Abstract
Background Gastroesophageal reflux disease (GERD) is among the most common complications of bariatric surgery. This study aimed to analyse the risk factors affecting the worsening of GERD symptoms after laparoscopic sleeve gastrectomy (LSG), and to establish and validate a related nomogram model. Methods The study recruited 236 participants and randomly divided them into training and validation sets in a ratio of 7:3. LASSO regression technique was used to select the optimal predictive features, and multivariate logistic regression was used to construct the column line graphs. The performance of the nomogram was evaluated and validated by analyzing the area under the receiver operating characteristic (ROC) curve, calibration curve, and decision curve. Results In this study, Lasso-logistic regression was applied to select 5 predictors from the relevant variables, which were body mass index (BMI), diabetes, hiatal hernia, GERD, and triglyceride levels. These 5 predictor variables constructed a model with moderate predictive power, with an area under the ROC curve of 0.779 for the training set and 0.796 for the validation set. Decision curve analysis showed that in external validation, if the risk thresholds were between 4 and 98% and 14–95%, then the nomogram can be applied to the clinic. Conclusions We have developed and validated a nomogram that effectively predicts the risk of worsening gastroesophageal reflux symptoms following LSG.
Keywords: Laparoscopic sleeve gastrectomy, GERD, Nomogram
Subject terms: Medical research, Experimental models of disease, Gastrointestinal diseases, Gastrointestinal system
Introduction
By 2035, it is projected that more than 4 billion people worldwide will meet the criteria for being overweight or obese (body mass index(BMI) ≥ 25 kg m2), representing approximately 51% of the global population1. Gastroesophageal reflux disease (GERD) is a common condition that causes symptoms such as heartburn and regurgitation when acidic stomach contents from the stomach enter the esophagus. GERD can cause inflammation of the esophageal mucosa and increase the risk of esophageal cancer2. Patients with GERD account for more than one-half of the obese population3. In addition, according to a Mendelian randomization study, factors such as obesity, diabetes and smoking are causally associated with the development of GERD4. Bariatric surgery, with its favorable safety profile compared to medication, diet, and exercise, offers substantial and long-lasting weight loss while reducing the morbidity and mortality associated with obesity-related diseases5. Laparoscopic sleeve gastrectomy (LSG) has become one of the most commonly performed bariatric surgeries worldwide, with lower complications and mortality rates compared to other weight loss surgeries6. GERD is one of the postoperative complications of bariatric surgery, while bariatric surgery may also lead to worsening of reflux symptoms in patients with preexisting preoperative GERD. In a study that included 10,451 patients undergoing bariatric surgery, the investigators conducted a follow-up survey of GERD severity preoperatively and one year after bariatric surgery, and new GERD symptoms or worsening of pre-existing symptoms occurred in up to 40% after sleeve gastrectomy (SG), compared with only 6% for Roux-en-Y gastric bypass (RYGB)7. Persistent GERD may lead to severe complications, including esophageal strictures, Barrett’s esophagus, and esophageal adenocarcinoma8. Therefore, this study will develop a nomogram to predict the risk of worsening GERD symptoms after LSG, leading to timely interventions to reduce the incidence of worsening GERD symptoms after surgery and prevent further progression of the disease.
Material and method
Study population
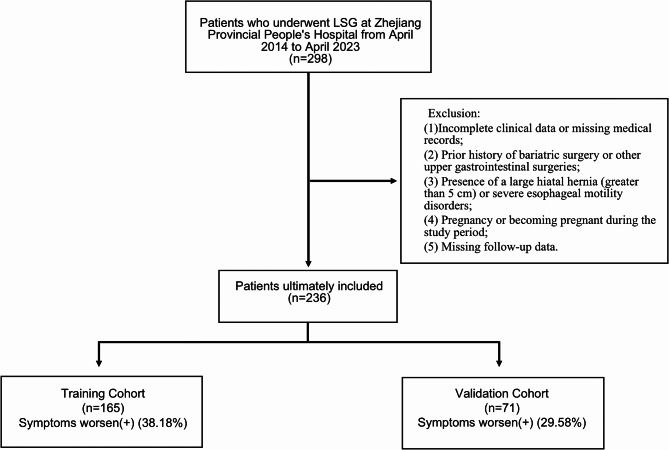
The primary study population in this study was from 236 patients who underwent LSG from April 2014 to April 2023 in Zhejiang Provincial People’s Hospital. All patients undergoing surgery in this study completed the GERD-Health-Related Quality of Life (GERD-HRQL) questionnaire preoperatively and 6 months postoperatively, respectively9. The inclusion criteria were as follows: (1) Patients with a BMI ≥ 30 kg/m2 who underwent laparoscopic sleeve gastrectomy at our center; (2) Adults aged 18–65 years; (3) Signed informed consent to participate in the study. The exclusion criteria were: (1) Incomplete clinical data or missing medical records; (2) Prior history of bariatric surgery or other upper gastrointestinal surgeries; (3) Presence of a large hiatal hernia (greater than 5 cm) or severe esophageal motility disorders; (4) Pregnancy or becoming pregnant during the study period; (5) Missing follow-up data. The diagnostic criteria for diabetes in this study included hemoglobin A1c ≥ 6.5%, fasting plasma glucose ≥ 7.0 mmol/L, or a 2-hour blood glucose level ≥ 11.1 mmol/L. The preoperative diagnosis of GERD is primarily based on medical history, endoscopy, and the GERD-Health Related Quality of Life questionnaire (GERD-HRQL). Figure 1 illustrates the flowchart for this research.
Fig. 1.
Research flowchart After a rigorous screening process, 236 participants were finally included, 165 in the training cohort and 71 in the validation cohort. The percentage of positivity was 38.18% in the training cohort and 29.58% in the validation cohort.
