Abstract
The presence or absence of the receptor CD4 and the coreceptors CCR5 and CXCR4 restrict the cell tropism of human immunodeficiency virus type 1 (HIV-1). Despite the importance of thymic infection by HIV-1, conflicting reports regarding the expression of HIV-1 coreceptors on human thymocytes have not been resolved. We assayed the expression and function of the major HIV-1 coreceptors, CCR5 and CXCR4, as well as CCR4 and CCR7 as controls, on human thymocytes. We detected CCR5 on 2.5% of thymocytes, CXCR4 on 53% of the cells, and CCR4 on 16% and CCR7 on 11% of human thymocytes. Moreover, infection by R5 HIV-1 did not significantly induce expression of CCR5. We found that two widely used anti-CCR5 monoclonal antibodies cross-reacted with CCR8, which may account for discrepancies among published reports of CCR5 expression on primary cells. This cross-reactivity could be eliminated by deletion of amino acids 2 through 4 of CCR8. Chemotaxis assays showed that SDF-1, which binds CXCR4; MDC, which binds CCR4; and ELC, which binds CCR7, mediated significant chemotaxis of thymocytes. In contrast, MIP-1β, whose receptor is CCR5, did not induce significant chemotaxis. Our results indicate that CXCR4, CCR4, CCR7, and their chemokine ligands may be involved in thymocyte migration during development in the thymus. CCR5 and its ligands, however, are likely not involved in these processes. Furthermore, the pattern of CCR5 and CXCR4 expression that we found may explain the greater susceptibility of human thymocytes to infection by HIV-1 isolates capable of using CXCR4 in cell entry compared to those that use only CCR5.
Certain chemokines and their receptors play an important role in the biology of human immunodeficiency virus type 1 (HIV-1). Chemokines are 70- to 100-amino-acid polypeptides that stimulate leukocyte migration and are involved in development, inflammation, and infectious diseases (reviewed in references 20 and 27). Chemokines are classified based on the arrangement and number of their amino-terminal cysteines as C, CC, CXC, or CX3C chemokines. The majority of known chemokines are in the CC or CXC category. All chemokines have structurally similar G protein-coupled receptors, which have seven α-helical transmembrane domains. In addition to their roles in inflammation and development, a number of chemokine receptors have been shown to be coreceptors for HIV-1, HIV-2, and simian immunodeficiency virus (SIV). HIV-1 requires a coreceptor in addition to its primary receptor, CD4, for productive infection. Ten human chemokine receptors or related molecules can perform this function in vitro. The most important HIV-1 coreceptors in infected individuals, however, are CCR5 and CXCR4 (reviewed in reference 20).
Nearly all HIV-1 isolates derived from newly infected patients or during the first few years following infection are exclusively CCR5 tropic (R5 HIV-1). The selective pressures which favor R5 HIV-1 isolates following transmission and early in the course of infection have not been well characterized but may relate to their greater ability to infect resting memory T cells (18, 34). During later stages of infection in a significant proportion of individuals, HIV-1 isolates evolve which gain the ability to use CXCR4 in addition to or instead of CCR5 (R5X4 or X4 HIV-1 [6, 31]). HIV-1 interaction with CCR5 may be a rate-limiting step in viral replication in infected individuals, since individuals who are heterozygous for a nonfunctional allele of CCR5 (CCR5Δ32) progress more slowly to AIDS (8, 9, 15, 22, 26). Furthermore, viral evolution to the R5X4 or X4 phenotype is associated with rapid replication in tissue culture and with high viral load and rapid progression to disease in infected individuals (6, 32). We and others have shown that R5X4 or X4 HIV-1 isolates are also more cytopathic and replicate to higher levels than R5 isolates in severe combined immune deficient (SCID) mice bearing human thymus-liver grafts (SCID-hu mice) (4, 16, 17, 30). Similarly, in SCID mice injected with human peripheral blood mononuclear cells (PBMC) and in spleen or tonsil culture, R5X4 and X4 isolates are more cytopathic than R5 HIV-1 isolates (12, 25, 28). These results may be explained by the more frequent expression of CXCR4 than of CCR5 by primary CD4+ thymocytes and mature T cells reported in several studies (3, 19, 23, 24). Other reports, however, do not show significant differences in the fraction of thymocytes bearing these two important HIV-1 coreceptors (2, 7, 38).
