Abstract
The emergence of nanogenerators, which have the ability to capture mechanical energy from the environment and to collect and transmit tiny energy, is rapidly becoming a hot research topic. The performance of electrode materials is the key to the efficiency of nanogenerators. Covalent organic skeletons (COFs), a class of crystalline organic porous materials with the advantages of large specific surface area, high porosity, tunable structure, and flexible tailorability, have very significant advantages in being used as nanogenerator materials. In this paper, we synthesised two COF materials to investigate the effect of the introduction of active metals on the friction power generation performance of COFs without changing their topology, COF-2 containing zinc ions is capable of generating a short-circuit current of 107.5 µA during friction. The porous structure increases the effective contact area to form a larger charge density, and the introduction of metal ions can accelerate the charge separation and transport. The two bidirectional synergistic effects of the materials significantly improve the output performance of the nanogenerator, and a simple and efficient method is explored for the enhancement of the output performance of COF-based triboelectric nanogenerators.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77287-9.
Keywords: M-COFs, TENGs, Bidirectional enhancement, Nanomaterials, Anti-corrosion of steel
Subject terms: Materials for energy and catalysis, Nanoscale materials, Chemical physics
Introduction
In response to the current social progress, industrial production and people’s daily lives have a greater demand for energy, but the energy crisis and environmental pollution problems associated with traditional fossil energy, forcing people to develop more green and sustainable energy1,2,3,4. The friction nanogenerator (TENG) is a new type of energy storage and output device, first discovered by Zhonglin Wang’s team in 2012, that can recover almost any type of mechanical energy in the environment and efficiently convert it into electrical energy5,6. Mechanical motions can be found everywhere in our daily lives, such as human body movements, water currents, air currents, and vibrations, which have the potential to drive friction nanogenerators, and thus TENG is considered a highly desirable energy conversion technology7,8,9. The basic structure of TENG consists of a pair of friction layers with opposite friction polarity, which generate friction charge and potential difference through physical contact of two friction materials, forming a coupling of contact initiation and electrostatic induction to realize energy conversion10,11,12. As a renewable energy source, TENG has attracted much attention due to its simple structure, wide choice of materials and cost effectiveness. The output power of TENG has been shown to be proportional to the square of the charge density of the electrode material, so increasing the charge density of the friction contact surface is the key to improving the output performance of TENG. Recently reported strategies to improve the output performance of TENG, including the selection of suitable friction electric materials, charge injection, morphology and structural design, where the most fundamental way to improve the output performance of TENG is to increase the friction charge by selecting suitable materials13,14,15,16.
Covalent organic frameworks (COFs) are a class of crystalline porous polymers with well-defined organic unit arrangements and intrinsic porosity, consisting of a variety of organic structural units connected by covalent bonds, which are capable of integrating a variety of molecular building blocks into highly ordered periodic arrays. As a new type of porous material, COF structure is highly ordered and easy to regulate, and COF can be pre-designed and regulated by reticulation chemistry and dynamic covalent chemistry17,18,19,20.The diversity of organic structural units as well as synthetic reactions facilitates us to obtain regular porous structure crystals with specific structures and functions, while the strong covalent bonding connectivity endows COFs with excellent chemical stability. In addition, COF has the advantages of large conjugated structure, high specific surface area, and tunable energy band structure, so it is considered as one of the most promising materials in gas adsorption and eparation21,22, sensing23, ionic conductor24,25, catalysis26,27, and energy storage and conversion28,29,30. Another advantage of COFs is their densely arranged p-columns and arrays on the backbone, which can be used as pre-arranged paths to facilitate charge transport, and the regular crystal structure reduces the number of charge-complexation points and increases the likelihood of generating electrons and holes, a distinctive feature that is difficult to achieve with other materials. Thus, COFs can provide an attractive structure-function platform for the development of suitable TENG systems.