Surgical technique
LSG is a minimally invasive weight-loss surgery commonly used to treat obesity. During the procedure, approximately 75–80% of the stomach is removed, which limits food intake and aids in weight loss. The surgery begins with the patient in a supine position under general anesthesia. After sterilizing and draping the surgical site, four to five small incisions are made in the abdomen to insert the laparoscope and surgical instruments. An ultrasonic scalpel is used to dissect the greater curvature, the fundus, and the posterior wall of the stomach, starting about 3 cm from the pylorus and continuing to the fundus. The resection is then performed with a cutting stapler, removing most of the greater curvature and the fundus, starting 3 cm beyond the pylorus. Once the staple line is confirmed to be free of leaks, barbed sutures are used to continuously close the remaining stomach’s staple line, preventing bleeding and leakage. Compared to traditional open surgery, laparoscopic sleeve gastrectomy offers shorter recovery times and less post-operative pain, making it a preferred option for many patients.
Outcome
The primary outcome was to assess the worsening of gastroesophageal reflux symptoms after LSG. The evaluation was conducted using the GERD-Health Related Quality of Life questionnaire (GERD-HRQL). The secondary outcome was to assess changes in BMI following bariatric surgery.
Data collection
In this study, we collected preoperative data on all patients undergoing bariatric surgery, including personal characteristics (gender, age, BMI; past medical history (diabetes, depression, antidepressant treatment, hypertension, hypertriglyceridemia, sleep apnea syndrome(SAS), GERD, hiatal hernia, smoking history, previous peptic ulcer, history of abdominal surgery); laboratory tests (white blood cell(WBC), lymphocyte(LYM), neutrophilic granulocyte(NEUT), hemoglobin(Hb), platelet(PLT), fasting insulin(FI), fasting c-peptid (FCP), total protein(TP), albumin(ALB), alanine aminotransferase(ALT), aspartate aminotransferase(AST), gamma-glutamyl transpeptidase(GGT), alkaline phosphatase(ALP), fasting plasma glucose(FPG), total serum bilirubin(TSB), direct bilirubin(DBIL), total bile acid(TBA), creatinine(Cr), uric acid(UA), total cholesterol(TC), triglycerides(TG), high density lipoprotein(HDL), low density lipoprotein(LDL), hemoglobin A1(HbA1) ,hemoglobin A1C(HbA1c), prothrombin time(PT)), and the results of the GERD-HRQL questionnaire. We defined an increase in GERD-HRQL questionnaire scores after bariatric surgery compared with preoperative scores as a worsening of GERD symptoms.
Data analysis
All statistical analyses were conducted using RStudio (version 4.2.3). Quantitative variables are presented as means (SD), and categorical variables as counts (%). In this study, Lasso penalized regression was first used for variable selection, applying Lasso regression to the initial 55 variables in the training set. Using Lasso with internal 5-fold cross-validation, the model reduced to 6 variables when it reached 1 standard error (1 SE) from the minimum distance. The variables included BMI, diabetes, hiatal hernia, GERD, hemoglobin A1, and triglycerides. These 6 variables selected by Lasso regression were then further modeled using logistic regression. The study confirmed that, except for HbA1, all variables were independent, with no multicollinearity (P < 0.05, VIF < 2). Since HbA1 was not an independent risk factor for postoperative worsening of GERD symptoms (P > 0.05), it was excluded from the model. Finally, we developed a nomogram for predicting postoperative GERD symptoms based on the logistic model, incorporating 5 preoperative predictors: BMI, diabetes, hiatal hernia, GERD, and triglycerides. This method is called Lasso-logistic regression. The model’s performance was evaluated using the area under the receiver operating characteristic curve (AUC). Decision curve analysis (DCA) was used to assess the model’s net benefit and clinical utility, and the calibration curve was used to evaluate the model’s predictive ability. A statistically significant difference was defined as P < 0.05.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (QT2024149). Written informed consent was obtained from all participants before inclusion.
Results
Baseline characteristics
Data from 236 patients were collected and randomly divided into training (165 patients) and validation (71 patients) cohorts at a ratio of 7:3. The study cohort included 81 males and 155 females, with an average age of 28.98 ± 7.69 years. The preoperative average BMI was 38.93 ± 7.52 kg/m2. Preoperative comorbidities included 9 patients with depression (3.18%), 52 with hypertension (22.03%), 47 with diabetes (19.92%), and 124 with hypertriglyceridemia (52.54%). Additionally, 36 patients had GERD (15.52%), 20 had hiatal hernia (8.47%), 38 were smokers (16.10%), and 11 were alcohol users (4.66%). Six months postoperatively, the average BMI was 30.44 ± 6.17 kg/m2, and the percentage of excess BMI loss (%EBMIL) was 80.22 ± 49.10%. The rate of postoperative worsening of gastroesophageal reflux symptoms was 38.18% in the training cohort and 29.58% in the validation cohort. Detailed information is presented in Table 1.
Table 1.
Differences in characteristics between the training cohort and the validation cohort.
