Abstract
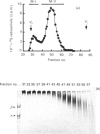
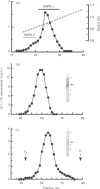
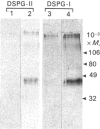
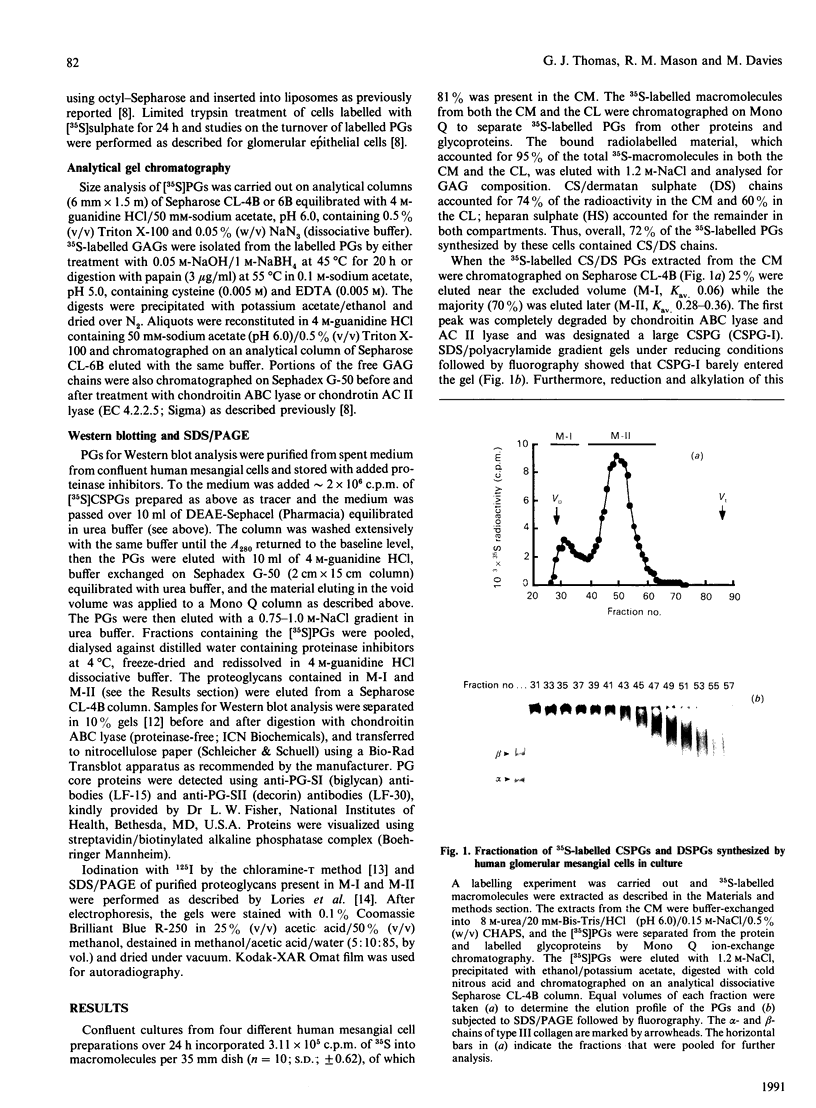
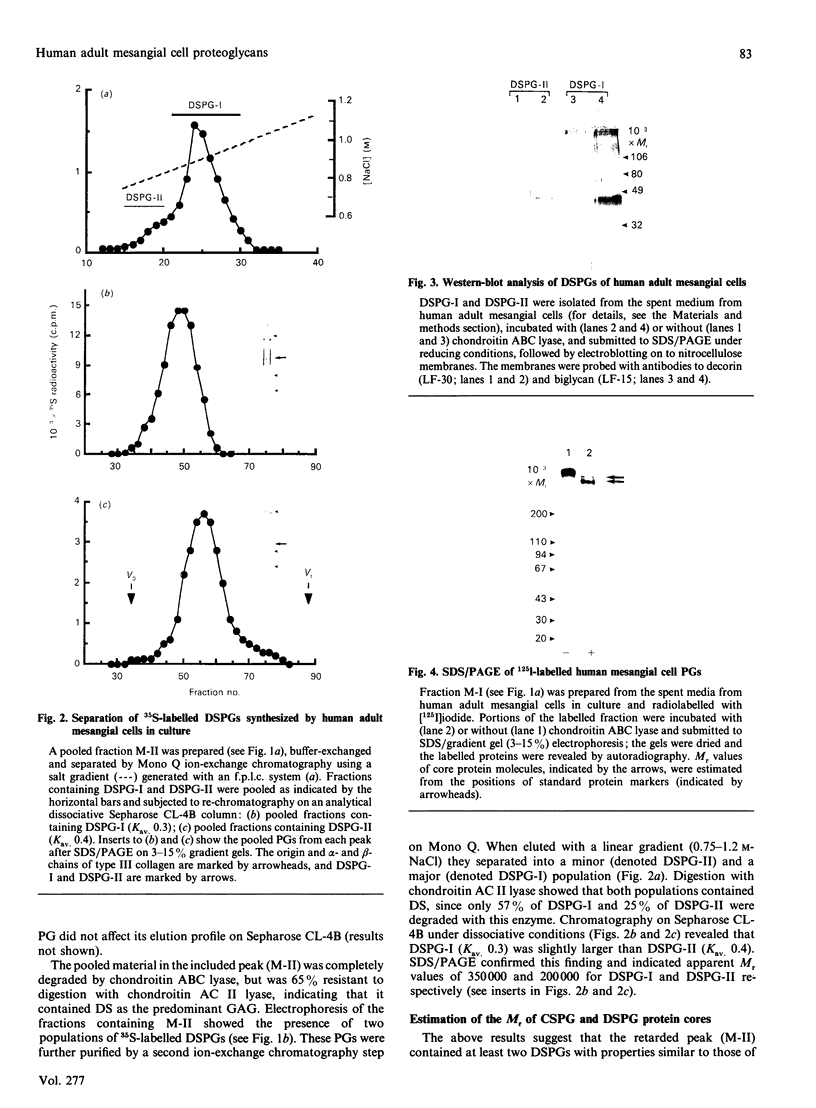
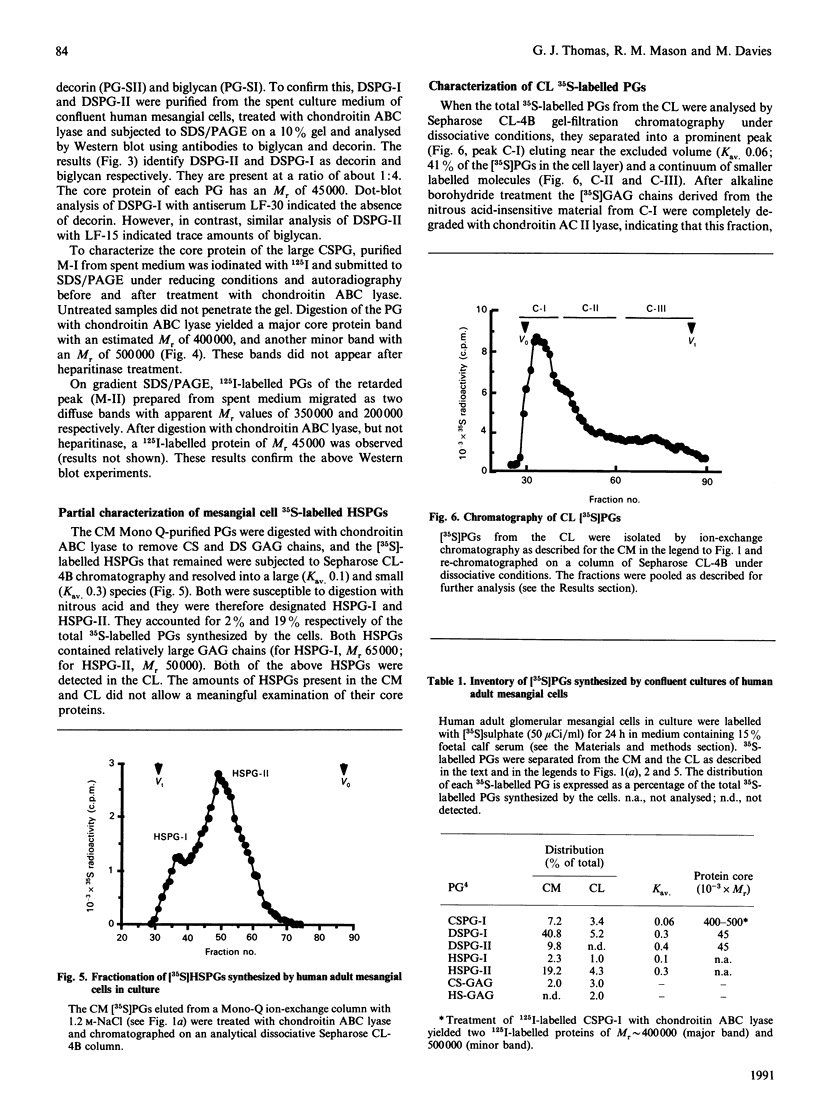
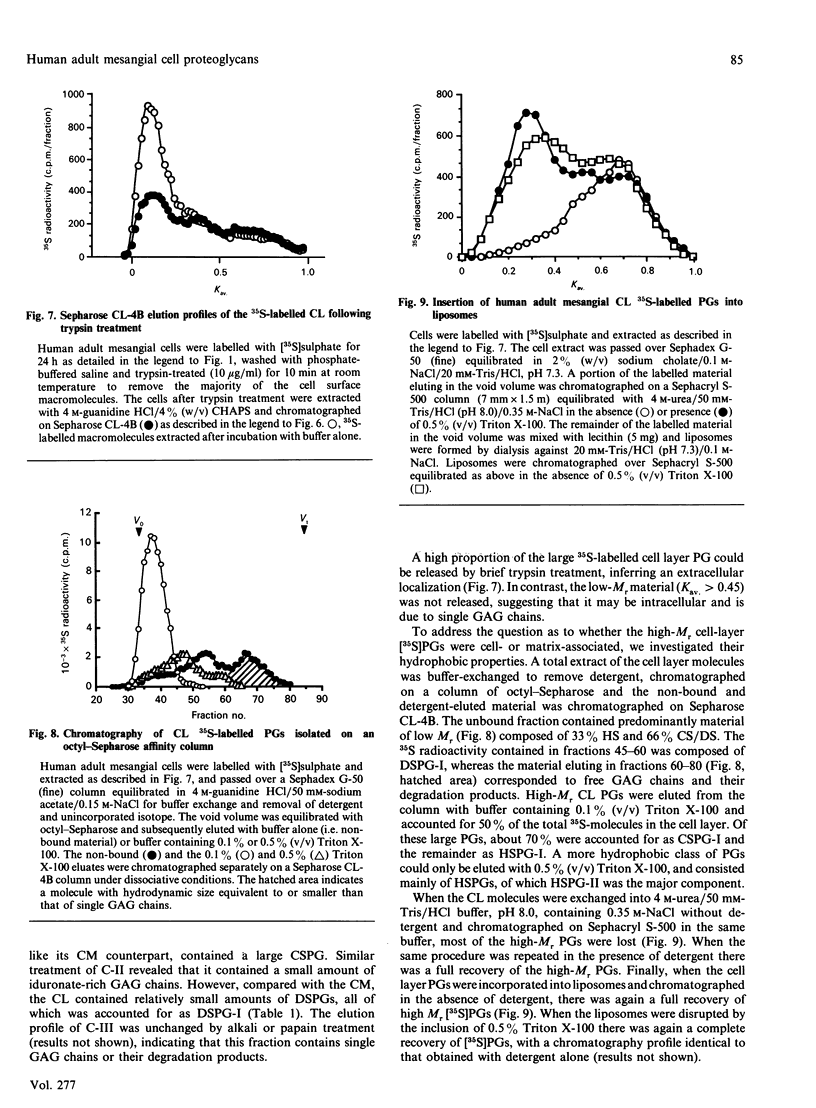
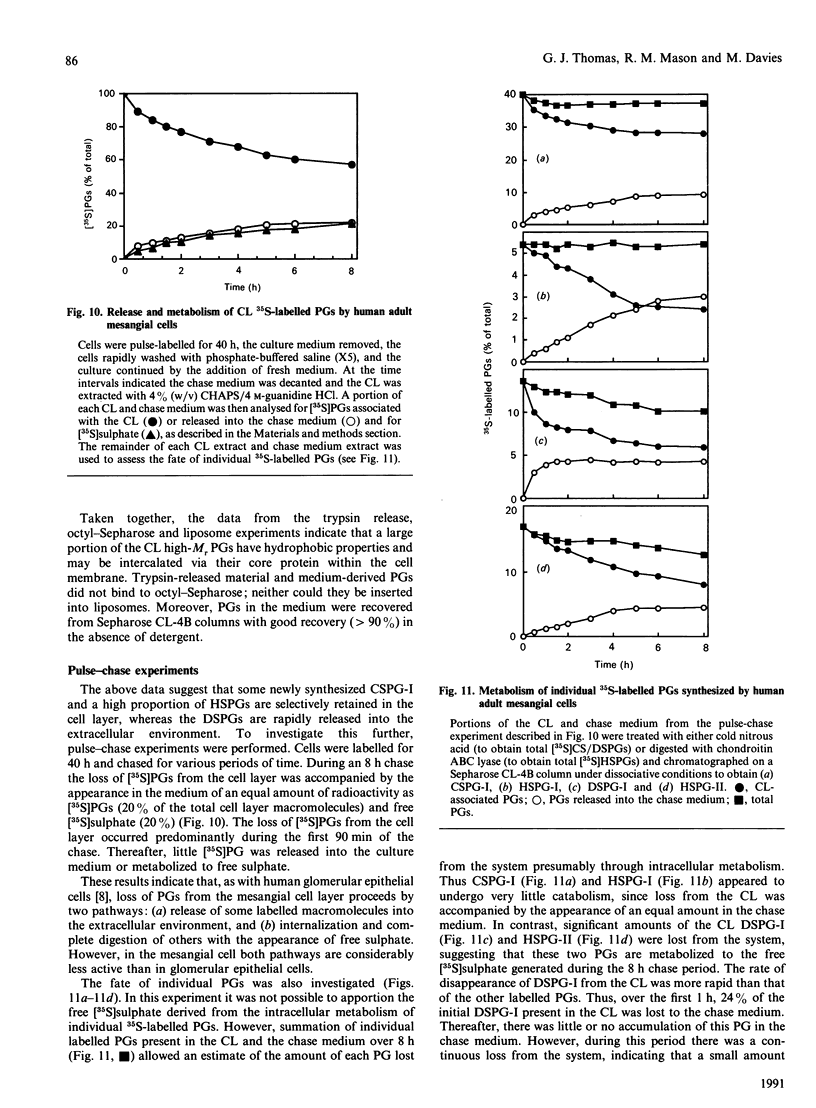
1. The newly synthesized proteoglycans from human adult glomerular mesangial cells labelled in vitro for 24 h with [35S]sulphate have been characterized using biochemical and immunological techniques. 2. The following proteoglycans were identified (% of total synthesized). (i) A large chondroitin sulphate proteoglycan, CSPG-I, Mr approximately 1 x 10(6) (10.6%). This proteoglycan consisted of a protein core of Mr approximately 4 x 10(5) and glycosaminoglycan chains of Mr 2.5 x 10(4), and was present in both the cell layer and the culture medium. (ii) A major small dermatan sulphate proteoglycan, DSPG-I, Mr 3.5 x 10(5) (46%), which was mainly located in the culture medium. (iii) A second minor small dermatan sulphate, DSPG-II, Mr approximately 2 x 10(5) (9.8%). This molecule was exclusively located in the culture medium. (iv) A large heparan sulphate