Abstract
Background and Objectives
Older adults (≥65 years) are the largest consumers of over-the-counter (OTC) medications and exceptionally vulnerable to the risks of these medications, including adverse drug events (ADEs). However, little is known about how older adults select and use OTCs. This is the first multisite study designed to prospectively quantify the type and intended use of OTCs selected by older adults in community pharmacies where products are purchased.
Research Design and Methods
Older adults (n = 144) were recruited from 10 community pharmacies from a Midwestern health system. Participants were given hypothetical symptoms and asked to select one or more OTCs for self-treatment. They were asked to report how they would use the products at symptom onset and when symptoms persisted or worsened. They also reported their current medication list and health conditions. Participants’ OTC selections were evaluated for 4 types of misuse: drug-age, drug-drug, drug-disease, and drug-label.
Results
Of the 144 participants, 114 (79%) demonstrated at least one type of misuse when describing how they would use their OTC selections at symptom onset. Drug-drug and drug-label misuse had the highest prevalence. Overall, 26 (18%) and 28 (19%) participants showed only drug-drug or drug-label misuse, respectively. Notably, 55 (38%) of participants demonstrated misuse in 2 or more misuse categories. Misuse potential was exacerbated when participants described treating persistent or worsening symptoms.
Discussion and Implications
The results highlight the high prevalence and complexity of OTC misuse in older adults and the need for additional work to improve OTC safety.
Keywords: Community pharmacy, Inappropriate medication use, Medication safety, Self-care, Self-treatment
Translational Significance: This is the first study to demonstrate and characterize significant potential misuse of over-the-counter (OTC) medications in older adults. Importantly, it provides a clear direction for future research, including assessing the clinical impact of OTC misuse and developing and implementing interventions that promote safe OTC selection and use without limiting patient autonomy.
Background and Objectives
Over-the-counter (OTC) medications are drug products sold directly to the public without a prescription, including medications that previously required a prescription (U.S. Food and Drug Administration, 2021, 2023). Today, there are over 300,000 OTCs available in the United States (U.S. Food and Drug Administration, 2022). Older adults (≥65 years) are the largest consumers of OTCs, accounting for 30% of OTC use despite comprising 17% of the population (National Council on Patient Information and Education, 2017; U.S. Census Bureau, 2023). Almost 50% of older adults use an OTC daily to weekly (Qato et al., 2008). Because OTCs do not require a prescription, there is little knowledge of or even consideration for how these medications are used, including the extent of their misuse (Chui et al., 2017).
Over-the-counter misuse poses a patient safety risk and can lead to adverse drug events (ADEs; Center for Disease Control and Prevention, 2024, April 17). ADEs associated with OTCs contribute to 178,000 hospitalizations annually (United Health Foundation, 2005). Older adults are exceptionally vulnerable to ADEs due to various age-related factors, including changes in pharmacokinetics and pharmacodynamics, multimorbidity, polypharmacy, health condition complexity, declining cognition, and general frailty (Woo et al., 2020). For older adults, high-risk OTC medications include sleep and pain medications, such as aspirin, ibuprofen, acetaminophen, and diphenhydramine. These OTCs are the most frequently used by older adults and implicated in ADEs leading to emergency department visits (Kaufman et al., 2002).
In the face of OTC-related ADEs, nearly a decade ago the Gerontological Society of America (GSA) and Consumer Healthcare Products Association (CHPA) convened a National Summit. The purpose of the Summit was to set an agenda for research in OTC behaviors among older adults, which was published in a white paper. The agenda included understanding the ways older adults select OTC medications and how they decide to start, use, and stop them (Albert et al., 2014). However, since this paper’s publication, minimal work has been done to answer these central questions.
This is the first multisite study designed to understand how older adults select and use OTCs in community pharmacies, where products are typically sold. By asking older adults to make hypothetical decisions, this study sought to understand how older adults would use OTCs at symptom onset and when symptoms persisted or worsened, quantify the OTC misuse prevalence, and characterize different misuse types.
Research Design and Methods
Recruitment
Older adults were recruited from 10 community pharmacies located within clinics of a large Midwestern health system between June 2022 and October 2022. The clinics were geographically dispersed to ensure engagement with diverse populations. Posters and fliers were available within the clinics to create awareness. Patients in the facilities approached researchers, who were stationed outside of the pharmacies, to inquire about the study and were recruited in real time. Eligible participants were ≥65 years old, would consider using an OTC product for self-care, and passed a six-item screener used to identify individuals with cognitive impairment (Callahan et al., 2002). The Institutional Review Board at the University of Wisconsin-Madison approved this study.
Data Collection
After giving informed consent, participants were asked to select whether they had the most experience with treating cough/cold/allergy, pain, or sleep symptoms, which are common health issues for older adults and associated with high-risk OTCs. Experience was defined as having a history of these symptoms. Participants were then given one of the following hypothetical scenarios:
Cough/cold/allergy: “Over the last couple of days, you have been having symptoms related to a cold or allergies, like a runny nose, stuffy nose, cough, or congestion. You have not taken any medications for your symptoms yet, and it’s not bad enough to call your doctor.”