Chemical modification can effectively increase the charge density of friction materials, and the treated crystals can obtain higher or lower electron density, which can be used to improve their friction electrical properties. Without changing its topology, active metal sites are introduced into the COF material to modulate its electronic structure.In this paper we have chosen zinc ions, which are relatively inexpensive and stable in terms of safety and activity, as ligand metal ions to obtain the target porous materials. No porous material with good crystallinity was obtained when the same batch of copper, iron, cobalt and manganese ions were tried for coordination. By exploring the crystal structure, the influence of microstructure on the COF material is deeply investigated, which provides a theoretical basis for improving its friction power generation performance.
Experimental
In this study, we proceeded to the crystal structure to analyze the factors affecting the charge density of TENG, for which we synthesized two COF materials (Figs. 1 and S1) to explore the effect of the introduction of active metals on the friction power generation performance of COFs without altering their topology, providing a simple and efficient way to improve the output performance of COF-based friction nanogenerators. The experimental results aptly confirm our speculation that the metal-containing COF exhibits a higher charge density as well as a better output capability compared to ordinary COF materials. COF-2 containing zinc ions is capable of generating current when undergoing friction, with a maximum instantaneous output voltage of 1450 V, a short-circuit current of 107.5 µA, and a power density of 4928.4 mW·m−2. Meanwhile, we found that COFs with metal sites exhibit better crystallinity, which suggests that the metal plays an auxiliary role for the molding of COF crystals. In addition, we introduced it into a self-powered cathodic protection system to explore the ability of COF materials to resist corrosion in externally connected circuits. The TENG device was connected to the steel through a circuit to simulate the corrosion of the steel by seawater. After 7 h of testing, the protected steel did not show any obvious corrosion phenomenon, which demonstrates the effectiveness of the external circuit protection we designed. In summary, this study reveals the important role of metals in enhancing the friction generation performance of COF materials and also explores the application of COF materials in self-powered cathodic protection systems.
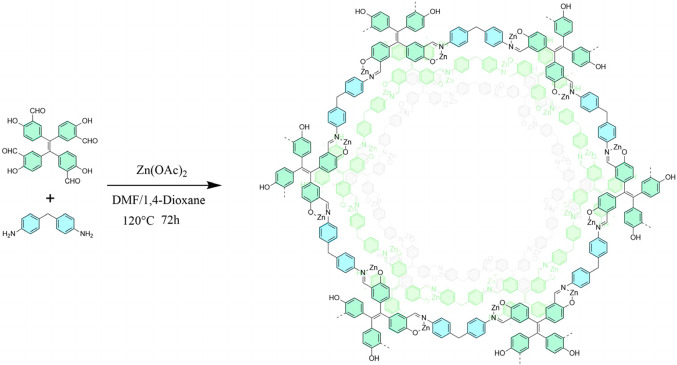
Figure 1.
Synthesis of COF-2.
Results and discussion
We have synthesized COF-2 in about 76% yield by condensation of Benzaldehyde, 3,3’,3’’,3’’’-(1,2-ethenediylidene) tetrakis [6-hydroxy- with 4,4’-Methylenedianiline (MDA) under solvent-heated conditions (Fig. 1) in the presence of Zn(OAc)2·2H2O. Catalyzed by acetic acid, the aldehyde group (-CHO) reacts with the amino group (-NH2) in a Schiff base reaction, which generates the imine bond (C = N) under solvent-heated conditions, and as the reaction proceeds, the C = N bond extends in a two-dimensional direction, gradually forming the layered structure of COF-2.