| Variables | Total cohort (n = 236) |
Training cohort (n = 165) | Validation cohort (n = 71) |
P value |
|---|---|---|---|---|
| Educationalbackground (%) | 0.728 | |||
| Unschooled | 1(0.42) | 1(0.61) | 0(0.00) | |
| Primary school degree | 6(2.54) | 3(1.82) | 3(4.23) | |
| Junior high school degree | 25(10.59) | 19(11.52) | 6(8.45) | |
| Senior high school degree | 51(21.61) | 36(21.82) | 15(21.13) | |
| University degree degree | 153(64.83) | 106(64.24) | 47(66.20) | |
| Married (%) | 0.610 | |||
| No | 122(51.69) | 83(50.30) | 39(54.93) | |
| Yes | 114(48.31) | 82(49.70) | 32(45.07) | |
| Age, mean (SD), year | 28.98(7.69) | 28.98(7.56) | 29.00(8.06) | 0.982 |
| Genders (%) | 0.576 | |||
| Female | 155(65.68) | 106(64.24) | 49 (69.01) | |
| Male | 81(34.32) | 59(35.76) | 22(30.99) | |
| Height, mean (SD), cm | 167.42(8.41) | 167.40(8.02) | 167.46(9.32) | 0.962 |
| Weight, mean (SD), cm | 110.27(27.72) | 111.07(27.67) | 108.39(28.01) | 0.498 |
| BMI, mean (SD), kg/m2 | 38.93(7.52) | 39.25(7.64) | 38.21(7.24) | 0.332 |
| BMI classification (%) | 0.887 | |||
| 0(25.0-29.9) | 16(6.78) | 11(6.67) | 5(7.04) | |
| 1(30.0-34.9) | 55(23.31) | 39(23.64) | 16(22.54) | |
| 2(35.0-39.9) | 75(31.78) | 50(30.30) | 25(35.21) | |
| 3(≥ 40) | 90(38.14) | 65(39.39) | 25(35.21) | |
| Depression (%) | 0.557 | |||
| No | 227(96.19) | 160(96.97) | 67(94.37) | |
| Yes | 9(3.81) | 5(3.03) | 4(5.63) | |
| Antidepressant treatment (%) | 0.742 | |||
| No | 229(97.03) | 161(97.58) | 68(95.77) | |
| Yes | 7(2.97) | 4(2.42) | 3(4.23) | |
| Hypertension (%) | 0.961 | |||
| No | 184(77.97) | 128(77.58) | 56(78.87) | |
| Yes | 52(22.03) | 37(22.42) | 15(21.13) | |
| Hepatitis B (%) | 0.371 | |||
| No | 227(96.19) | 157(95.15) | 70(98.59) | |
| Yes | 9(3.81) | 8(4.85) | 1(1.41) | |
| Diabetes (%) | 0.560 | |||
| No | 189(80.08) | 130(78.79) | 59(83.10) | |
| Yes | 47(19.92) | 35(21.21) | 12(16.90) | |
| Hypertriglyceridemia (%) | 0.956 | |||
| No | 112(47.46) | 79(47.88) | 33(46.48) | |
| Yes | 124(52.54) | 86(52.12) | 38(53.52) | |
| Nonalcoholic Fatty Liver Disease (%) | 0.458 | |||
| No | 16(6.78) | 13(7.88) | 3(4.23) | |
| Yes | 220(93.22) | 152(92.12) | 68(95.77) | |
| Obstructive sleep apnea hypopnea syndrome (%) | 0.086 | |||
| No | 196(93.05) | 132(80.00) | 64(90.14) | |
| Yes | 40(16.95) | 33(20.00) | 7(9.86) | |
| Gastroesophageal reflux disease (%) | 0.510 | |||
| No | 200(84.75) | 142(86.06) | 58(81.69) | |
| Yes | 36(15.25) | 142(86.06) | 58(81.69) | |
| Hiatal hernia (%) | 0.806 | |||
| No | 216(91.53) | 152(92.12) | 64(90.14) | |
| Yes | 20(8.47) | 13(7.88) | 7(9.86) | |
| Helicobacter pylori (%) | 0.120 | |||
| No | 192(81.36) | 139(84.24) | 53(74.65) | |
| Yes | 44(18.64) | 26(15.76) | 18(25.35) | |
| Pepticuler (%) | 0.910 | |||
| No | 217(91.95) | 151(91.52) | 66(92.96) | |
| Yes | 19(8.05) | 14(8.48) | 5(7.04) | |
| Gastric ulcer (%) | 0.898 | |||
| No | 225(95.34) | 158(95.76) | 67(94.37) | |
| Yes | 11(4.66) | 7(4.24) | 4(5.63) | |
| Duodenal ulcers (%) | 0.477 | |||
| No | 228(96.61) | 158(95.76) | 70(98.59) | |
| Yes | 8(3.39) | 7(4.24) | 1(1.41) | |
| Gallbladder stones (%) | 1.000 | |||
| No | 217(91.95) | 152(92.12) | 65(91.55) | |
| Yes | 19(8.05) | 13(7.88) | 6(8.45) | |
| Gallbladder polyp (%) | 0.612 | |||
| No | 229 (97.03) | 159 (96.36) | 70(98.59) | |
| Yes | 7(2.97) | 6(3.64) | 1(1.41) | |
| Smoking (%) | 0.198 | |||
| No | 198(83.90) | 138(83.64) | 60(84.51) | |
| Yes | 38(16.10) | 27(16.36) | 11(15.49) | |
| Drinking (%) | 1.000 | |||