Pain: “Over the last couple of days, you have been having soreness and muscle aches from an activity like snow shoveling, gardening, or hiking. You have not taken any medication to help with these aches yet, and it’s not bad enough to call your doctor.”
Sleep: “Over the last couple of days, you have been having difficulty falling asleep or staying asleep. You have not taken any medication to help with this sleep problem, and it’s not bad enough to call your doctor.”
Participants were asked to go into the pharmacy and choose one or more OTCs to treat their hypothetical symptoms. To better replicate a real-world experience, they were given a pretend credit card to “buy” their selected products, and they could interact with the staff if desired. Participants were not given a time limit for this process. Prior to data collection, pharmacy staff were instructed to interact with the participants as they normally would.
Participants were told to bring their selected OTC products to the researcher to complete a short, follow-up interview. During the interview, participants could refer to the package when answering questions. Participants were asked to describe how they would use their OTC selection(s), including dose, frequency, and duration, at symptom onset. In instances where participants selected more than one OTC, they were also asked to describe if and how they would use the products in combination. Participants were then told to imagine they were experiencing persistent or worsening symptoms and asked what they would do. If the participant indicated that they would call the doctor or use a non-pharmacological treatment option (such as icing or seeing a chiropractor), the interview was concluded. If the participant indicated that they would continue using their initial selections, use a different dose of the same products, or use different OTCs, they were once again asked how they would use the selected OTC(s), as described above. The full symptom scenarios and interview questions are included in Supplementary Material Section 1. All interviews were audio recorded, and OTC product photographs were taken for clinical interpretation purposes.
After the in-person interview, a 20-min phone interview was scheduled with the participant. Participants were asked to report home medications they used in the last 30 days, including prescriptions, OTCs, vitamins, and herbal and alternative medications. For each medication, the interviewer collected the name, dose/strength, frequency, and where they purchased their medications. Participants completed the three-question Brief Health Literacy Screen (BHLS), with low health literacy defined as a summative score ≤9, and adequate health literacy defined as a score >9 (max score of 15; Chew et al., 2004). Participants responded yes or no to a list of health conditions modified from Older Americans Resources and Services (OARS; Duke Aging Center, 2005). Lastly, participants reported demographic information. The phone interview guide is included in Supplementary Material Section 2. All phone interviews were recorded. After both interviews were completed, participants were mailed $40 for participating. The participants’ selected OTC products and all interview responses were entered and verified in REDCap, a web-based software platform for data management (Harris et al., 2009, 2019).
Misuse Classifications
Analysis aimed to identify 4OTC misuse types: (1) drug-age, (2) drug-drug, (3) drug-disease, and (4) drug label. Drug-age and drug-drug misuse could be determined without clinical judgment and were independently evaluated by three PharmD students and verified by a pharmacist researcher. Drug-disease and drug-label misuse were independently evaluated by three clinical pharmacists with experience treating older adult patients.
Drug-age misuse was based on the 2019 Beers Criteria (American Geriatrics Society, 2019). The Beers Criteria included two main OTC classes. The first was oral nonsteroidal anti-inflammatory drugs (NSAIDs) used chronically (>90 days). Oral NSAIDs included aspirin (>325 mg), ibuprofen, naproxen, and magnesium salicylate tetrahydrate. Although magnesium salicylate tetrahydrate is not listed on the Beers Criteria, it was included as an NSAID. The second class was anticholinergics, including chlorpheniramine, triprolidine, doxylamine, diphenhydramine, and brompheniramine. For each OTC, the active ingredients were compared to the Beers Criteria. For OTCs with multiple active ingredients, each ingredient was evaluated separately. Additionally, OTCs categorized as NSAIDS were assessed for chronic use by reviewing the patient-reported duration of use from the in-person interview.
Drug-drug misuse was defined as using two or more medications that have a drug–drug interaction. Interactions were determined by comparing each selected OTC to the participant’s home medications using Lexicomp (Lexicomp, 2023b). Lexicomp does not include entries for generic combination medications. For these OTCs, a brand equivalent was selected. If a brand equivalent was not available, selections were made for each individual ingredient. If there was not an entry in Lexicomp that matched (e.g., a homeopathic product), the OTC selection or home medication was labeled, “no match in Lexicomp,” and excluded from evaluation. The Lexicomp Drug Interactions Module (Lexicomp, 2023a) was used to identify drug–drug interactions. Interactions with the following domains carried enough risk to be considered misuse: C (monitor therapy), D (consider therapy modification), and X (avoid combination). Any interactions with an A (no known interaction) or B (no action needed) risk rating were considered safe use (Lexicomp, 2023a).