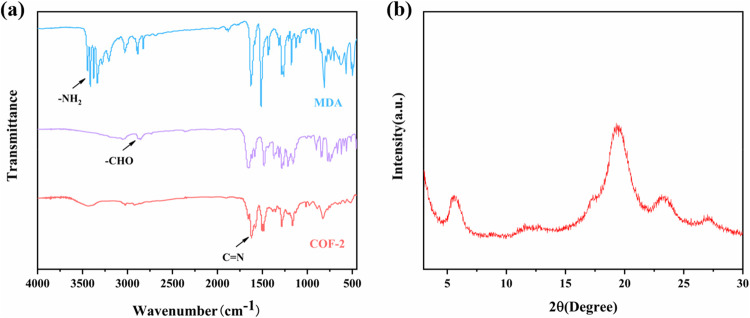
We investigated the structure of these COFs using various characterization techniques. Fourier transform infrared spectroscopy (FT-IR) of COF-1 and − 2 makes it evident that the condensation reactions were both carried out completely (Figs. 2a and S4a), MDA showed stretching and bending vibrations of the -NH2 group near 3400 cm−1; the -CHO group had stretching vibrations at 2850 cm−1; the stretching vibrations of -CHO and -NH2 were significantly hourly in the FT-IR spectra of COF-2, whereas the stretching vibration band of the imine bond (C = N) was shown at 1620 cm−1, which indicated that the condensation reaction was success. Powder X-ray diffraction (PXRD) spectra showed well-defined peaks demonstrating a highly ordered framework structure of the COFs (Figs. 2b and S4b), as evidenced also by scanning electron microscopy (SEM), and the COF particle sizes all reached the micrometer scale (Figs. S5 and S6). The solid-state 13C cross-polarization/magic-angle spinning nuclear magnetic resonance (CP/MAS/NMR) signals of 2 can be definitely assigned as the proposed structure (Fig. S17). Obviously, the signal at 166 ppm, which corresponds to typical C = N resonances, clearly indicatives the formation of imine bond.
Figure 2.
(a) Fourier transform infrared spectrum of COF-2. (b) Powder X-ray diffraction of COF-2.
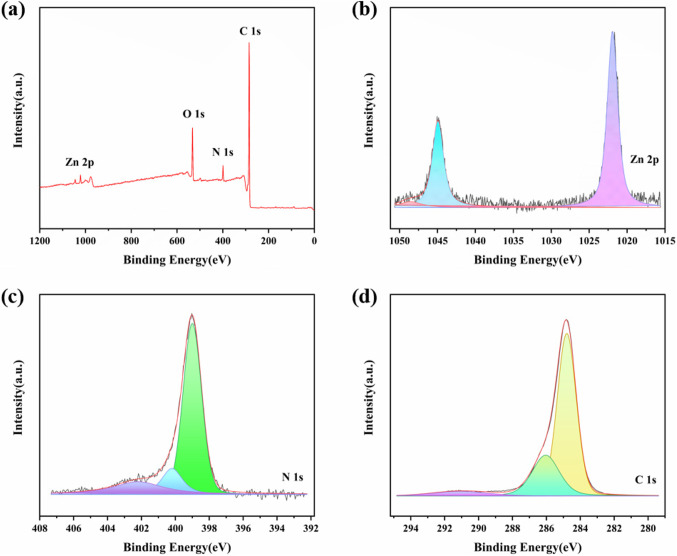
The elements and structures were further verified by X-ray photoelectron spectroscopy (XPS), and the full spectrum of COF-2 (Fig. 3a) identified C 1s, N 1s (Fig. 3c), O 1s (Fig. 3d), and Zn 2p (Fig. 3b) as shown in Fig. 3. The presence of C = N (399.0 eV) and N-H (400.2 eV) groups can be seen in the N 1s XPS spectra of COF-2; in the C 1s XPS spectra, 284.8 and 386.0 eV corresponded to C = C and C = N groups, respectively, which further proved the synthesis of imine bonding, which was in good agreement with the FT-IR spectral results of COF-2. The Zn surface chemical state can be seen in its Zn 2p XPS spectra fitted with spin-orbit 2p3/2 and satellite signals, indicating the presence of Zn in the Zn2+ state.
Figure 3.
X-ray photoelectron spectra of COF-2. (a) Full spectrum. (b) Zn 2p. (c) N 1s. (d) O 1s.