| No | 225(95.34) | 157(95.15) | 68(95.77) | |
| Yes | 11(4.66) | 8(4.85) | 3(4.23) | |
| History of abdominalsurgery (%) | 0.255 | |||
| No | 173(73.31) | 125(75.76) | 48(67.61) | |
| Yes | 63(26.69) | 40(24.24) | 23(32.39) | |
| White blood cell, mean (SD), 109/L | 8.38(2.04) | 8.39(2.05) | 8.34(2.05) | 0.865 |
| Lymphocyte, mean (SD), 109/L | 2.74(1.62) | 2.76(1.87) | 2.68(0.79) | 0.703 |
| Neutrophilic granulocyte, mean (SD), 109/L | 5.11(1.66) | 5.12(1.71) | 5.09(1.54) | 0.870 |
| Hemoglobin, mean (SD), g/L | 142.68(16.54) | 142.70(16.29) | 142.63(17.22) | 0.977 |
| Platelet, mean (SD), 109/L | 295.01(209.17) | 299.05(246.70) | 285.63(64.56) | 0.652 |
| Fasting insulin, mean (SD), µ IU/mL | 37.78(30.35) | 36.70(25.11) | 40.27(40.06) | 0.409 |
| Fasting c-peptide, mean (SD), ng/ml | 4.56(2.26) | 4.47(2.19) | 4.76(2.42) | 0.362 |
| Total protein, mean (SD), g/L | 74.37(5.24) | 74.02(5.59) | 75.17(4.26) | 0.125 |
| Albumin, mean (SD), g/L | 43.18(3.43) | 43.00(3.51) | 43.59(3.21) | 0.228 |
| Alanine aminotransferase, mean (SD), U/L | 59.20(49.07) | 57.80(50.08) | 62.45(46.84) | 0.516 |
| Aspartate aminotransferase, mean (SD), U/L | 38.04(28.22) | 37.02(27.37) | 40.42(33.17) | 0.397 |
| Gamma-glutamyl transpeptidase, mean (SD), U/L | 49.85(33.14) | 49.89(33.37) | 49.76(32.82) | 0.978 |
| Alkaline phosphatase, mean (SD), U/L | 82.86(25.89) | 84.07(26.38) | 80.07(24.67) | 0.278 |
| Fasting plasma glucose, mean (SD), mmol/L | 5.81(2.11) | 5.88(2.19) | 5.65(1.93) | 0.451 |
| Total serum bilirubin, mean (SD), µmol/L | 12.65(5.48) | 12.45(5.42) | 13.11(5.62) | 0.393 |
| Direct Bilirubin, mean (SD), µmol/L | 2.57(1.75) | 2.48(1.52) | 2.79(2.18) | 0.209 |
| Total bile acid, mean (SD), µmol/L | 4.75(7.82) | 4.33(3.32) | 5.74(13.35) | 0.206 |
| Creatinine, mean (SD), µmol/L | 71.65(32.11) | 72.67(37.04) | 69.29(15.40) | 0.459 |
| Uric Acid, mean (SD) ,µmol/L | 427.91(96.60) | 426.59(95.35) | 430.96(100.05) | 0.751 |
| Total cholesterol, mean (SD), mmol/L | 7.61(37.30) | 8.65(44.61) | 5.20(1.12) | 0.515 |
| Triglyceride, mean (SD), mmol/L | 2.04(1.57) | 2.13(1.73) | 1.84(1.12) | 0.209 |
| High density lipoprotein, mean (SD), mmol/L | 1.13(0.26) | 1.12(0.26) | 1.15(0.25) | 0.418 |
| Low density lipoprotein, mean (SD), mmol/L | 3.23(0.85) | 3.22(0.82) | 3.25(0.92) | 0.839 |
| Hemoglobin A1, mean (SD),% | 8.31(1.52) | 8.35(1.51) | 8.22(1.54) | 0.551 |
| Hemoglobin A1C, mean (SD),% | 6.02(1.23) | 6.06(1.20) | 5.93(1.29) | 0.465 |
| Prothrombin time, mean (SD), s | 11.11(0.67) | 11.10(0.68) | 11.14(0.64) | 0.724 |
| Symptoms worsen | ||||
| No | 152(64.41) | 102(70.42) | 50(70.42) | 0.264 |
| Yes | 84(35.59) | 63(38.18) | 21(29.58) |
Independent risk factors in the training set
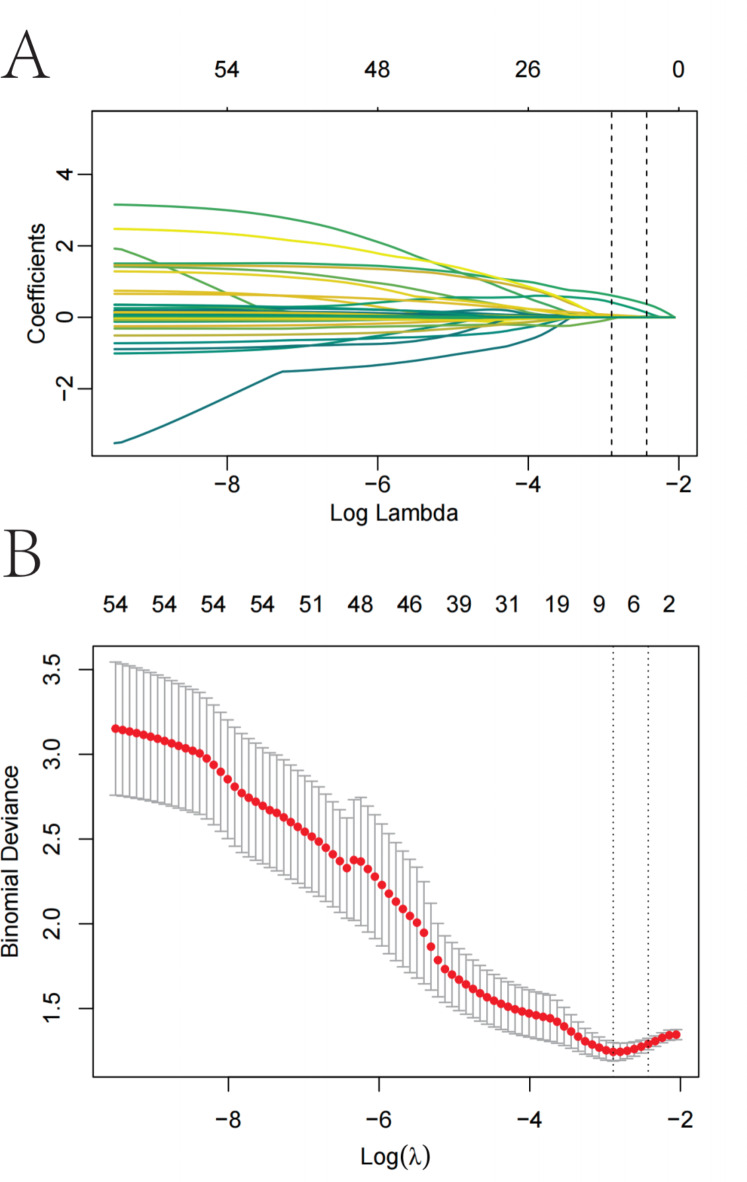
From the 55 relevant feature variables, we selected 6 potential predictors with non-zero coefficients in the LASSO regression model (Fig. 2; Table 2). These predictors include BMI, diabetes, hiatal hernia, GERD, hemoglobin A1c, and triglyceride levels.
Fig. 2.
Variable selection by LASSO binary logistic regression model. A coefficient profile plot was produced against the log(lambda) sequence (Fig. 2A). Six variables with nonzero coefficients were selected by optimal lambda. By verifying the optimal parameter (lambda) in the LASSO model, the partial likelihood deviance (binomial deviance) curve was plotted versus log(lambda) and dotted vertical lines were drawn based on 1 standard error criteria (Fig. 2B).
Table 2.