proteoglycan, HSPG-I, Mr 8 x 10(5) (3.3%). (v) A second large heparan sulphate proteoglycan HSPG-II, Mr approximately 6 x 10(5) (23%). HSPG-I and HSPG-II were extracted from both the culture medium and the cell layer. 3. Western blot analysis of the core proteins released by chondroitin ABC lyase treatment of DSPG-I and DSPG-II identified these dermatan sulphate proteoglycans as biglycan and decorin respectively. Both DSPG-I and DSPG-II had core proteins of Mr 45,000. 4. The cell-layer-associated forms of CSPG-I, HSPG-I and HSPG-II were accessible to limited trypsin treatment, bound to octyl-Sepharose and could be inserted into liposomes, indicating a possible cell membrane location. 5. Pulse-chase experiments indicated that the cell-layer-associated [35S]proteoglycans undergo limited metabolism to inorganic [35S]sulphate, the majority of which is accounted for by the degradation of HSPG-II and to a lesser extent DSPG-I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Chang Y., Yanagishita M., Hascall V. C., Wight T. N. Proteoglycans synthesized by smooth muscle cells derived from monkey (Macaca nemestrina) aorta. J Biol Chem. 1983 May 10;258(9):5679–5688. [PubMed] [Google Scholar]
- Couchman J. R., Caterson B., Christner J. E., Baker J. R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984 Feb 16;307(5952):650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L., Rosenzweig L. J. Distribution of sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Eur J Cell Biol. 1983 Sep;31(2):290–295. [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Isolation and characterization of dermatan sulfate proteoglycans synthesized by cultured bovine aortic endothelial cells. J Biol Chem. 1988 Dec 15;263(35):19222–19231. [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Kim Y., Oegema T. R., Jr Proteoglycans synthesized by human glomerular mesangial cells in culture. J Biol Chem. 1990 Jun 5;265(16):9533–9543. [PubMed] [Google Scholar]
- Klein D. J., Oegema T. R., Jr, Fredeen T. S., van der Woude F., Kim Y., Brown D. M. Partial characterization of proteoglycans synthesized by human glomerular epithelial cells in culture. Arch Biochem Biophys. 1990 Mar;277(2):389–401. doi: 10.1016/0003-9861(90)90595-p. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lories V., De Boeck H., David G., Cassiman J. J., Van den Berghe H. Heparan sulfate proteoglycans of human lung fibroblasts. Structural heterogeneity of the core proteins of the hydrophobic cell-associated forms. J Biol Chem. 1987 Jan 15;262(2):854–859. [PubMed] [Google Scholar]