Isothermal adsorption experiments were carried out at 77 K. The specific surface areas (BET) of COF-1 and − 2 were 74.86 m2/g and 131.52 m2/g, respectively, which were both type II adsorption-desorption isotherms as can be seen in Fig. S8, and the presence of micropores in them can also be seen. Fig. S9 shows the thermogravimetric analysis curves of COF-1 and − 2. Both COF-1 and COF-2 started to lose weight significantly after 450 °C, and both showed good thermal stability.
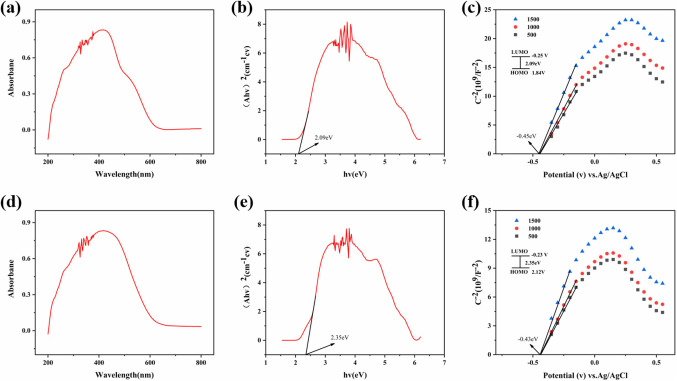
Prior to the TENG output test, we investigated the band gap energy (Eg) of both materials using UV-Vis diffuse reflectance spectroscopy (UV/Vis) (Fig. 4a and d) and calculated their highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) using the Mott-Schottky method, which allowed us to determine the semiconductor type of the COF materials and predict their TENG output properties. The UV-Vis diffuse reflectance spectra of COF-1 and − 2 show bandgap energies of 2.09 eV and 2.35 eV, respectively; the Mott-Schottky curves confirm that both materials are n-type semiconductors (Fig. 4b and e). The lowest unoccupied molecular orbital (LUMO) flat bands of COF-1 and − 2 were − 0.25 eV and − 0.43 eV, respectively, when Ag/AgCl was used as a reference (obtained at -0.45 eV and − 0.63Ev binding, respectively), and their highest occupied molecular orbitals (HOMOs) were 1.84 eV and 2.12 eV, respectively (Fig. 4c and f). From the test results, we know that the HOMO orbitals of COF-2 are higher than the HOMO orbitals of COF-1, which means that the electron leaping ability inside COF-2 is stronger, and the electrons are more likely to be transferred and transported in it, and the output performance is higher; therefore, we speculate that the output capability of TENG-2, which is based on COF-2 as a material, will be better. In addition to the electrode material has a large impact on the TENG output performance, the contact tightness between the materials also has a relationship on the output performance impact as well. Therefore, we tested the performance of the two COFs under the same experimental conditions by taking equal amounts of COF-1 and − 2 in the same mortar for the same amount of time and coating the powder uniformly on the copper tape to minimize the effect of other errors on the output performance of TENG.
Figure 4.
(a), (d) UV-Vis diffuse reflectance spectra of COF-1 and − 2. (b), (e) Tauc plots corresponding to (a) and (d). (c), (f) Mott-Schottky plots of COF-1 and − 2 in 0.2 M Na2SO4 solution.