AIDS-associated R5 HIV-1 isolates replicate to higher levels and are more cytopathic than pre-AIDS R5 isolates in tissue culture and in SCID-hu mice (29, 33). Nevertheless, no R5 isolate studied to date is as cytopathic for human thymocytes as R5X4 patient isolates or the X4 molecular clone NL4-3 (4, 29). To more fully understand the mechanism(s) of R5 and X4 HIV-1 pathogenesis in the human thymus, we sought to reconcile the discrepant published results regarding the fraction of human thymocytes that express CCR5 (2, 7, 19, 24, 38). We assayed the expression of CCR5 on human thymocytes by flow cytometry with five different anti-CCR5 monoclonal antibodies (MAb). We also assayed the chemotaxis of thymocytes to CCR5 ligands, since we reasoned that low levels of CCR5, below the limit of detection by flow cytometry, might be detected by the chemotaxis assay. Moreover, such low levels of CCR5 expression might be sufficient to allow HIV-1 entry. Concurrently, we assayed the expression and function in chemotaxis assays of CCR4, CCR7, and CXCR4. CXCR4 was chosen because it is the other major coreceptor for HIV-1. CCR4 and CCR7 were chosen as controls for these experiments because EBI1 ligand chemokine (ELC) elicited abundant thymocyte chemotaxis and macrophage-derived chemokine (MDC) elicited moderate chemotaxis.
MATERIALS AND METHODS
Antibodies and chemokines.
The anti-CCR4 MAb 328B was provided by Carol Raport, David Chantry, and Patrick Gray of ICOS Corporation (Seattle, Wash.). Anti-CCR5 MAb 5C7 and 3A9 (unconjugated) have been described previously (36, 37). Anti-CCR5 MAb 2D7-APC, 2D7-fluorescein isothiocyanate (FITC), and 3A9-phycoerythrin (PE) were form Pharmingen (San Diego, Calif.). Anti-CCR5 MAb 182-biotin and 183-FITC were from R&D Systems (Minneapolis, Minn.). Hitoshi Hasegawa, Ehime University, Ehime, Japan, provided the anti-CCR7 MAb CCR7.6B3 (13). The anti-CXCR4 MAb 12G5-PE was from Biosource International (Camarillo, Calif.). CD3, CD4, CD8, and isotype control MAb, goat anti-mouse immunoglobulin G (IgG)-PE and streptavidin-PE were all purchased from Caltag Laboratories (Burlingame, Calif.). Recombinant human chemokines MDC, macrophage inflammatory protein 1β (MIP-1β), stromal cell-derived factor 1 (SDF-1), and ELC were provided by David Chantry and Patrick Gray of ICOS Corporation or obtained from R&D Systems.
Preparation and titration of HIV-1 stocks.
HIV-1 biological clones were obtained from Hanneke Schuitemaker of the Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands. Virus stocks were amplified by infection of 2-day phytohemagglutinin (PHA)- and interleukin-2 (IL-2)-stimulated healthy donor PBMC. One half of each virus-containing supernatant was removed every 2 days and replaced with fresh medium containing IL-2. Fresh stimulated PBMC were added 7 days postinfection if viral titers of the collected supernatants had not peaked. Virus-containing supernatants were aliquotted and frozen at −80°C until needed. The titer of virus in each supernatant was measured by limiting dilution infection of 2-day PHA- and IL-2-stimulated healthy donor PBMC.
Preparation and HIV-1 infection of SCID-hu mice.
