Abstract
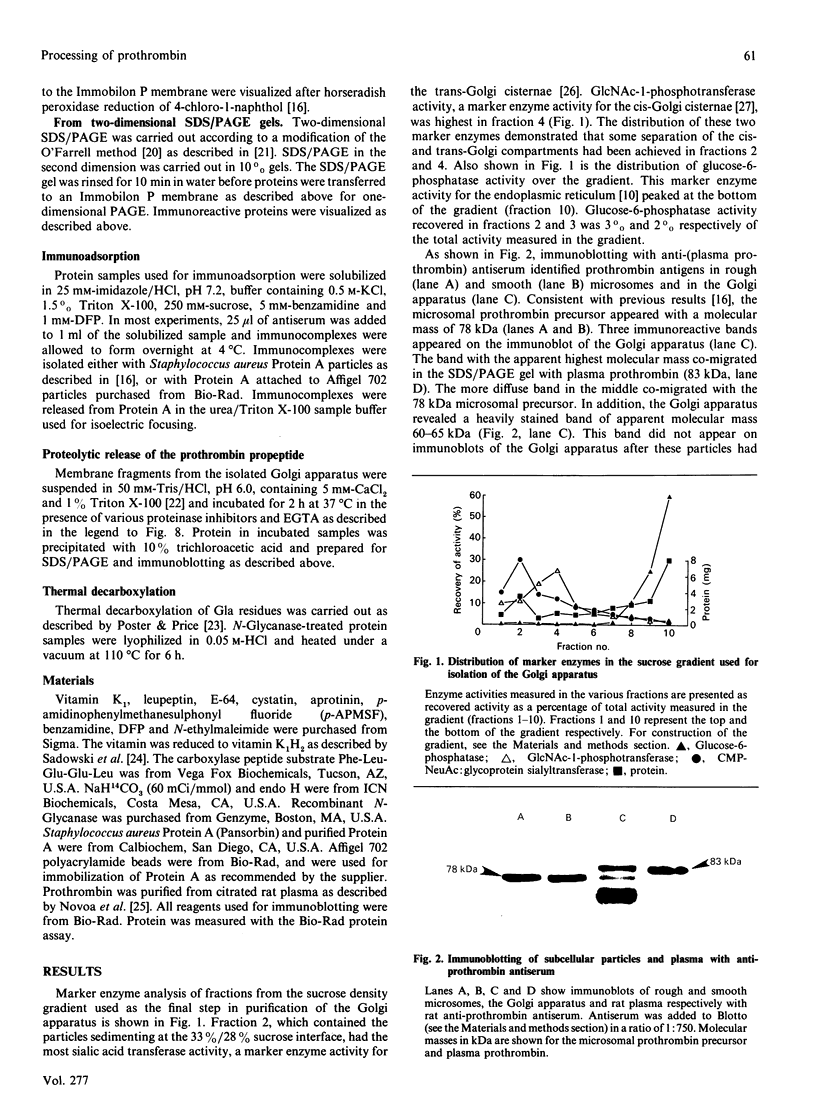
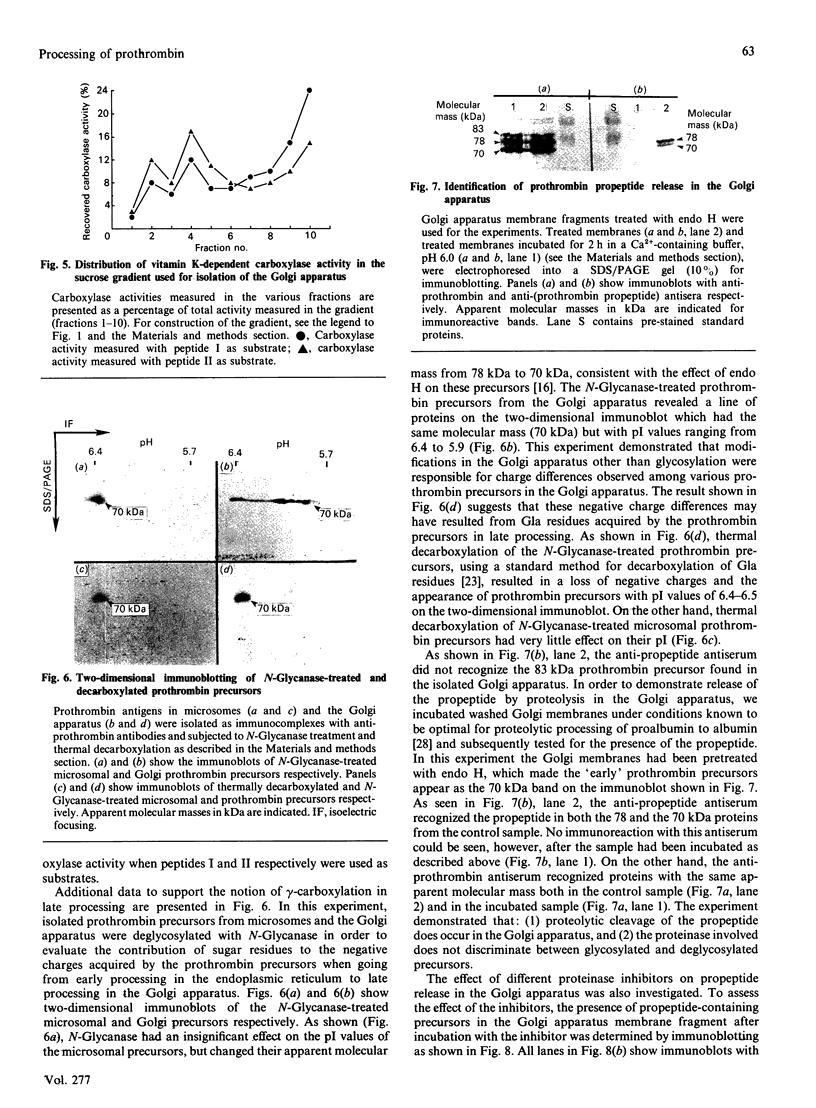
Antibodies raised against plasma prothrombin and the prothrombin propeptide were used to identify prothrombin precursors in rough and smooth microsomes and in the Golgi apparatus. The data demonstrate that the propeptide is part of the prothrombin molecule when undergoing a variety of modifications in the Golgi apparatus. It is shown that these modifications result in an increase in the apparent molecular mass of the prothrombin precursor from 78 kDa in early processing to 83 kDa in late processing. The 83 kDa prothrombin precursor was not recognized by the anti-propeptide antiserum and most likely represents the final product of the precursor in the secretory pathway. Evidence is presented that the propeptide is released from the parent molecule in the Golgi apparatus by a membrane-bound Ca(2+)-dependent serine proteinase(s) with characteristics similar to those of the proalbumin-to-albumin-converting enzyme. Vitamin K-dependent carboxylase activity was measured in membrane fragments obtained from the Golgi apparatus preparation. Sucrose-density-gradient centrifugation and the use of marker enzymes showed that carboxylase activity was highest in fractions enriched in cis-Golgi cisternae. Two different synthetic peptides were used as substrates for the carboxylase. These peptides were from the N-terminal and the C-terminal part of the gamma-carboxyglutamic acid (Gla) region of prothrombin. It is shown that the N-terminal and the C-terminal peptides were preferred as substrates for the carboxylase in the microsomal and the Golgi apparatus preparations respectively. It is also shown that the prothrombin precursor acquires negative charges in the Golgi apparatus that do not result from addition of sugars in late processing. These negative charges could be eliminated by thermal decarboxylation, suggesting that Gla residues may also be synthesized in late processing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Preparation of monoclonal antibodies to preselected protein regions. Methods Enzymol. 1986;121:69–95. doi: 10.1016/0076-6879(86)21010-1. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Hesford F. J. Localization of galactosyl- and sialyltransferase by immunofluorescence: evidence for different sites. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4736–4739. doi: 10.1073/pnas.82.14.