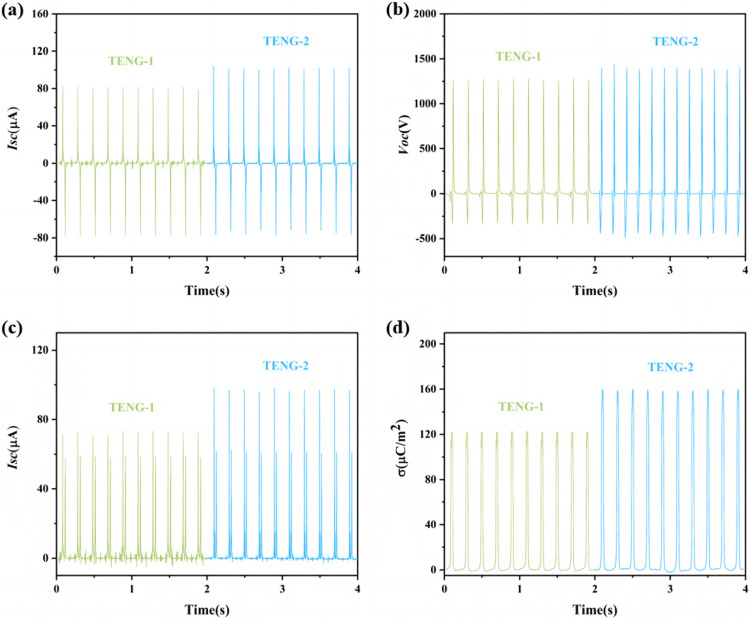
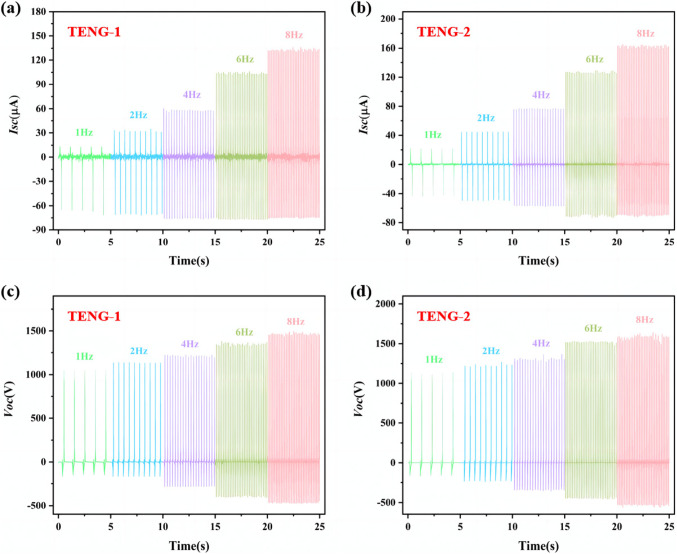
The working principle of TENG based on COF material is to utilize the coupling of contact initiation and electrostatic induction, and the vertical contact-detachment mode is used in this experiment.The positive electrode materials of the TENG device are COF-1 and COF-2, respectively, and the negative electrode materials are polyvinylidene difluoride (PVDF), which are named as TENG-1 and TENG-2, respectively. In the output performance test results of TENG, the short-circuit current and open-circuit voltage at 5 Hz are 82.8 µA and 1260 V and 107.3 µA and 1450 V, respectively (Fig. 5a and b), and the Isc and Voc of TENG − 2 are larger compared to TENG-1. At 5 Hz, the DC currents obtained by TENG-1 and TENG-2 through the rectifier bridge are 73.5 µA and 99.5 µA, respectively (Fig. 5c); and the charge densities are 122.6 µC·m-2,and 161.3 µC·m-2, respectively (Fig. 5d), according to the friction nanogenerator under different load resistances power density and short-circuit current, the instantaneous power peaks at a load resistance of 100 MΩ, which is 2822.4 mW·m-2and 4928.4 mW·m-2, respectively (Figs. S10f and S11f). We also tested the output performance of compounds 1 and 2 under different contact frequency conditions and found that Isc and Voc increased with increasing frequency (Fig. 6a and d), and the output performance of TENG − 2 was higher than that of TENG-1 at 1–8 Hz. When TENG − 2 is operated at 8 Hz, the current and voltage can reach 165.6 µA(Fig. 6b) and 1600 V(Fig. 6d). The test results show that the magnitude of the output performance of the compounds is TENG-2 > TENG-1, which suggests that the incorporation of the metal sites, which can modulate the electronic structure of the COFs, significantly improves the TENG output performance of the compounds. The specific output performance data of TENG-1 and TENG-2 are shown in Figs. S10 and S11.
Figure 5.
Comparison of output performance of TENG-1 and TENG-2. (a) Isc at 5 Hz. (b) Voc at 5 Hz. (c) DC Isc at 5 Hz. (d) Charge density.