The inclusion of interactions with “C” risk ratings was decided through discussions with a panel of clinical pharmacists. All interactions fall into one of three severity ratings: minor (effects would be considered tolerable in most cases—no need for medical intervention), moderate (medical intervention needed to treat effects), or major (effects may result in death, hospitalization, permanent injury, or therapeutic failure). Only “C” interactions with “moderate” or “major” severity were determined to constitute misuse. Although a “C” risk rating typically indicates that concomitant use of two medications outweighs the risks, those with “moderate” or “major” severity may lead to the need for medical intervention or serious health consequences (Lexicomp, 2023a).
Only interactions between an OTC selection and home medication were reported. Interactions between two OTC selections or two home medications were excluded. In some cases, an OTC selection interacted with multiple home medications, or had multiple interactions with the same home medication. In these cases, each interaction was reported separately.
Drug-disease misuse was defined as medication use that is contraindicated with a participant’s health conditions. Potential contraindications included those between the selected OTCs and disease states designated as high risk per the 2019 Beers Criteria, a health condition listed on the product labeling, or a condition determined to be clinically significant by the evaluators (American Geriatrics Society, 2019). Certain melatonin products included a warning to, “not use [the] product, unless advised by a physician, if you are pregnant, attempting to become pregnant, nursing, taking any medications, or have any chronic medical conditions.” For these cases, drug-disease misuse was not automatically assumed if the participant affirmed any health conditions, and clinical judgment determined misuse. Discrepancies were identified and sent back to the pharmacists to reexamine the data and add comments or update their response if needed. Final misuse determination was based on any health condition identified by at least two pharmacists. If multiple health conditions were identified, each condition was considered a separate drug-disease misuse instance.
Drug-label misuse was defined as medication use not conforming to label instructions. This definition was compartmentalized into five categories: (1) exceeds daily dosage (exceeds threshold consumption amount for a 24-h window), (2) exceeds single dose (maximum amount recommended for a single dose), (3) dose timing/frequency (second dose taken earlier than the recommended time span between doses), (4) use duration (taken longer than the maximum number of days), and (5) inappropriate indication (used to treat symptoms that the product is not intended to treat). For drug-label misuse, the participants’ reported use of each OTC product was evaluated. If a participant indicated a dose and frequency range, the higher dose and higher frequency were assumed for evaluation. The clinical pharmacists reported a decision for each drug-label misuse category, and final decisions were determined by agreement between at least two pharmacists. If a participant’s reported use met multiple categories, each category was considered a separate drug-label misuse instance.
Data Analysis
For each type of misuse, prevalence was analyzed. Prevalence was first calculated at the participant level, using individual participants as the unit of measurement. Next, misuse prevalence was calculated with OTC selections as the unit of measurement. Because participants could select more than one OTC to treat their hypothetical symptoms, the sample size for OTC selections was larger than the participant sample. Not all OTC selections could be evaluated for each misuse category due to insufficient product information or missing phone interviews. Additionally, if a participant discussed using a prescription medication or was unable to provide the exact medication name, it was excluded from analysis. The total number of misuse instances was also calculated. This included an OTC selection that demonstrated drug-age misuse, a drug–drug interaction, a drug–disease interaction, or an instance of drug-label misuse in any category. Misuse prevalence at the participant and selection levels and total instances were also calculated for participants who stated they would change their medication use or use/add a different OTC when symptoms persisted or worsened. For the purposes of this manuscript, OTC selections used to treat symptoms at onset are referred to as “typical OTC selections.” OTC selections that were changed or added to treat persistent and worsening symptoms are referred to as “persistent/worsening OTC selections.”
Results
In total, 144 older adults participated in the in-person interview, with all but three completing the follow-up phone interview. On average, participants who completed the phone interview were 72 years old (range of 65–91 years old), used 11 home medications, and had 5 health conditions. The prevalence of each health condition is further outlined in Table 1. Overall, 136 (96%) participants identified as White, 5 (4%) as Black or African American, 5 (4%) as American Indian or Alaska Native, and 1 (1%) chose not to identify. None of the 141 participants reported Hispanic or Latino origin or descent. A total of 83 (59%) participants identified as female, and 57 (41%) identified as male. Lastly, 132 (94%) received a score >9 on the BHLS.
Table 1.