Variables screened by Lasso penalized regression.
| Best λ value | Variables in Lasso model | Coefficient | OR |
|---|---|---|---|
| 0.088 | BMI | 2.00E-02 | 1.02 |
| Diabetes | 3.72E-01 | 1.45 | |
| Hiatal hernia | 3.81E-01 | 1.46 | |
| Triglyceride | 6.11E-03 | 1.01 | |
| Gastroesophageal reflux disease | 1.33E-01 | 1.14 | |
| Hemoglobin A1 | 1.24E-02 | 1.01 |
Prediction model development
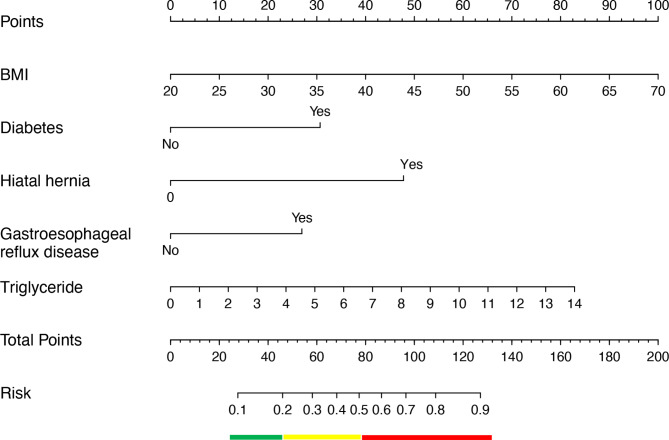
Following logistic regression analysis, the 5 predictors, BMI (OR = 1.09, 95% CI 1.04–1.15), diabetes (OR = 3.88, 95% CI 1.62–9.55), hiatal hernia (OR = 8.25, 95% CI 2.03–42.64), GERD (OR = 3.28, 95% CI 1.15–9.86), and triglyceride (OR = 1.30, 95% CI 1.03–1.72), all showed statistically significant differences (Table 3). Then, we introduced the 5 independent predictors mentioned above to develop a nomogram associated with worsening of GERD symptoms after LSG and presented in Fig. 3.
Table 3.
Independent variables included in the nomogram based on the Lasso-logistic regression in the training cohort.
| Nomogram model | VIF | OR | 95% CI | P value |
|---|---|---|---|---|
| BMI | 1.07 | 1.09 | (1.04–1.15) | < 0.001 |
| Diabetes | 1.06 | 3.88 | (1.62–9.55) | 0.003 |
| Gastroesophageal reflux disease | 1.02 | 3.28 | (1.15–9.86) | 0.028 |
| Hiatal hernia | 1.04 | 8.25 | (2.03–42.64) | 0.005 |
| Triglyceride | 1.06 | 1.30 | (1.03–1.72) | 0.036 |
Fig. 3.
The nomogram for predicting worsening of gastroesophageal reflux symptoms after LSG based on the Lasso-logistic regression.
Prediction model validation
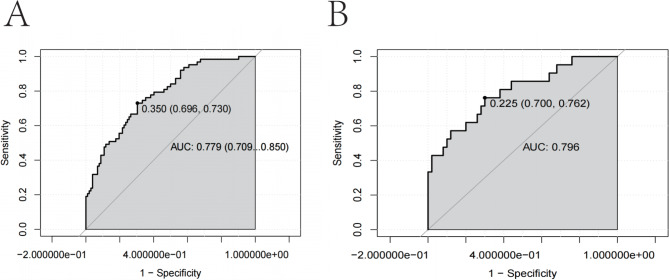
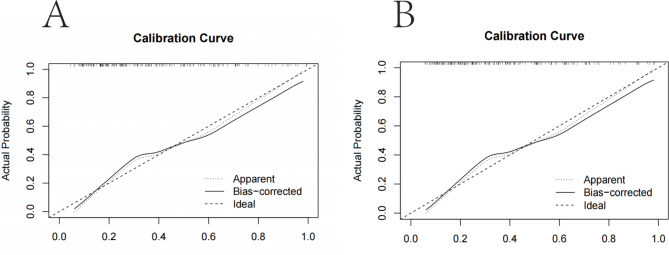
For the prediction model, the area under the ROC curve for the nomogram was 0.779 in the training set and 0.796 in the validation set, indicating moderate performance (Fig. 4A and B). The calibration curves of the nomogram for predicting the risk of worsening postoperative GERD symptoms in patients undergoing LSG also showed good consistency (Fig. 5A and B). In conclusion, the nomogram of the model have good predictive ability.
Fig. 4.
ROC validation of the nomogram prediction of the risk of worsening GERD symptoms after LSG. The y-axis represents the rate of true positives for the risk prediction. The x-axis represents false positives for the risk prediction. The area under the curve represents the performance rate of the nomogram. Figure 4A is from the training set and Fig. 4B is from the validation set.
Fig. 5.
Calibration curves of the risk nomogram prediction of worsening postoperative GERD symptoms after LSG. The y-axis meant the actual postoperative worsening of GERD symptoms. The x-axis meant the predicted risk of worsening postoperative GERD symptoms. The diagonal dotted line meant a perfect prediction by an ideal model. The solid line represented the performance of the training set (Fig. 5A) and validation set (Fig. 5B), which indicated that a closer fit to the diagonal dotted line represented a better prediction.
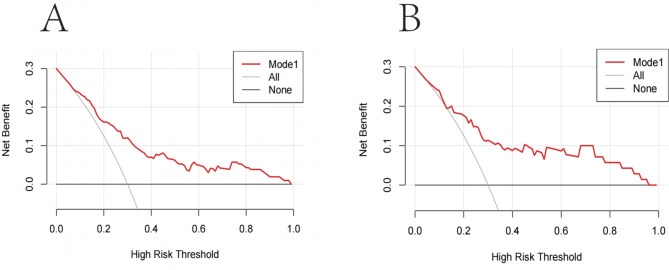
Decision curves showed that the risk of worsening postoperative GERD symptoms was more accurately predicted using this nomogram when the risk threshold probability was between 4 and 98% in the current study, and between 14% and 95% in the validation set (Fig. 6A and B). According to the nomogram, the net benefit was comparable to several overlapping values in this range.
Fig. 6.
Decision curve analysis for the risk nomogram for worsening of GERD symptoms after LSG. The y-axis measured the net benefit. The thick solid line represented the assumption that all patients had no worsening of GERD symptoms after LSG. The thin solid line represented the assumption that all patients had worsening of GERD symptoms after LSG. The red line represents the risk nomogram. (Fig. 6A) From the training set and (Fig. 6B) from the validation set.