SCID-hu thymus/liver mice were created by implantation of human fetal thymus and liver fragments under the kidney capsule of C.B-17 SCID mice as originally described by McCune and colleagues (21). SCID and SCID-hu mice were maintained in microisolator cages on racks with HEPA-filtered air blown into each cage (Allentown Caging, Allentown, Pa.). The mice were implanted with 1-mm3 pieces of human fetal thymus and liver when they were 6 to 8 weeks old. Sixteen- to twenty-four-week gestational age tissue was obtained from Advanced Bioscience Resources (Alameda, Calif.). One piece of fetal thymus and two of fetal liver were inserted under the left kidney capsule of each mouse using a 16-gauge cancer implant needle set (Popper and Sons, New Hyde Park, N.Y.). The grafts were left undisturbed for 4 to 6 months prior to infection with HIV-1. Mice were anesthetized with ketamine and xylazine (8 and 0.8 μg per g of body weight, respectively) injected intraperitoneally prior to all surgical procedures. Methoxyflurane was used if additional anesthesia was necessary, and buprenone or bupivacaine was administered to minimize postoperative discomfort for all surgical procedures. Thymus-liver grafts were exteriorized and measured with a caliper. Only grafts larger than or equal to 0.5 cm in diameter were infected with HIV-1. Freshly titered HIV-1 stocks were diluted in Iscove's medium with 2% fetal calf serum, and 2,000 50% tissue culture infective doses (TCID50) were injected directly into the thymus-liver grafts in a volume of 50 μl. SCID-hu mice were biopsied at 3, 6, 9, and 12 weeks postinfection. For each biopsy, the grafts were again exteriorized, and one quarter to one half of the tissue, depending on the size of the graft, was removed.
Immunohistochemistry.
Thymus-liver tissue derived from SCID-hu mice was frozen in optimal-cutting-temperature compound (Miles Inc., Elkhart, Ind.) on dry ice. Thin sections (5 μm) were cut with a Leica CM3050 S cryostat microtome, deposited onto polylysine-coated slides, and then immediately fixed in cold acetone. Frozen sections on slides were stored at −75°C until needed for immunohistochemical staining. Sections were treated with an avidin-biotin blocking kit (Vector Labs, Burlingame, Calif.) and then incubated with isotype control MAb or with anti-CCR5 or anti-CXCR4 MAb. The staining was developed with a Vectastain Elite ABC kit with 3-amino-9-ethylcarbazole substrate (Vector Labs). Sections were counterstained with hematoxylin and mounted with Crystal/Mount (both from Biomeda Corp., Foster City, Calif.). Slides were viewed with an Olympus BHS microscope, and digital photographs were taken with a Dage-MTI DC-330 camera. The digital images were captured with a Scion 7 video capturing board and processed with Adobe PhotoShop.
Cell preparation.
Thymocytes were prepared from SCID-hu mice bearing human thymus-liver grafts or from pediatric thymus tissue obtained from patients undergoing cardiac surgery. A single-cell suspension was made by mincing the tissue in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Rockville, Md.), with 0.5% bovine serum albumin (BSA; Intergen, Purchase, N.Y.). Cells were strained through nylon mesh, washed twice in phosphate-buffered saline (PBS), and resuspended in IMDM with 0.5% BSA for chemotaxis assay or in PBS with 0.02% NaN3 (PBSA) with 2% fetal bovine serum for flow cytometry. GHOST cells were obtained from the NIH AIDS Research and Reference Reagent Program. L1.2 cells expressing CCR4, CCR5, CCR7, and CXCR4, used as controls in the chemotaxis assays, were provided by D. Chantry and P. Gray of ICOS Corporation and grown in RPMI 1640 with 10% fetal bovine serum and 0.4 mg of geneticin (Life Technologies) per ml. L1.2 cells and L1.2 cells expressing CCR5, CCR8, and CCR8Δ2–4, used for flow cytometric analyses of anti-CCR5 MAb reactivity, were derived and maintained as previously described (35, 37).
Chemotaxis assays.
Thymocytes (100 μl, 107/ml) or L1.2 cells expressing chemokine receptors (100 μl, 106/ml) were placed into 3- or 5-μm-pore-size membrane inserts of 24-well Transwell cell culture chambers (Costar, Cambridge, Mass.). Chemokines in chemotaxis assay medium (600 μl; 0.1 ng/ml to 1 μg/ml) were added to six lower wells of each Transwell plate. Chambers were incubated at 37°C for 1.5 h. The contents of the lower wells were pooled, washed twice with PBS, resuspended in 100 μl of PBSA with 2% fetal bovine serum, and stained with appropriate antibodies. Cell migration was quantified by counting cells gated by low angle and 90° light scatter on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Flow cytometry.