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Peach R. J. Calcium-dependent KEX2-like protease found in hepatic secretory vesicles converts proalbumin to albumin. FEBS Lett. 1988 Feb 29;229(1):167–170. doi: 10.1016/0014-5793(88)80819-6. [DOI] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze E. M., Morré D. J. Isolation of plasma membrane, golgi apparatus, and endoplasmic reticulum fractions from single homogenates of mouse liver. J Cell Physiol. 1984 Apr;119(1):46–57. doi: 10.1002/jcp.1041190109. [DOI] [PubMed] [Google Scholar]
- Dallner G. Isolation of rough and smooth microsomes--general. Methods Enzymol. 1974;31:191–201. [PubMed] [Google Scholar]
- Degen S. J., Davie E. W. Nucleotide sequence of the gene for human prothrombin. Biochemistry. 1987 Sep 22;26(19):6165–6177. doi: 10.1021/bi00393a033. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Creek K. E., Merion M., Hirschberg C. B. Subfractionation of rat liver Golgi apparatus: separation of enzyme activities involved in the biosynthesis of the phosphomannosyl recognition marker in lysosomal enzymes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3938–3942. doi: 10.1073/pnas.80.13.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Liebman H. A., Furie B. Molecular basis of hemophilia B: a defective enzyme due to an unprocessed propeptide is caused by a point mutation in the factor IX precursor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5803–5807. doi: 10.1073/pnas.83.16.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Foster D. C., Sprecher C. A., Holly R. D., Gambee J. E., Walker K. M., Kumar A. A. Endoproteolytic processing of the dibasic cleavage site in the human protein C precursor in transfected mammalian cells: effects of sequence alterations on efficiency of cleavage. Biochemistry. 1990 Jan 16;29(2):347–354. doi: 10.1021/bi00454a007. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990 May 1;75(9):1753–1762. [PubMed] [Google Scholar]
- Howell K. E., Ito A., Palade G. E. Endoplasmic reticulum marker enzymes in Golgi fractions--what does this mean? J Cell Biol. 1978 Nov;79(2 Pt 1):581–589. doi: 10.1083/jcb.79.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard B. R., Ulrich M. M., Jacobs M., Vermeer C., Walsh C., Furie B., Furie B. C. Vitamin K-dependent carboxylase: affinity purification from bovine liver by using a synthetic propeptide containing the gamma-carboxylation recognition site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6893–6897. doi: 10.1073/pnas.86.18.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J. E., Suttie J. W. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987 Nov 15;262(32):15334–15337. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Hirani S., Rasmussen J. A vesicular intermediate in the transport of hepatoma secretory proteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1987 Feb;104(2):221–230. doi: 10.1083/jcb.104.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P. Peptide precursor processing enzymes within secretory vesicles. Ann N Y Acad Sci. 1987;493:292–307. doi: 10.1111/j.1749-6632.1987.tb27214.x. [DOI] [PubMed] [Google Scholar]
- Novoa E., Seegers W. H., Hassouna H. I. Improved procedures for the purification of selected vitamin K-dependent proteins. Prep Biochem. 1976;6(5):307–338. doi: 10.1080/00327487608061622. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oda K., Ikehara Y. Putative convertase involved in the proteolytic conversion of rat proalbumin to serum albumin. J Biochem. 1988 Aug;104(2):159–161. doi: 10.1093/oxfordjournals.jbchem.a122432. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Reitman M. L., Kornfeld S. Lysosomal enzyme targeting. N-Acetylglucosaminylphosphotransferase selectively phosphorylates native lysosomal enzymes. J Biol Chem. 1981 Dec 10;256(23):11977–11980. [PubMed] [Google Scholar]
- Sadowski J. A., Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J Biol Chem. 1976 May 10;251(9):2770–2776. [PubMed] [Google Scholar]
- Suttie J. W., Jackson C. M. Prothrombin structure, activation, and biosynthesis. Physiol Rev. 1977 Jan;57(1):1–70. doi: 10.1152/physrev.1977.57.1.1. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Trinchera M., Ghidoni R. Two glycosphingolipid sialyltransferases are localized in different sub-Golgi compartments in rat liver. J Biol Chem. 1989 Sep 25;264(27):15766–15769. [PubMed] [Google Scholar]
- Ulrich M. M., Furie B., Jacobs M. R., Vermeer C., Furie B. C. Vitamin K-dependent carboxylation. A synthetic peptide based upon the gamma-carboxylation recognition site sequence of the prothrombin propeptide is an active substrate for the carboxylase in vitro. J Biol Chem. 1988 Jul 15;263(20):9697–9702. [PubMed] [Google Scholar]
- Wallin R., Culp E. N., Coleman D. B., Goodman S. R. A structural model of human erythrocyte band 2.1: alignment of chemical and functional domains. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4095–4099. doi: 10.1073/pnas.81.13.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Early processing of prothrombin and factor X by the vitamin K-dependent carboxylase. J Biol Chem. 1988 Jul 15;263(20):9994–10001. [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem J. 1987 Jan 15;241(2):389–396. doi: 10.1042/bj2410389. [DOI] [PMC free article] [PubMed] [Google Scholar]