Prevalence of Participant (n = 144) Health Conditions From Adapted Older Americans Resources and Services List
| Health conditions | n (%) |
|---|---|
| Arthritis or rheumatism | 89 (62%) |
| Allergies or sinus problems | 83 (58%) |
| High blood pressure | 80 (56%) |
| Other joint or muscle problems | 47 (33%) |
| Heart trouble or disease | 46 (32%) |
| Unsteadiness/balance difficulties | 43 (30%) |
| Diabetes | 35 (24%) |
| Thyroid or other gland disorders | 35 (24%) |
| Other urinary tract disorders (including prostate trouble) | 33 (23%) |
| Asthma or wheezing | 32 (22%) |
| History of insomnia (described as lasting one month or longer) | 31 (22%) |
| Bronchitis/emphysema/chronic obstructive pulmonary disease | 28 (19%) |
| Glaucoma or cataracts | 28 (19%) |
| History of falls (described as two or more falls in one year) | 27 (19%) |
| Circulation trouble in arms or legs | 25 (17%) |
| Kidney disease | 18 (13%) |
| Cancer or leukemia | 12 (8%) |
| Effects of stroke | 9 (6%) |
| Stomach or duodenal ulcers | 9 (6%) |
| Liver problems or disease | 4 (3%) |
| Alzheimer’s disease or dementia | 1 (1%) |
| Parkinson’s disease | 1 (1%) |
| Seizures or epilepsy | 1 (1%) |
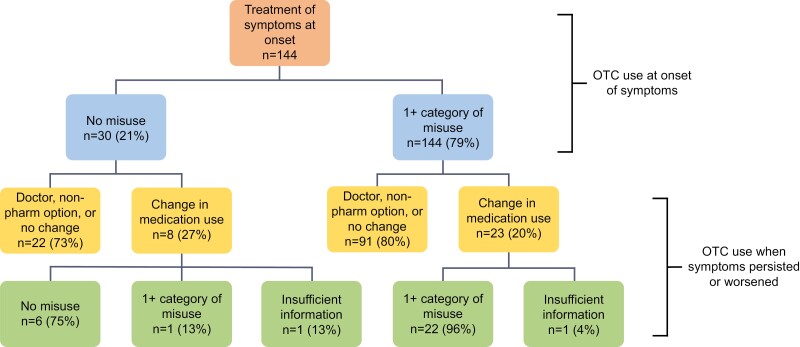
Of the 144 participants, 114 (79%) demonstrated misuse in at least one category when describing how they would use their OTC selections at symptom onset. Overall, 365 misuse instances were identified among these 114 participants ( = 3.20 instances per participants). When the 144 participants described how they would use their selections when symptoms persisted or worsened, 113 (78%) indicated that they would discontinue their OTC selection and call the doctor, use a non-pharmacological treatment option, or continue using their selection in the same way. However, 31 (22%) indicated that they would use their initial selection differently (increase dose, frequency, or duration) or add a new product. Of these 31 participants, 23 indicated a change that would contribute to further misuse. In these 23 individuals, 110 misuse instances were identified ( = 4.78 instances per participant). These results are further outlined in Figure 1.
Figure 1.
Participant self-described over-the-counter (OTC) use outcomes.
Participant Level
As shown in Table 2, 114 participants (79%) demonstrated at least one misuse type when describing how they would treat symptoms at onset, with most exhibiting drug-drug and drug-label misuse. Additionally, 55 (38%) of these participants demonstrated misuse in more than one category, and 6 (4%) in all four categories. When discussing how they would treat persistent or worsening symptoms, 31 (22%) participants said that they would change their OTC use. This included changing the dose, frequency, or duration of use of their initial OTC selections, or changing to or adding a new OTC product. Of these 31 participants, 23 (74%) demonstrated misuse in at least one category, with 15 (48%) having misuse in more than one category, and 3 (10%) exhibiting misuse in all four. Again, most of these participants demonstrated drug-drug and drug-label misuse.
Table 2.
Prevalence of Misuse by Category for Participants With Typical and Persistent/Worsening OTC Selections
| Misuse categories | n (%) |
|---|---|
| Participants with typical OTC selections (n = 144) | |
| No misuse | 30 (21%) |
| 1 misuse category | 59 (41%) |
| Drug | 26 (18%) |
| Age | 5 (3%) |
| Label | 28 (19%) |
| Disease | 0 (0%) |
| 2 misuse categories | 35 (24%) |
| Drug and age | 8 |
| Drug and label | 16 |
| Drug and disease | 5 |
| Age and label | 3 |
| Age and disease | 2 |
| Disease and label | 1 |
| 3 misuse categories | 14 (10%) |
| Drug, age, and label | 5 |
| Drug, age, and disease | 4 |
| Drug, label, and disease | 5 |
| Age, label, and disease | 0 |
| 4 misuse categories | 6 (4%) |
| Insufficient information | 0 (0%) |
| Participants with persistent/worsening OTC selections (n = 31) | |
| No misuse | 6 (19%) |
| 1 misuse category | 8 (26%) |
| Drug | 3 (10%) |
| Age | 0 (0%) |
| Label | 5 (16%) |
| Disease | 0 (0%) |
| 2 misuse categories | 6 (19%) |
| Drug and age | 1 |
| Drug and label | 4 |
| Drug and disease | 0 |
| Age and label | 1 |
| Age and disease | 0 |
| Disease and label | 0 |
| 3 misuse categories | 6 (19%) |
| Drug, age, and label | 3 |
| Drug, age, and disease | 0 |
| Drug, label, and disease | 3 |
| Age, label, and disease | 0 |
| 4 misuse categories | 3 (10%) |
| Insufficient information | 2 (6%) |
Note: OTC = over-the-counter.