Flow cytometry was used to characterize cell surface expression of CD4, CD8, CCR4, CCR5, CCR7, and CXCR4 on thymocytes derived from pediatric cardiac surgical patients or SCID-hu mice and on GHOST and L1.2 cells. Cells were incubated with MAb for 30 to 60 min on ice and then washed twice with PBSA. Where appropriate, cells were incubated with goat anti-mouse IgG-PE or streptavidin-PE on ice for an additional 30 to 60 min and washed twice with PBSA. Cells were then spun down and resuspended in PBS with 2% formaldehyde. Cells were analyzed using a FACSCalibur flow cytometer and Cellquest software (BDIS). Cell populations analyzed were defined based on their low-angle and 90° light-scattering properties or on low-angle light scatter and exclusion of 7-amino-actinomycin D. Isotype control MAb were used to set markers defining positive reactivity.
RESULTS
Expression of chemokine receptors on human thymocytes.
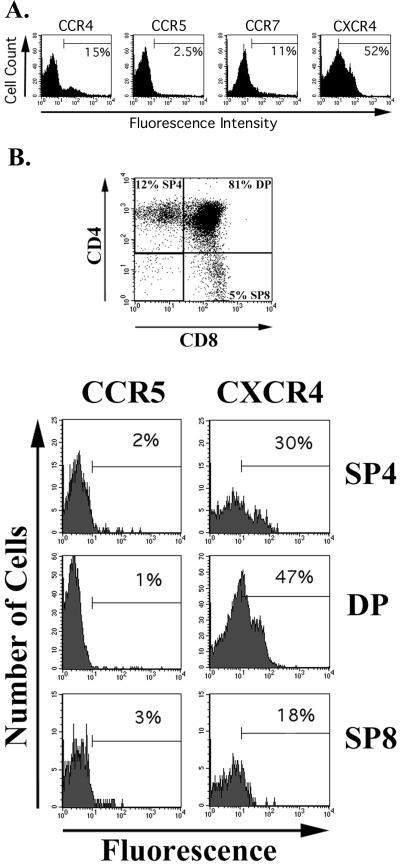
Human thymocytes derived from SCID-hu mice were stained with MAb directed to the chemokine receptors CCR4, CCR5, CCR7, and CXCR4. These experiments were repeated 6 to 21 times, depending on the antibody, using SCID-hu mice created with tissue from nine different donors. Nonthymocytes were excluded from these analyses by stringent gating based on the low angle and 90° light scatter of the cells. Gated cells were greater than 99% CD7-positive thymocytes. An average of 16% of light scatter-gated thymocytes expressed CCR4, while only 2.5% expressed CCR5 detected by MAb 2D7 (Table 1). Similarly, 11% of the cells expressed CCR7, but expression of this receptor exhibited greater variability than that of the other chemokine receptors assayed. In contrast, 53% of human thymocytes derived from SCID-hu mice expressed CXCR4. A representative set of results is shown in Fig. 1A, while the average, standard deviation, and range of all assays are shown in Table 1. Because of the widely divergent reports of CCR5 expression on human thymocytes, we used four additional anti-CCR5 MAb to measure the cell surface expression of CCR5 (2, 7, 19, 24, 38). For each MAb, substantial agreement in the percent and intensity of positively staining cells was observed with the results obtained with MAb 2D7. This was true for MAb 3A9, which stained 3.4% of the thymocytes; MAb 5C7, which stained 4.2% of the cells; MAb 182, which reacted with 2.1%; and MAb 183, which identified 1.7% of human thymocytes as CCR5 positive (Table 1).
TABLE 1.