Discussion
This study investigated factors contributing to the worsening of GERD symptoms following LSG. The findings identified BMI, diabetes, hiatal hernia, GERD, and triglyceride levels as independent risk factors for the exacerbation of GERD symptoms post-surgery. Using these five risk factors, we developed a nomogram prediction model, which demonstrated strong predictive accuracy, discrimination, and clinical utility.
GERD is one of the postoperative complications of bariatric surgery3. Compared to bypass and other bariatric procedures, LSG is associated with a higher incidence of postoperative GERD. A meta-analysis of 46 studies, including 10,718 patients, found that the incidence of GERD increased by 19% after LSG, with new-onset reflux occurring in 23% of cases. Long-term complications included a 28% incidence of esophagitis and an 8% incidence of Barrett’s esophagus. Furthermore, 4% of patients required conversion to RYGB due to severe reflux10. GERD is strongly linked to the development of esophageal malignancies, as it induces inflammatory esophagitis that can lead to mucosal damage. Persistent GERD can result in Barrett’s esophagus and eventually esophageal cancer11. Previous studies have identified several risk factors for postoperative GERD, though these studies were often limited by small sample sizes and the use of retrospective or inadequate statistical methods12. In this study, we employed Lasso-logistic regression modeling, which allowed for the integration of multiple potential risk factors into a single predictive tool, providing greater prognostic accuracy than traditional single and multifactorial logistic regression analyses13. Five significant risk factors, including BMI, were identified as predictors of postoperative GERD risk. Our study found an overall incidence of GERD symptom worsening of 35.59%, aligning with the results of other studies7. Based on these findings, we developed and validated a new predictive tool using five key variables: previous diabetes, GERD, hiatal hernia, elevated BMI, and high triglyceride levels. These factors are critical in determining the risk of postoperative GERD in patients undergoing LSG.
In this study, BMI was identified as a significant risk factor for the worsening of GERD symptoms following LSG, with a positive correlation between higher BMI and increased GERD risk (OR = 1.09, 95% CI 1.04–1.15). This finding is consistent with results from other studies14–16. The mechanism may involve increased acid exposure in the esophagus, linked to elevated intra-abdominal pressure and delayed gastric emptying associated with abdominal obesity17. Additionally, heightened intra-abdominal pressure raises the risk of gastroesophageal reflux and the development of hiatal hernia18. Reduced lower esophageal sphincter (LES) pressure and more frequent transient LES relaxation may further explain how obesity contributes to gastroesophageal reflux19.This process could be driven by adipose tissue overproducing pro-inflammatory cytokines (e.g., IL-6, TNF-α), leading to LES relaxation and insulin resistance, which exacerbate reflux symptoms20.
Hiatal hernia is a major cause of GERD, with a prevalence of 40-70% in the obese population and 20-53% for hiatal hernia specifically3. Kermansaravi et al. found a significant association between hiatal hernia and GERD (P= 0.012), with moderate and large hiatal hernias being significant risk factors for new-onset GERD21. Hiatal hernia occurs when the esophageal hiatus in the diaphragm enlarges, causing the LES to shift into the thoracic cavity, leading to LES relaxation or dysfunction. This condition predisposes the LES to negative intrathoracic pressure and the formation of a gastric acid pocket. Moreover, hiatal hernia disrupts the ligament, causing LES laxity and reduced reinforcement19. It may also prolong the retention of acid in the stomach, increasing the risk of gastric acid and digestive fluids entering the esophagus22. Preoperative endoscopic evaluation of a hiatal hernia is essential to prevent gastroesophageal reflux following bariatric surgery23. For patients at high risk of postoperative reflux who also have a hiatal hernia, simultaneous hiatal hernia repair during bariatric surgery can help reduce this risk24. In a study of 134 patients who underwent LSG, 66 (49.2%) were diagnosed with GERD preoperatively, and 34 (25.3%) were found to have an esophageal hiatal hernia during surgery. Among the patients who had LSG combined with hiatal hernia repair, only 2 (1.5%) developed GERD symptoms after 6 to 12 months of follow-up25. Further studies are needed to explore the potential benefits of bariatric surgery combined with hiatal hernia repair.
Diabetes is a significant factor in the development of GERD. In this study, diabetes was present in up to one-third of the patients. A Mendelian randomization study identified a causal relationship between diabetes and GERD4. Additionally, a meta-analysis of nine observational studies demonstrated a strong association between the two conditions (OR = 1.61; 95% CI 1.36–1.91; P = 0.003)26. Our findings further support this relationship. Although the exact pathophysiological mechanism remains unclear, autonomic neuropathy, particularly damage to the vagus nerve in diabetic patients, may play a key role in the development of GERD26.
Patients with a preoperative diagnosis of GERD are more likely to experience worsening GERD symptoms postoperatively. In a study of 217 patients undergoing robotic-assisted sleeve gastrectomy, a higher percentage of postoperative GERD patients had preoperative GERD symptoms (40.5% vs. 23.9%; P = 0.04)12. Additionally, a recent study of 164 bariatric surgery patients identified preoperative GERD symptoms as an independent risk factor for postoperative GERD (OR = 2.489, P = 0.013)27. Another study found that all LSG patients with preoperative GERD continued to exhibit GERD symptoms after surgery28. These findings align with the results of our study. This may be due to the loss of the anti-reflux barrier postoperatively, dysfunction at the gastroesophageal junction, increased intragastric pressure, and poor lifestyle habits29.
In this study, elevated triglyceride levels were identified as a significant predictor of worsening GERD symptoms following LSG. A case-control study of 372 patients demonstrated a positive association between triglycerides and reflux esophagitis (OR = 2.07, 95% CI 1.12–3.82)30. This finding was further supported by a cross-sectional case-control study of 7,078 participants, which also identified elevated triglycerides as an independent predictor of reflux esophagitis (OR = 1.20, 95% CI 1.05–1.36)31. These results are consistent with our findings. The underlying mechanism may involve IL-6 production by adipose tissue, as IL-6 has been shown to reduce esophageal circular muscle contraction in animal models of esophagitis32,33.