In participants with typical OTC selections demonstrating drug-drug misuse, an average of 2.5 drug–drug interactions was seen. This average increased to 2.6 drug–drug interactions in participants with persistent/worsening selections demonstrating drug-drug misuse. In those with typical selections demonstrating drug-disease misuse, selections interacted with an average of 1.3 health conditions per participant. This average decreased to 1.0 for those with persistent/worsening selections exhibiting drug-disease misuse.
Selection Level
The following results focus on each individual OTC product as the unit of analysis. Due to the limitations described in the “Data Analysis” section above, some OTC products could not be evaluated for every misuse category. As a result, each category had the following sample size:
Drug-age: 197 typical, 34 persistent/worsening OTCs
Drug-drug: 191 typical, 30 persistent/worsening OTCs
Drug-disease: 192 typical, 32 persistent/worsening OTCs
Drug-label: 194 typical, 34 persistent/worsening OTCs
Table 3 shows misuse prevalence (categorized by misuse type and symptom scenario) for typical OTC selections. Table 3 also shows misuse for persistent/worsening OTC selections. Across all typical and persistent/worsening misuse categories and symptom scenarios, the following OTCs contributed to the most misuse: ibuprofen, acetaminophen, diphenhydramine, naproxen, doxylamine, and combination products containing at least one of these five active ingredients.
Table 3.
Misuse for Typical and Persistent/Worsening OTC Selections by Symptom Scenario
| Misuse categories | Typical OTC selections n (%) |
Persistent or worsening OTC selections n (%) |
|---|---|---|
| Drug-age evaluation (typical n = 197; persistent/worsening n = 34) | 17 (24%) | |
| Cough/cold/allergy | ||
| Drug-age misuse | 8 (8%) | 4 (57%) |
| Pain | ||
| Drug-age misuse | 10 (37%) | 4 (20%) |
| Sleep | ||
| Drug-age misuse | 0 (0%) | |
| Drug-drug evaluation (typical n = 191; persistent/worsening n = 30) | 28 (41%) | |
| Cough/cold/allergy | ||
| Drug-drug misuse | 43 (44%) | 4 (80%) |
| Pain | ||
| Drug-drug misuse | 11 (42%) | 12 (60%) |
| Sleep | ||
| Drug-drug misuse | 1 (20%) | |
| Drug-disease evaluation (typical n = 192; persistent/worsening n = 32) | 6 (9%) | |
| Cough/cold/allergy | ||
| Drug-disease misuse | 13 (13%) | 2 (40%) |
| Pain | ||
| Drug-disease misuse | 5 (19%) | 4 (20%) |
| Sleep | ||
| Drug-disease misuse | 0 (0%) | |
| Drug-label evaluation (typical n = 194; persistent/worsening n = 34) | 20 (29%) | |
| Cough/cold/allergy | ||
| Drug-label misuse | 47 (47%) | 4 (57%) |
| Pain | ||
| Drug-label misuse | 10 (40%) | 14 (70%) |
| Sleep | ||
| Drug-label misuse | 3 (43%) |
Note: OTC = over-the-counter.
Overall, typical selections demonstrated the highest drug-drug misuse prevalence, as 82 (43%) had a drug–drug interaction. These 82 selections created 189 total drug–drug interactions, which were classified into the following risk ratings: 141 (75%) C (moderate severity), 4 (2%) C (major severity), 28 (15%) D, and 16 (8%) X. Additionally, these drug–drug interactions were most common with home medications from the following drug classes: cardiovascular (44%), supplements (12%), neurological (12%), and psychiatric (12%). Drug-label misuse showed the next highest prevalence, with 77 (40%) typical OTC products demonstrating misuse. Table 4 shows drug-label misuse for each category. When broken down by symptom scenario, the highest prevalence was seen with drug-label misuse of pain products. For drug-disease misuse, misuse was most commonly due to contraindications with hypertension, unsteadiness/balance difficulties, kidney disease, and history of falls (two or more falls in one year).
Table 4.
Prevalence of Each Drug-Label Misuse Category for Typical and Persistent/Worsening OTC Selections
| Drug-label misuse categories | n (%) |
|---|---|
| Typical OTC selections with drug-label misuse (n = 77) | |
| Over daily dosage | 2 (3%) |
| Inappropriate timing/frequency | 9 (12%) |
| Exceeds recommended single dose | 5 (6%) |
| Inappropriate duration of use | 33 (43%) |
| Inappropriate indication | 0 (0%) |
| Multiple drug-label categories | 28 (36%) |
| Persistent/worsening OTC selections with drug-label misuse (n = 21) | |
| Over daily dosage | 1 (5%) |
| Inappropriate timing/frequency | 0 (0%) |
| Exceeds recommended single dose | 2 (10%) |
| Inappropriate duration of use | 5 (24%) |
| Inappropriate indication | 0 (0%) |
| Multiple drug-label categories | 13 (62%) |
Note: OTC = over-the-counter.
