDV p24 and amphoterin suggests a mechanism for the neuropathogenesis of BDV in infected animal brains. Analyses using BDV-infected cultured cells demonstrated that the BDV infection, as well as bacterially expressed p24 protein, can efficiently inhibit cell process outgrowth and migration of neural cells (Fig. 3 to 5) and can also reduce the leading-edge localization of amphoterin in the cells grown on laminin (Fig. 6). Furthermore, expression of RAGE mRNA was not comparably enhanced between the BDV-infected and uninfected neural cells (Fig. 7). These results suggest that intracellular binding of p24 protein with amphoterin inhibits the secretion or movement of amphoterin in infected neuronal cells. As secretion from the cell surface must be critical for the function of amphoterin (11, 15, 20, 29, 30, 39), reduction of the secreted amphoterin may affect neuronal maturation or neural cell communication, especially during the early stages of brain development. This may result in developmental damage or disturbance of neuronal cells observed in neonatally BDV-infected rats. Investigation of the developmental process of the brains of BDV-infected neonatal animals, including vertically infected neonates, will be of interest not only from the biological perspective regarding amphoterin but also with regard to the pathogenesis of BDV p24 in CNS development.
Previous study has demonstrated a high level of expression of amphoterin mRNA in the areas of the cerebral cortex, hippocampus, and cerebellum of the developing CNS (18, 31). There is evidence that granule cell neurons of the dentate gyrus and cerebellum of rat brains, as well as Purkinje cell neurons, are positive for amphoterin mRNA for at least 14 days after birth (W. Kamitani and K. Tomonaga, unpublished data). The CNS areas of neuronal degeneration observed in BDV-infected rats are consistent with those of amphoterin distribution in the developing brains, indicating the possibility that the functional disturbance of amphoterin is directly involved in neuronal degeneration or developmental damage in infected rat brains. The observation that BDV infection of postnatal day-15 rats does not cause significant signs of hippocampal neuron degeneration (42) supports this possibility. On the other hand, some of the cells most severely affected by the developmental damage in BDV-infected brains are not known to be infected. Therefore, it is also possible that extracellular p24 protein or infected Purkinje cells that connect with granule cell neurons play a role in the degeneration or developmental damage of neurons. Furthermore, the p24 protein in infected mature brains may influence the survival of neuronal cells from damage to immune responses or viral infections. Interestingly, it has been reported that expression of amphoterin is enhanced by proinflammatory cytokines, such as interleukin-1 and tumor necrosis factor (50; Kamitani and Tomonaga, unpublished). Injury to neuronal cells by accumulation of BDV p24 antigen should be investigated further to determine the neuropathogenesis of BDV. Experiments are currently in progress in our laboratory to detect interactions between amphoterin and p24 proteins in BDV-infected animal brains and to investigate the direct effects of p24 expression in the development of neonatal animal brains.
ACKNOWLEDGMENTS
W.K. and Y.S. contributed equally to this work.
This work was supported by Gakujusu-frontier Cooperative Research in Rakuno-gakuen University, the Special Coordination Funds for Science and Technology from the Science and Technology Agency (STA), and the Grants-in-Aid for BDV Research from the Ministry of Health, Labour and Welfare and from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bause-Niedrig I, Pauli G, Ludwig H. Borna disease virus-specific antigens: two different proteins identified by monoclonal antibodies. Vet Immunol Immunopathol. 1991;27:293–301. doi: 10.1016/0165-2427(91)90027-a. [DOI] [PubMed] [Google Scholar]
- 3.Bause-Niedrig I, Jackson M, Schein E, Ludwig H, Pauli G. Borna disease virus-specific antigens. II. The soluble antigen is a protein complex. Vet Immunol Immunopathol. 1992;31:361–369. doi: 10.1016/0165-2427(92)90022-i. [DOI] [PubMed] [Google Scholar]
- 4.Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Dev Brain Res. 1995;90:45–53. doi: 10.1016/0165-3806(96)83485-7. [DOI] [PubMed] [Google Scholar]
- 5.Baxevanis A D, Landsman D. The HMG-1 box protein family: classification and functional relationship. Nucleic Acids Res. 1995;23:1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode L, Durrwald R, Ludwig H. Borna virus infections in cattle associated with fatal neurological disease. Vet Rec. 1994;135:283–284. doi: 10.1136/vr.135.12.283. [DOI] [PubMed] [Google Scholar]
- 7.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 8.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone K M, Park S W, Rubin S A, Waltrip II R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daston M M, Ratner N. Expression of P30, a protein with adhesive properties, in Schwann cells and neurons of the developing and regenerating peripheral nerve. J Cell Biol. 1991;112:1229–1239. doi: 10.1083/jcb.112.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Torre J C, Bode L, Durrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 14.Eisenman L M, Brothers R, Tran M H, Kean R B, Dickson G M, Dietzschold B, Hooper D C. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J Neurovirol. 1999;5:181–189. doi: 10.3109/13550289909022000. [DOI] [PubMed] [Google Scholar]