Compared to other bariatric procedures, LSG offers several advantages. It is relatively simple to perform and does not significantly alter the normal digestive and absorption pathways, reducing the risk of nutritional deficiencies. Additionally, LSG preserves the pylorus and antrum, which lowers the incidence of dumping syndrome and facilitates postoperative endoscopic examinations34. However, GERD has emerged as a serious complication of LSG. Beyond health consequences such as esophagitis, Barrett’s esophagus, and esophageal cancer, GERD can also significantly reduce the patient’s quality of life, with severe symptoms potentially leading to anxiety or depression15. Previous studies have demonstrated that RYGB is a protective factor for GERD (OR = 0.19,95%CI 0.12–0.30, P< 0.001).RYGB is more effective than SG in the treatment of GERD in obese patients and has a lower incidence of new GERD after RYGB35. Additionally, LSG combined with anti-reflux surgery has been shown to prevent the development of GERD36. Therefore, for patients who are eligible for LSG and at high risk of GERD, incorporating a GERD prediction model into the preoperative assessment is recommended. For patients at high risk of worsening GERD symptoms post-surgery, RYGB or combined anti-reflux surgery should be considered.
This study has several limitations. First, the GERD-HRQL questionnaire was used to assess GERD symptoms after bariatric surgery, which is more subjective and less rigorous compared to High-Resolution Manometry and Upper Gastrointestinal Endoscopy. Second, our analysis focused primarily on preoperative factors, without considering intraoperative and postoperative recovery, related complications, or lifestyle habits. Lastly, the study may lack generalizability due to its relatively small sample size and short follow-up period. Future studies should involve larger sample sizes, multicenter prospective studies, or randomized controlled trials (RCTs) incorporating advanced algorithms, such as machine learning, to further validate our findings.
Conclusion
In this study, we analyzed the risk factors that may contribute to the worsening of postoperative GERD symptoms in patients undergoing LSG. Five factors were identified as significant risk contributors: BMI, diabetes, hiatal hernia, preoperative GERD, and elevated triglyceride levels. Based on these findings, we developed and validated a simple and reliable nomogram to predict the risk of GERD symptom exacerbation in LSG patients. The nomogram demonstrated strong predictive accuracy, discriminative power, and clinical utility in both the training and validation sets, indicating its potential effectiveness in practical applications. With this tool, clinicians and patients can implement timely interventions based on the risk assessment.
Abbreviations
- BMI
Body mass index
- SAS
Sleep apnea syndrome
- WBC
White blood cell
- LYM
Lymphocyte
- NEUT
Neutrophilic granulocyte
- Hb
Hemoglobin
- PLT
Platelets
- FI
Fasting insulin
- FCP
Fasting C-peptide
- TP
Total protein
- ALB
Albumin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma-glutamyl transpeptidase
- ALP
Alkaline phosphatase
- FPG
Fasting plasma glucose
- TSB
Total serum bilirubin
- DBIL
Direct bilirubin
- TBA
Total bile acid
- Cr
Creatinine
- UA
Uric acid
- TC
Total cholesterol
- TG
Triglyceride
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
- HbA1c
Hemoglobin A1c
- HbA1
Hemoglobin A1
- PT
Prothrombin time
- LSG
Laparoscopic sleeve gastrectomy
- EBMIL
Excess body mass index loss
Author contributions
L. J, X.-K.H, Z.-Y. G, J.G, Z.Z, F.-Q.X, Y.L, H.-P. Z, C.-F. D, J.-W. L, L. L, X.-D. S, Z.-F. W, Z.-Q. X and Y.-J. W. was involved in the design of the study protocol, data collection, and follow-up; L.J, Z.Z and F.-Q.X was involved in the analysis of the data and the production of the pictures and tables; L.J. completed the manuscript. X.-D. S, Z.-Q. X and Y.-J. W reviewed and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Funding
This study was funded by the General Project of Medicine and Health Science and Technology Programme of Zhejiang Province (2021KY486), the General Research Project of Zhejiang Provincial Department of Education (No. Y202249323), and the Basic Scientifc Research Funds of Department of Education of Zhejiang Province (KYQN202118).
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (QT2024149). Written informed consent was obtained from all participants before inclusion.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Jin, Xiao-Kun Huang and Zhen-Yu Gao contributed equally.
References
- 1.World Obesity Federation, World Obesity Atlas. (2023). https://data.worldobesity.org/publications/?cat=19
- 2.Maret-Ouda, J., Markar, S. R. & Lagergren, J. Gastroesophageal reflux disease. Jama324, 2565. 10.1001/jama.2020.21573 (2020). [DOI] [PubMed] [Google Scholar]
- 3.King, K. et al. Assessment and management of gastroesophageal reflux disease following bariatric surgery. Surg. Obes. Relat. Dis.17, 1919–1925. 10.1016/j.soard.2021.07.023 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Yuan, S. & Larsson, S. C. Adiposity, diabetes, lifestyle factors and risk of gastroesophageal reflux disease: A mendelian randomization study. Eur. J. Epidemiol.37, 747–754. 10.1007/s10654-022-00842-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiassi, S. & Morton, J. M. Safety and efficacy of bariatric and metabolic surgery. Curr. Obes. Rep.9, 159–164. 10.1007/s13679-020-00377-y (2020). [DOI] [PubMed] [Google Scholar]