As seen with the typical selections, drug-label and drug-drug misuse showed the highest prevalence for persistent/worsening selections, with 21 (62%) and 17 (57%) demonstrating misuse, respectively. Table 4 also shows the prevalence for each drug-label misuse category for the persistent/worsening selections. For drug-drug misuse, 45 total drug–drug interactions were identified and classified into the following risk ratings: 33 (73%) C (moderate severity), 1 (2%) C (major severity), 8 (18%) D, and 3 (7%) X. Persistent/worsening OTC selections most commonly interacted with home medications from the cardiovascular drug class (53%). When classified by symptom scenario, the highest overall prevalence was for drug-drug misuse of cough/cold/allergy products. Finally, for participants making a persistent/worsening selection, drug-disease misuse was most commonly seen in those with hypertension.
Discussion and Implications
To date, this is the first and largest study to evaluate older adult OTC selection and use in a naturalistic environment where older adults typically select these medications. This is in comparison to the numerous lab-based simulations and self-reported surveys that have been done. Results highlight the high prevalence and complexity of OTC misuse in older adults, as nearly 4 out of 5 participants demonstrated at least one type of misuse when treating symptoms at onset, with half exhibiting misuse in multiple categories. This is consistent with literature indicating that OTC misuse is widespread among older adults (Chui et al., 2017). Misuse potential was exacerbated when participants made a change in OTC use to treat worsening symptoms. Misuse was also present, to varying extents, across all three symptom scenarios. Overall, this issue can contribute to serious health consequences and hospitalizations (Donneyong et al., 2023; Karlowicz-Bodalska et al., 2023).
Most drug–drug interactions were classified as a “C” risk rating with “moderate” or “major” severity. This means that the benefits of concomitant medication use typically outweigh the risks, assuming that both physicians and pharmacists are monitoring and documenting that potential risk. Providers should consider a monitoring plan to prevent negative patient outcomes and adjust medication doses accordingly—even for OTCs, which are typically not considered in clinical decision-making.
The drug-label misuse analysis demonstrates that older adults are often using OTCs for an inappropriate amount of time. Long-term OTC use further stresses the importance of monitoring and potential intervention. Unfortunately, pharmacists and other healthcare providers are often not aware of the OTCs being used by their patients, especially if they are excluded from electronic health records, making it difficult to monitor therapies (Kebodeaux, 2019; Serper et al., 2013). Overall, this stresses the importance of considering interactions with a “C” risk rating as misuse, especially those with “moderate” or “major” severity that could deteriorate a patient’s conditions or cause permanent harm if not acknowledged.
These findings have several implications. First, next steps should include increasing awareness of risks associated with OTC selections, including educational programming to share these results with older adult advocacy groups or organizations working directly with this patient population. Future research should also examine the clinical impact of OTC misuse, including its contribution to ADEs and subsequent outcomes for older adults, and focus on reducing its prevalence in older adults by developing and implementing interventions that guide OTC-selection decisions without limiting patient autonomy. These interventions could also help providers improve older adult communication and monitor OTC selections.
At the policy level, this study has implications for prescription-to-nonprescription medication switches. Applications for these switches require safety data demonstrating that the drug product is safe to use in the nonprescription setting. It also requires data demonstrating that consumers can understand how to use the drug safely without provider supervision (U.S. Food and Drug Administration, 2022). Our results, especially the high prevalence of drug-label misuse, suggest that these data should ensure that prescription-to-nonprescription switches are safe for all populations, including older adults. This study also has potential implications for OTC labeling. The Food and Drug Administration’s OTC Drug Facts Label regulation aimed to redesign OTC labels in an easy-to-read format that can help patients navigate medication selections (U.S. Food and Drug Administration, 2015). The high prevalence of drug-label misuse demonstrates that OTC labels may still be insufficient for older adults’ safe use. This is especially concerning considering that many patients are not told how to use OTC products, even when providers tell them what OTCs to purchase. Additionally, in the United States, select medications, including pseudoephedrine, are placed behind the counter as a public safety measure (U.S. Food and Drug Administration, 2017). Future work could explore the expansion of this policy to other high-risk products. Lastly, other countries’ OTC policies, especially those requiring certain OTCs to be stored behind the counter, could be examined to evaluate how these decisions affect OTC product selection and use, which could inform U.S. policy (López et al., 2023).
Limitations
OTC misuse prevalence may be underreported for several reasons. First, if participants selected more than one OTC product to treat their hypothetical symptoms, potential interactions between these selections were considered out of scope and not included in the analysis. It is possible that concomitant use of multiple OTCs may have resulted in additional misuse. Second, participants self-reported medications and health conditions. It is possible that these lists were incomplete and additional misuse was not identified. Third, our study was conducted in community pharmacies, but OTCs can be purchased at non-pharmacy locations, including convenience stores and gas stations. These locations do not provide direct access to healthcare providers in real time, requiring older adults to make decisions independently and potentially leading to greater misuse. Fourth, we evaluated OTC selections to treat hypothetical symptoms, rather than evaluating OTCs selected by older adults to treat existing symptoms in real time. However, we assumed the higher dose and higher frequency for participants who provided a range for their OTC selections, so some misuse may be overreported. Lastly, our patient population was homogenous and had adequate health literacy.