- Martin J., Davies M., Thomas G., Lovett D. H. Human mesangial cells secrete a GBM-degrading neutral proteinase and a specific inhibitor. Kidney Int. 1989 Nov;36(5):790–801. doi: 10.1038/ki.1989.264. [DOI] [PubMed] [Google Scholar]
- McCarthy K. J., Accavitti M. A., Couchman J. R. Immunological characterization of a basement membrane-specific chondroitin sulfate proteoglycan. J Cell Biol. 1989 Dec;109(6 Pt 1):3187–3198. doi: 10.1083/jcb.109.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H., Takeuchi T., Suzuki S., Maeda K., Yamada K., Eguchi G., Kimata K. Aortic endothelial cells synthesize a large chondroitin sulphate proteoglycan capable of binding to hyaluronate. Biochem J. 1990 Jan 1;265(1):61–68. doi: 10.1042/bj2650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Suzuki S. Proteinase activity in chondroitin lyase (chondroitinase) and endo-beta-D-galactosidase (keratanase) preparations and a method to abolish their proteolytic effect on proteoglycan. Biochem J. 1980 Oct 1;191(1):203–207. doi: 10.1042/bj1910203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 1989 Sep 15;262(3):823–827. doi: 10.1042/bj2620823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Stein H. D., Kashgarian M. The mesangium and glomerulonephritis. Klin Wochenschr. 1982 Sep 15;60(18):1077–1094. doi: 10.1007/BF01715838. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jenner L., Mason R. M., Davies M. Human glomerular epithelial cell proteoglycans. Arch Biochem Biophys. 1990 Apr;278(1):11–20. doi: 10.1016/0003-9861(90)90224-m. [DOI] [PubMed] [Google Scholar]
- Timpl R. Recent advances in the biochemistry of glomerular basement membrane. Kidney Int. 1986 Sep;30(3):293–298. doi: 10.1038/ki.1986.183. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Hascall V. C. Proteoglycans in primate arteries. III. Characterization of the proteoglycans synthesized by arterial smooth muscle cells in culture. J Cell Biol. 1983 Jan;96(1):167–176. doi: 10.1083/jcb.96.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M., Yamada K. M., Yoneda M., Suzuki S., Kimata K. Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem. 1986 Oct 15;261(29):13526–13535. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]