Chemokine receptor detection by MAb on human thymocytes derived from SCID-hu mice
| Antigen | Antibody | % Positive cells
|
|||
|---|---|---|---|---|---|
| Avg | SD | Range | n | ||
| CCR4 | 328B | 16 | 4 | 11–23 | 8 |
| CCR5 | 2D7 | 2.5 | 1.3 | 0.5–4.3 | 21 |
| CCR5 | 3A9 | 3.4 | 2.2 | 0.4–8 | 12 |
| CCR5 | 5C7 | 4.2 | 2.3 | 1.7–8 | 8 |
| CCR5 | 182 | 2.1 | 0.8 | 1.4–3 | 3 |
| CCR5 | 183 | 1.7 | 0.2 | 1.6–1.9 | 3 |
| CCR7 | 6B3 | 11 | 8 | 3–28 | 11 |
| CXCR4 | 12G5 | 53 | 8 | 46–68 | 6 |
FIG. 1.
Chemokine receptor expression on human thymocytes. (A) Anti-CCR4 MAb 328B, anti-CCR5 MAb 2D7-APC, anti-CCR7 MAb 6B3, anti-CXCR4 MAb 12G5-PE, and isotype control MAb were incubated with freshly isolated cells derived from SCID-hu mice. Cells were washed and resuspended in PBS plus 2% formaldehyde (CCR5 and CXCR4 stains) or incubated with goat anti-mouse IgG-FITC (CCR4 and CCR7 stains) prior to washing and fixation with PBS–2% formaldehyde. Data were collected and analyzed with a FACSCalibur flow cytometer and CellQuest software. Thymocytes were analyzed by excluding other cells based on their low angle and 90° light scatter. Isotype control MAb were used to define the marker denoting positive staining. (B) SCID-hu thymus-liver graft cells were isolated and incubated with CD4-peridinin chlorophyll protein, CD8-FITC, anti-CCR5-APC, anti-CXCR4-PE, or isotype control MAb conjugated to each of the same fluorochromes. The cells were prepared, run, and analyzed as for panel A except that the CD4 and CD8 stains were used to differentiate the anti-CCR5 and anti-CXCR4 immunofluorescence of the major thymocyte subsets.
When the major developmental subsets of thymocytes defined by expression of CD4 and CD8 were analyzed, different patterns of expression were seen for CCR5 and CXCR4 (Fig. 1B). CCR5 was consistently expressed on an equal or slightly greater percentage of mature thymocytes, singly positive for CD4 (SP4) or CD8 (SP8), compared to the predominant immature thymocytes, which are doubly positive for both CD4 and CD8 (DP). In contrast, CXCR4 was detected on the surface of a significantly greater percentage of DP cells than either SP4 or SP8 thymocytes.
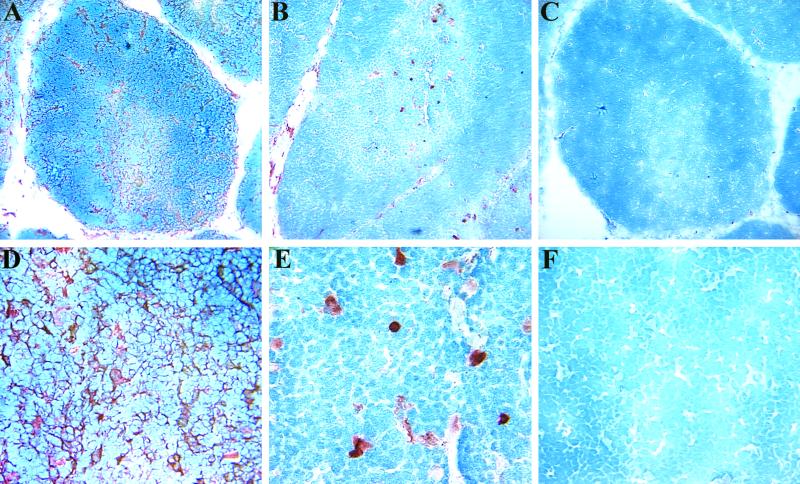
Immunohistochemical analyses of 5-μm frozen sections of human thymus-liver grafts derived from SCID-hu mice gave results that were consistent with our flow cytometric analyses (Fig. 2). We observed positive staining with the anti-CXCR4 MAb 12G5 on a large fraction of thymus-liver graft cells in both the thymic cortex and medulla (Fig. 2A). At higher magnification, CXCR4 was evident on thymocyte processes as well as cell bodies (Fig. 2D). In contrast, CCR5, which was detected with MAb 2D7, was present on few cells in the medulla and fewer still in the cortex of the thymus-liver grafts (Fig. 2B and 2E). Staining with an isotype control MAb yielded very little reactivity, confirming that the CCR5 and CXCR4 reactivity we observed was specific (Fig. 2C and 2F).