Conclusions
Overall, results indicate that nearly 80% of older adults using OTC products demonstrated probable misuse. There are currently over 55.8 million older adults in the United States (United States Census Bureau, 2023), and research shows that around 50% use an OTC daily to weekly (Qato et al., 2008). If we extrapolate our findings to the U.S. population, over 22.3 million older adults are potentially misusing these medications, representing a population health problem that has gone undetected. Beyond that, the number of Americans aged 65 and older will continue to grow at an increasing rate (U.S. Census Bureau, 2019). Over the last decade, millions of dollars have been allocated for research to address older adult prescription medication safety, but little has been done to address OTC use. This first study focusing on this gap has highlighted that older adults’ ability to safely self-treat with OTC products is overestimated. Shedding light on this problem is crucial, as we cannot afford to wait another decade to understand and improve the safe use of OTCs among older adults.
Supplementary Material
Acknowledgments
The authors thank the individuals who assisted their team with data collection, especially Laurel Legenza, Martha Maurer, and Ashley Morris. The authors also thank Hannah Moses and Amanda Lopez for their assistance with data entry, verification, and evaluation. Lastly, the authors would like to acknowledge and thank Olayinka Shiyanbola, Nora Jacobson, Richard Holden, Kenneth Walker, and Joel Gollhardt for their contributions in conceptualizing the manuscript.
Contributor Information
Jason S Chladek, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA.
Aaron M Gilson, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; Sonderegger Research Center for Improved Medication Outcomes, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Jamie A Stone, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; Sonderegger Research Center for Improved Medication Outcomes, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Maria E Berbakov, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; Sonderegger Research Center for Improved Medication Outcomes, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Taylor L Watterson, Department of Pharmacy Systems, Outcomes, and Policy, University of Illinois Chicago College of Pharmacy, Chicago, Illinois, USA.
Elin C Lehnbom, Sonderegger Research Center for Improved Medication Outcomes, University of Wisconsin-Madison, Madison, Wisconsin, USA; Department of Pharmacy, Uppsala University, Uppsala, Sweden.
Emily L Hoffins, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA.
Katherine A Hemesath, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA.
Jukrin Moon, Department of Industrial and Systems Engineering, University of Iowa, Iowa City, Iowa 52242, USA.
Lauren L Welch, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Denise L Walbrandt Pigarelli, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Edward C Portillo, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Stephanie M Resendiz, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA.
Shiying Mai, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA.
Michelle A Chui, Division of Social and Administrative Sciences, University of Wisconsin-Madison School of Pharmacy, Madison, Wisconsin, USA; Sonderegger Research Center for Improved Medication Outcomes, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Funding
This work was supported by the Agency for Healthcare Research and Quality (R18HS027737) and the Clinical and Translational Science Award program through the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002373, TL1TR002375, and UL1TR002373).
Conflict of Interest
None.
Data Availability
This study is not preregistered, and the data are not available because the authors have not completed their original work with the data set.
References
- Albert, S. M., Bix, L., Bridgeman, M. M., Carstensen, L. L., Dyer-Chamberlain, M., Neafsey, P. J., & Wolf, M. S. (2014). Promoting safe and effective use of OTC medications: CHPA-GSA National Summit. Gerontologist, 54(6), 909–918. 10.1093/geront/gnu034 [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society Beers Criteria Update Expert Panel. (2019). American Geriatrics Society 2019 updated AGS beers criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 67(4), 674–694. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- Callahan, C. M., Unverzagt, F. W., Hui, S. L., Perkins, A. J., & Hendrie, H. C. (2002). Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care, 40(9), 771–781. 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2024, April 17). FastStats: Medication Safety Data. Medication Safety Program. https://www.cdc.gov/medicationsafety/adult_adversedrugevents.html [Google Scholar]
- Chew, L. D., Bradley, K. A., & Boyko, E. J. (2004). Brief questions to identify patients with inadequate health literacy. Family Medicine, 36(8), 588–594. [PubMed] [Google Scholar]
- Chui, M. A., Stone, J. A., & Holden, R. J. (2017). Improving over-the-counter medication safety for older adults: A study protocol for a demonstration and dissemination study. Research in Social & Administrative Pharmacy, 13(5), 930–937. 10.1016/j.sapharm.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donneyong, M. M., Oyarzún-González, X., & Conversation, T. (2023). There’s a small but very real chance that over-the-counter medication could send you to the emergency room, says team that studies medications. Fortune Well. https://fortune.com/well/2023/04/21/are-over-the-counter-medications-safe-pharmacy-hospitalization/ [Google Scholar]