Figure 6.
Comparison of Isc and Voc of TENG-1 and TENG-2 at different frequencies from 1 to 8 Hz.
When TENG − 2 is operated at 8 Hz, the current and voltage are 165.6 µA and 1600 V, which outperforms most of the COF materials, and some of the MOF materials, and shows a very good output performance when compared with the materials that have been reported (see SI Table 1). In addition, the peak instantaneous power of TENG − 2 at a load resistance of 100 MΩ is 4928.4 mW·m-2, which outperforms the peak instantaneous power of the reported materials (see SI Table 1), demonstrating that the device has a great potential for friction power generation capability output.
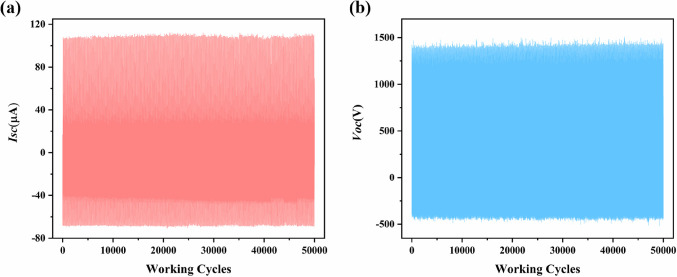
In addition we tested the stability and durability of TENG-2 at 5 Hz. The cycling test results show that after 50,000 cycles, the Isc and Voc values of TENG-2 did not show significant changes and remained in a stable state (Fig. 7a and b), while the Isc and Voc values of TENG-1 did not show significant changes (Fig. S12), which proves that the samples all have good stability and lays the foundation for their practical applications. To further verify the stability of the friction electric materials, we observed the morphology of compounds 1 and 2 by scanning electron microscopy (SEM) and the elemental distributions of compounds 1 and 2 by characteristic K spectral lines (EDX) (Fig. S13-S16), and the SEM results showed that the morphology of compounds 1 and 2 was almost unchanged before and after the experiments, which confirmed their stability.
Figure 7.
Isc and Voc of TENG-2 at 50,000 cycles.
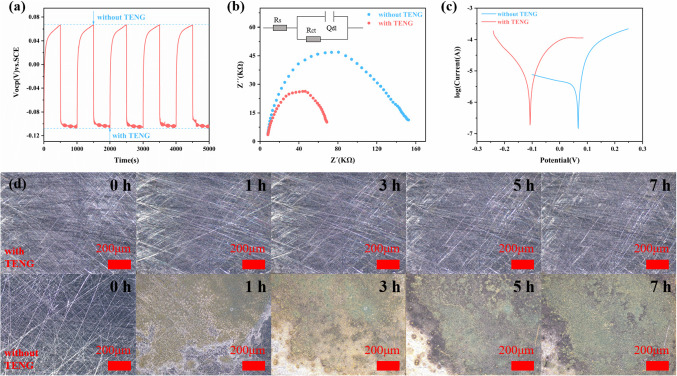
Based on the excellent output performance of TENG-2, we introduced it into self-powered cathodic protection systems to explore the potential of COF materials for metal corrosion protection. We simulated the chemical corrosion reaction of carbon steel in seawater by connecting the protected carbon steel and platinum electrodes to the negative and positive terminals of the TENG-2 after the TENG-2 is connected to the rectifier bridge.The microcurrent generated by the TENG-2 after friction provides electrons passing through the surface of the carbon steel, which restricts the electrochemical corrosion reaction of the carbon steel in the seawater itself, and thus realizes the protection of carbon steel. The properties, degree of corrosion and protective effect of TENG-2 connected and unconnected carbon steel were determined by measuring the open circuit potential (OCP) of the carbon steel and by obtaining the electrochemical impedance spectra (EIS) and electrochemical polarization curves (Tafel) of the circuit system in a three-electrode system, respectively. Without the TENG-2 connected, the OCP of the protected carbon steel remained at 0.06 V. Once the carbon steel was connected to the TENG-2 circuit, the OCP rapidly decreased to -0.11 V and remained stable. This negative migration correlates with an increase in the amount of transferred charge, indicating that the TENG-2 provides effective cathodic protection, and when the TENG-2 is disconnected, the OCP once again returns to its original position (Fig. 8a). EIS is another important parameter to judge the anticorrosion circuit, Fig. 8b shows the impedance diagram of the circuit in both cases, the internal resistance of the circuit is lower when TENG-2 is connected than when it is not connected to TENG, which suggests that the microcurrent generated by TENG-2 is more likely to supply the electrons needed for the surface of the carbon steel, thus limiting the progress of its own electrochemical reaction, which further confirms its ability to protect the external cathode electrode. In addition, the test results of the polarization curves show that the polarization potential (Icorr) of the TENG-2 connected system is much lower than that of the unconnected TENG (Fig. 8c), which is in good agreement with the observed changes in OCP, attributed to the large number of transferred electrons originating from the TENG-2 system. And due to the lower internal resistance, the number of electrons supplied by TENG-2 to the carbon steel surface is high, limiting its own reaction, and thus the polarization current (Icorr) of the TENG-2-connected system is higher than that of the unconnected TENG.