- 15.Fages C, Nolo R, Huttunen H J, Eskelinen E, Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113:611–620. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Dunia D, Watanabe M, Syan S, Mallory M, Masliah E, de la Torre J C. Synaptic pathology in Borna disease virus persistent infection. J Virol. 2000;74:3441–3448. doi: 10.1128/jvi.74.8.3441-3448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 18.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen J X, Nagashima M, Lundh E R, Vijay S, Nitecki D, Morser J, Stern D, Schmidt A M. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 19.Hornig M, Weissenbnck H, Horscroft N, Lipkin W I. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huttunen H J, Kuja-Panula J, Sorci G, Agneletti A L, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan D J, Duncan C H. Full length cDNA sequence for bovine high mobility group 1 (HMG1) protein. Nucleic Acids Res. 1988;16:10375. doi: 10.1093/nar/16.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Shoya Y, Koda T, Takashima I, Lai P K, Ikuta K, Kakinuma M, Kishi M. Nuclear targeting activity associated with the amino terminal region of the Borna disease virus nucleoprotein. Virology. 1998;243:188–197. doi: 10.1006/viro.1998.9049. [DOI] [PubMed] [Google Scholar]
- 24.Landsman D, Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993;15:539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Qu X, Schmidt A M. Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J Biol Chem. 1998;273:30870–30878. doi: 10.1074/jbc.273.47.30870. [DOI] [PubMed] [Google Scholar]
- 26.Lipkin W I, Travis G H, Carbone K M, Wilson M C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipkin W I, Schneemann A, Solbrig M V. Borna disease virus: implications for human neuropsychiatric illness. Trends Microbiol. 1995;3:64–69. doi: 10.1016/s0966-842x(00)88877-0. [DOI] [PubMed] [Google Scholar]
- 28.Lundgren A L, Zimmermann W, Bode L, Czech G, Gosztonyi G, Lindberg R, Ludwig H. Staggering disease in cats: isolation and characterization of the feline Borna disease virus. J Gen Virol. 1995;76:2215–2222. doi: 10.1099/0022-1317-76-9-2215. [DOI] [PubMed] [Google Scholar]
- 29.Melloni E, Sparatore B, Patrone M, Pessino A, Passalacqua M, Pontremoli S. Extracellular release of the “differentiation enhancing factor,” a HMG1 protein type, is an early step in murine erythroleukemia cell differentiation. FEBS Lett. 1995;368:466–470. doi: 10.1016/0014-5793(95)00716-m. [DOI] [PubMed] [Google Scholar]
- 30.Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266:16722–16729. [PubMed] [Google Scholar]
- 31.Nair S M, Zhao Z, Chou D K, Tobet S A, Jungalwala F B. Expression of HNK-1 carbohydrate and its binding protein, SBP-1, in apposing cell surfaces in cerebral cortex and cerebellum. Neuroscience. 1998;85:759–771. doi: 10.1016/s0306-4522(97)00666-0. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y, Takahashi H, Shoya Y, Nakaya T, Watanabe M, Tomonaga K, Iwahashi K, Ameno K, Momiyama N, Taniyama H, Sata T, Kurata T, de la Torre J C, Ikuta K. Isolation of Borna disease virus from human brain tissue. J Virol. 2000;74:4601–4611. doi: 10.1128/jvi.74.10.4601-4611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 34.Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander E O, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268:19726–19738. [PubMed] [Google Scholar]
- 35.Planz O, Rentzsch C, Batra A, Batra A, Winkler T, Büttner M, Rziha H-J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pletnikov M V, Rubin S A, Schwartz G J, Carbone K M, Moran T H. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 37.Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262:16625–16635. [PubMed] [Google Scholar]
- 38.Rauvala H, Merenmies J, Pihlaskari R, Korkolainen M, Huhtala M L, Panula P. The adhesive and neurite-promoting molecule p30: analysis of the amino-terminal sequence and production of antipeptide antibodies that detect p30 at the surface of neuroblastoma cells and of brain neurons. J Cell Biol. 1988;107:2293–2305. doi: 10.1083/jcb.107.6.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauvala H, Huttunen H J, Fages C, Kaksonen M, Kinnunen T, Imai S, Raulo E, Kilpelainen I. Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol. 2000;19:377–387. doi: 10.1016/s0945-053x(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 40.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 41.Rubin S A, Sylves P, Vogel M, Pletnikov M, Moran T H, Schwartz G J, Carbone K M. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 42.Rubin S A, Bautista J R, Moran T H, Schwartz G J, Carbone K M. Viral teratogenesis: brain developmental damage associated with maturation state at time of infection. Dev Brain Res. 1999;112:237–244. doi: 10.1016/s0165-3806(98)00180-1. [DOI] [PubMed] [Google Scholar]
- 43.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 44.Sauder C, de la Torre J C. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 45.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 46.Shoya Y, Kobayashi T, Koda T, Ikuta K, Kakinuma M, Kishi M. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J Virol. 1998;72:9755–9762. doi: 10.1128/jvi.72.12.9755-9762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taguchi A, Blood D C, del Toro G, Canet A, Lee D C, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann M A, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern D M, Schmidt A M. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 48.Thierer J, Riehle H, Grebenstein O, Binz T, Herzog S, Thiedemann N, Stitz L, Rott R, Lottspeich F, Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992;73:413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- 49.Tsuda K, Kikuchi M, Mori K, Waga S, Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988;27:6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Bloom O, Zhang M, Vishnubhakat J M, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue K R, Faist E, Abraham E, Andersson J, Andersson U, Molina P E, Abumrad N N, Sama A, Tracey K J. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe M, Zhong Q, Kobayashi T, Kamitani W, Tomonaga K, Ikuta K. Molecular ratio between Borna disease viral-p40 and -p24 proteins in infected cells determined by quantitative antigen capture ELISA. Microbiol Immunol. 2000;44:765–772. doi: 10.1111/j.1348-0421.2000.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 52.Wen L, Huang J K, Johnson B H, Reeck G R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989;17:1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]