- Duke Aging Center. (2005). Older Americans resources and services. Duke University School of Medicine. https://agingcenter.duke.edu/oars [Google Scholar]
- Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O’Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., & Duda, S. N.; REDCap Consortium. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlowicz-Bodalska, K., Sauer, N., Jonderko, L., & Wiela-Hojeńska, A. (2023). Over the counter pain medications used by adults: A need for pharmacist intervention. International Journal of Environmental Research and Public Health, 20(5), 4505. 10.3390/ijerph20054505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, D. W., Kelly, J. P., Rosenberg, L., Anderson, T. E., & Mitchell, A. A. (2002). Recent patterns of medication use in the ambulatory adult population of the United States: The Slone survey. Journal of the American Medical Association, 287(3), 337–344. 10.1001/jama.287.3.337 [DOI] [PubMed] [Google Scholar]
- Kebodeaux, C. D. (2019). Prescription and over-the-counter medication record integration: A holistic patient-centered approach. Journal of the American Pharmacists Association, 59(2S), S13–S17. 10.1016/j.japh.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Lexicomp. (2023a). Drug interactions module. Wolters Kluwer. https://www.wolterskluwer.com/en/solutions/lexicomp/resources/facts-comparisons-user-academy/drug-interactions [Google Scholar]
- Lexicomp. (2023b). Wolters Kluwer. http://online.lexi.com [Google Scholar]
- López Vila, E. D., Buts, C., & Jegers, M. (2023). A quantitative classification of OTC medicines regulations in 30 European countries: Dispensing restrictions, distribution, pharmacy ownership, and pricing systems. Journal of Pharmaceutical Policy and Practice, 16(1), 19. 10.1186/s40545-023-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Council on Patient Information and Education. (2017).Medication management for older adults. https://www.bemedwise.org/medication-management-for-older-adults [Google Scholar]
- Qato, D. M., Alexander, G. C., Conti, R. M., Johnson, M., Schumm, P., & Lindau, S. T. (2008). Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. Journal of the American Medical Association, 300(24), 2867–2878. 10.1001/jama.2008.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serper, M., McCarthy, D. M., Patzer, R. E., King, J. P., Bailey, S. C., Smith, S. G., Parker, R. M., Davis, T. C., Ladner, D. P., & Wolf, M. S. (2013). What patients think doctors know: Beliefs about provider knowledge as barriers to safe medication use. Patient Education and Counseling, 93(2), 306–311. 10.1016/j.pec.2013.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Health Foundation. (2005, April 1). Exceeding the recommended dosage can do more than wipe out your pain. United States Food and Drug Administration. https://web.archive.org/web/20150511211923/http://www.fda.gov/downloads/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/UCM290318.pdf [Google Scholar]
- United States Census Bureau. (2019, December 10). By 2030, all baby boomers will be age 65 or older. https://www.census.gov/library/stories/2019/12/by-2030-all-baby-boomers-will-be-age-65-or-older.html [Google Scholar]
- United States Census Bureau. (2023, June 2). Older population and aging. https://www.census.gov/topics/population/older-aging.html [Google Scholar]
- United States Food and Drug Administration. (2022, June 28). Drug application process for nonprescription drugs. https://www.fda.gov/drugs/types-applications/drug-application-process-nonprescription-drugs [Google Scholar]
- United States Food and Drug Administration. (2017, November 24). Legal requirements for the sale and purchase of drug products containing pseudoephedrine, ephedrine, and phenylpropanolamine. https://www.fda.gov/drugs/information-drug-class/legal-requirements-sale-and-purchase-drug-products-containing-pseudoephedrine-ephedrine-and [Google Scholar]
- United States Food and Drug Administration. (2015, June 5). OTC drug facts label. https://www.fda.gov/drugs/information-consumers-and-patients-drugs/otc-drug-facts-label [Google Scholar]
- United States Food and Drug Administration. (2021, June 28). Understanding over-the-counter medicines. https://www.fda.gov/drugs/buying-using-medicine-safely/understanding-over-counter-medicines [Google Scholar]
- United States Food and Drug Administration. (2022, June 28). Prescription-to-nonprescription (Rx-to-OTC) switches. https://www.fda.gov/drugs/drug-application-process-nonprescription-drugs/prescription-nonprescription-rx-otc-switches [Google Scholar]
- United States Food and Drug Administration. (2023, July 17). Prescription to over-the-counter (OTC) switch list. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/prescription-over-counter-otc-switch-list [Google Scholar]
- Woo, S. D., Yoon, J., Doo, G. E., Park, Y., Lee, Y., Lee, S. H., Lee, Y. H., & Ye, Y. M. (2020). Common causes and characteristics of adverse drug reactions in older adults: A retrospective study. BMC Pharmacology & Toxicology, 21(1), 87. 10.1186/s40360-020-00464-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is not preregistered, and the data are not available because the authors have not completed their original work with the data set.