Figure 8.
(a) OCP changes for 5 cycles. (b) EIS with and without TENG-2 connected and equivalent circuit. (c) Tafel with and without TENG-2 connected. (d) Metallographic micrographs of carbon steel with and without TENG-2 connected after 0, 1, 3, 5, and 7 h of immersion in 3.5 wt% NaCl solution.
The above results show that TENG-2 has a good effect in the cathodic protection system of carbon steel. In order to visualize the cathodic protection effect of TENG-2, we simulated the surface morphology of carbon steel after immersion in seawater to evaluate its corrosion protection effect. By comparing the rust stains on the carbon steel after 0 h, 1 h, 3 h, 5 h and 7 h of immersion, it was found that the surface of the carbon steel protected by the connected TENG-2 system showed no obvious signs of corrosion even after 7 h of immersion in the solution (Fig. 8d), compared to the carbon steel not connected to the TENG which showed obvious rust stains after only 1 h of immersion, but the rust stains on the surface of the carbon steel got worse and worse with the prolongation of the immersion time. The results indicate that the COF-2-based TENG can be used as an effective external power source to protect carbon steel.
Conclusions
The chemical properties of the friction electrode materials deeply affect the triboelectric nanogenerator output performance, and the higher the charge density of the materials, when there is a significant charge separation effect upon friction, the stronger the friction power generation output performance of the compounds. In this paper, we design and synthesize porous COF materials to increase the contact area of the friction material while maintaining multiple channels, thus increasing the contact area and charge output during the operation of the TENGs device. There is a potential for mass production, but also greatly reduce the synthesis time, saving the synthesis cost. In addition, the organic monomer selection of symmetric molecules, the cost further reduces the cost of material preparation. In addition, the introduction of metal zinc ions is designed to further intensify the material charge separation, which is more conducive to the charge movement and output during friction, and the material bi-directionally enhances the friction power generation output performance. In practical applications, COF-2 has high stability and excellent output performance, and can be used as a self-powered cathodic protector to protect metallic materials from corrosion, and the anti-corrosion experiments as well as comparisons with literature reports confirm the material’s excellence. Therefore, this study not only reveals the effect of porous materials on the output performance of TENG after the introduction of metallic zinc ions, but also provides a simple method for designing new self-powered cathodic protection materials in the future.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by Natural Science Foundation of Henan Province of China (242300420210, 242300420208), Key Scientific Research Projects of Universities in Henan Province (24B150050), Research Project on Teaching Reform of Postgraduate Education in Zhongyuan University of Technology(JG202433), Supported by the Zhongyuan University of Technology Advantageous Discipline Strength Enhancement Program (SD202414, GG202408), Young Backbone Teacher of Zhongyuan University of Technology (2023XQG09), Key Projects of Science and Technology of Henan Province ( 242102241003 ), Natural Science Fund Project of Zhongyuan University of Technology (K2025YB014), Supported by Program for Innovative Research Team (in Science and Technology) in University of Henan Province(23IRTSTHN019).