- 6.Brajcich, B. C. & Hungness, E. S. Sleeve gastrectomy. Jama324, 908. 10.1001/jama.2020.14775 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, A. P. et al. Evaluation of patient reported gastroesophageal reflux severity at baseline and at 1-year after bariatric surgery. Ann. Surg.275, 1143–1148. 10.1097/sla.0000000000004533 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Mittal, R. & Vaezi, M. F. Esophageal motility disorders and gastroesophageal reflux disease. N. Engl. J. Med.383, 1961–1972. 10.1056/NEJMra2000328 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Velanovich, V. 25 years of the GERD-HRQL symptom severity instrument: An assessment of published applications. Surg. Endosc.37, 255–265. 10.1007/s00464-022-09463-9 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Yeung, K. T. D., Penney, N., Ashrafian, L., Darzi, A. & Ashrafian, H. Does Sleeve Gastrectomy expose the distal esophagus to severe reflux? A systematic review and Meta-analysis. Ann. Surg.271, 257–265. 10.1097/sla.0000000000003275 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Caspa Gokulan, R., Garcia-Buitrago, M. T. & Zaika, A. I. From genetics to signaling pathways: Molecular pathogenesis of esophageal adenocarcinoma. Biochim. Biophys. Acta Rev. Cancer1872, 37–48. 10.1016/j.bbcan.2019.05.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellorin, O., Senturk, J. C., Cruz, M. V., Dakin, G. & Afaneh, C. Predictive factors for developing GERD after Sleeve Gastrectomy: Is preoperative endoscopy necessary?. J. Gastrointest. Surg.26, 1015–1020. 10.1007/s11605-021-05207-7 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Tang, G. et al. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: a prospective two-center cohort study using LASSO-logistic regression. Int. J. Surg.89, 105948. 10.1016/j.ijsu.2021.105948 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Richter, J. E. & Rubenstein, J. H. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology154, 267–276. 10.1053/j.gastro.2017.07.045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Q. et al. Analyzing the correlation between gastroesophageal reflux disease and anxiety and depression based on ordered logistic regression. Sci. Rep.14, 6594. 10.1038/s41598-024-57101-2 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson, B. C., Somers, S. C., Fuchs, C. S., Kelly, C. P. & Camargo, C. A. Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N. Engl. J. Med.354, 2340–2348. 10.1056/NEJMoa054391 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Serag, H. B. et al. Obesity increases oesophageal acid exposure. Gut56, 749–755. 10.1136/gut.2006.100263 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polomsky, M., Peters, J. H. & Schwartz, S. I. Hiatal hernia and disorders of the spine: A historical perspective. Dis. Esophagus25, 367–372. 10.1111/j.1442-2050.2011.01263.x (2012). [DOI] [PubMed] [Google Scholar]
- 19.Fass, O. Z. & Mashimo, H. The effect of bariatric surgery and endoscopic procedures on gastroesophageal reflux disease. J. Neurogastroenterol. Motil.27, 35–45. 10.5056/jnm20169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh, Y. H. et al. What is the impact of metabolic syndrome and its components on reflux esophagitis? A cross-sectional study. BMC Gastroenterol.1910.1186/s12876-019-0950-z (2019). [DOI] [PMC free article] [PubMed]
- 21.Kermansaravi, M., Kabir, A., Mousavimaleki, A. & Pazouki, A. Association between hiatal hernia and gastroesophageal reflux symptoms after one-anastomosis/mini gastric bypass. Surg. Obes. Relat. Dis.16, 863–867. 10.1016/j.soard.2020.03.011 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Tack, J. & Pandolfino, J. E. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 154, 277–288. 10.1053/j.gastro.2017.09.047 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Chan, D. L. et al. Accuracy of hiatal hernia diagnosis in bariatric patients: Preoperative endoscopy versus intraoperative reference. JGH Open.4, 1074–1078. 10.1002/jgh3.12388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Chaar, M., Ezeji, G., Claros, L., Miletics, M. & Stoltzfus, J. Short-term results of laparoscopic sleeve gastrectomy in combination with hiatal hernia repair: Experience in a single Accredited Center. Obes. Surg.26, 68–76. 10.1007/s11695-015-1739-y (2016). [DOI] [PubMed] [Google Scholar]
- 25.Daes, J., Jimenez, M. E., Said, N., Daza, J. C. & Dennis, R. Laparoscopic sleeve gastrectomy: Symptoms of gastroesophageal reflux can be reduced by changes in surgical technique. Obes. Surg.22, 1874–1879. 10.1007/s11695-012-0746-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, X. M., Tan, J. C., Zhu, Y. & Lin, L. Association between diabetes mellitus and gastroesophageal reflux disease: A meta-analysis. World J. Gastroenterol.21, 3085–3092. 10.3748/wjg.v21.i10.3085 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaldi, M. et al. Role of preoperative high-resolution manometry in the identification of patients at high risk of postoperative GERD symptoms 1 year after Sleeve Gastrectomy. Obes. Surg.33, 2749–2757. 10.1007/s11695-023-06732-x (2023). [DOI] [PubMed] [Google Scholar]
- 28.Navarini, D. et al. Predictive factors of gastroesophageal reflux disease in bariatric surgery: A controlled trial comparing sleeve gastrectomy with gastric bypass. Obes. Surg.30, 1360–1367. 10.1007/s11695-019-04286-5 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Masood, M., Low, D., Deal, S. B. & Kozarek, R. A. Gastroesophageal reflux disease in obesity: bariatric surgery as both the cause and the cure in the morbidly obese Population. J. Clin. Med.1210.3390/jcm12175543 (2023). [DOI] [PMC free article] [PubMed]
- 30.Wu, P. et al. The association of metabolic syndrome with reflux esophagitis: A case-control study. Neurogastroenterol. Motil.23, 989–994. 10.1111/j.1365-2982.2011.01786.x (2011). [DOI] [PubMed] [Google Scholar]
- 31.Chung, S. J. et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: A cross-sectional case-control study of 7078 koreans undergoing health check-ups. Gut57, 1360–1365. 10.1136/gut.2007.147090 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Mohamed-Ali, V. et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab.82, 4196–4200. 10.1210/jcem.82.12.4450 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Cao, W. et al. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol.287, G1131–G1139. 10.1152/ajpgi.00216.2004 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Zhang, N. et al. Reduction in obesity-related comorbidities: Is gastric bypass better than sleeve gastrectomy?. Surg. Endosc.27, 1273–1280. 10.1007/s00464-012-2595-7 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Gu, L. et al. Relationship between bariatric surgery and gastroesophageal reflux disease: A systematic review and Meta-analysis. Obes. Surg.29, 4105–4113. 10.1007/s11695-019-04218-3 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Aili, A. et al. Gastroesophageal reflux related changes after sleeve gastrectomy and sleeve gastrectomy with fundoplication: A retrospective single center study. Front. Endocrinol. (Lausanne)13, 1041889. 10.3389/fendo.2022.1041889 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.