Author contributions
S.W. and Y.Z. synthesized and characterized the materials. Z.X., Y.Y., and Z.Z. conduct the experiments. Y.Y., and S.Z. characterized the samples. J.X., S.C. and Z.W. discussed the results and prepared the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia-Bin Xiong, Email: xjiabin@foxmail.com.
Si-Ru Chen, Email: siruchen@zut.edu.cn.
Zhuo Wang, Email: wz3530@126.com.
References
- 1.Mughal, N. et al. Energy Strateg. Rev.39, 100745–100751 (2022). [Google Scholar]
- 2.Mandal, B. ChemistrySelect4(28), 8301–8310 (2019). [Google Scholar]
- 3.Uçkun Kiran, E. et al. Waste Biomass Valoriz.5(6), 903–917 (2014). [Google Scholar]
- 4.Reddy, B. S. Renew. Sustain. Energy Rev.47, 198–212 (2015). [Google Scholar]
- 5.Wang, Z. et al. J. Mater. Chem. A6(18), 8549–8557 (2018). [Google Scholar]
- 6.Cui, S. et al. Chem. Sci.7(10), 6477–6483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, X., Tat, T. & Chen, J. Trends Chem.3(9), 765–778 (2021). [Google Scholar]
- 8.Karan, S. K. et al. Nano Energy49, 655–666 (2018). [Google Scholar]
- 9.Niu, Q. et al. Nano Energy74, 104837–104875 (2020). [Google Scholar]
- 10.Jiang, T. et al. Adv. Funct. Mater.25(19), 2928–2938 (2015). [Google Scholar]
- 11.Wang, Z. L. Mater. Today52, 348–363 (2022). [Google Scholar]
- 12.Niu, S. et al. Adv. Funct. Mater.24(22), 3332–3340 (2014). [Google Scholar]
- 13.Liu, Y. et al. Adv. Funct. Mater.30(50), 4714–4747 (2020). [Google Scholar]
- 14.Chen, J. et al. Adv. Energy Mater.11(44), 2106–2117 (2021). [Google Scholar]
- 15.Cui, S. et al. Nano Res.11(4), 1873–1882 (2018). [Google Scholar]
- 16.Yamamoto, S. et al. Chem. Sci.9(13), 3282–3289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, M. et al. Coord. Chem. Rev.435, 213778–213800 (2021). [Google Scholar]
- 18.Geng, K. et al. Chem. Rev.120(16), 8814–8933 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Jin, E. et al. Science357(6352), 673–676 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Jin, E. et al. Angew. Chem. Int. Ed.59(29), 12162–12169 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Li, J. et al. Chin. Chem. Lett.33(6), 3017–3020 (2022). [Google Scholar]
- 22.Zhang, J. et al. Nat. Commun.14(1), 4922–4932 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, X. et al. Chem. Soc. Rev.48(20), 5266–5302 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Sahoo, R. et al. Adv. Energy Mater.11(39), 2300–2334 (2021). [Google Scholar]
- 25.Yan, Q. L. et al. Acs Appl. Nano Mater.7(7), 7555–7561 (2024). [Google Scholar]
- 26.Prakash, K. et al. J. Mater. Chem. A11(27), 14489–14538 (2023). [Google Scholar]
- 27.Yu, H.-Y. et al. Chem. Eng. J.445, 136713–136724 (2022). [Google Scholar]
- 28.Li, J. et al. Chem. Soc. Rev.49(11), 3565–3604 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Yang, X. et al. Angew. Chem. Int. Ed.59(46), 20385-20389. (2020). [DOI] [PubMed] [Google Scholar]
- 30.Zhao, H. et al. J. Mater. Sci.57(22), 9980–9991 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].