FIG. 2.
Immunohistochemical analysis of thymus-liver graft sections. Thin sections (5 μm) were incubated with anti-CXCR4 MAb 12G5 (A and D) or anti-CCR5 MAb 2D7 (B and C) or without primary MAb (C and F). The staining was developed with a Vectastain Elite ABC kit with 3-amino-9-ethylcarbazole substrate. Sections were counterstained with hematoxylin and viewed with an Olympus BHS microscope, and digital photographs were taken with a Dage-MTI DC-330 camera. The overall magnification was X100 for panels A to C and X400 for panels D to F. The digital images were captured with a Scion 7 video capturing board and processed with Adobe PhotoShop.
HIV-1 infection does not induce CCR5 expression on human thymocytes in SCID-hu mice.
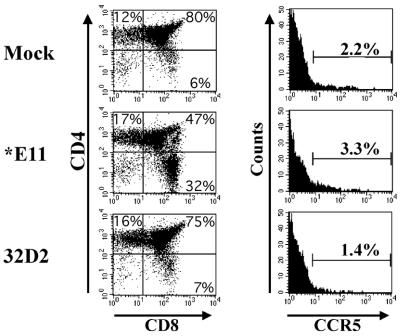
To test the possibility that CCR5 expression might be induced by infection with HIV-1, we infected SCID-hu thymus-liver grafts with two biological clones of HIV-1 derived from patient ACH142, who never developed X4 HIV-1 yet progressed rapidly to AIDS and death. We used 2,000 TCID50 of the pre-AIDS R5 HIV-1 clone ACH142-32D2 or the cytopathic, AIDS-associated R5 clone ACH142-∗E11 (29). Control grafts were mock infected. Six weeks postinfection, grafts were biopsied, incubated with CD4, CD8, and anti-CCR5 MAb, and analyzed by flow cytometry. Both the mock-infected and 32D2-infected grafts had normal SP4, SP8, and DP thymocyte subsets; 75 to 80% of the cells were DP for CD4 and CD8, while the ratio of SP4 to SP8 cells was between 2 and 3 (Fig. 3, upper and lower left panels). These values are within the normal range which we have previously observed for uninfected grafts and also for thymus-liver grafts infected with HIV-1 patient isolates from the early stages of infection (29). In contrast, infection by the R5 AIDS virus ∗E11 caused significant cytopathic effects on DP and SP4 cells in human thymus-liver grafts. The percentage of DP cells was significantly lower, as was the SP4 to SP8 cell ratio (Fig. 3, middle left panel). Our previous work with ∗E11 showed that significant depletion of CD4+ thymocytes was always accompanied by viral replication to greater than 1 copy of HIV-1 DNA for every 8 cells (29). Nevertheless, CCR5 levels were not significantly affected by infection with either HIV-1 clone 32D2 or ∗E11 compared to mock-infected grafts. In each case we detected CCR5 with MAb 2D7 on between 1 and 3.5% of the light scatter-gated thymocytes, which is within the normal range (Fig. 3, right panels).
FIG. 3.
R5 HIV-1 infection does not alter CCR5 expression in SCID-hu thymus-liver grafts. Human thymus-liver grafts in SCID mice were injected with 2,000 TCID50 of the R5-AIDS HIV-1 clone ∗E11 or the R5 pre-AIDS clone 32D2 or mock infected. Six weeks later, the grafts were biopsied, incubated with CD4-PE, CD8-PerCP, and anti-CCR5 (2D7)-APC, and analyzed by flow cytometry as described in the legend to Fig. 1.
Cross-reaction of anti-CCR5 MAb with CCR8.
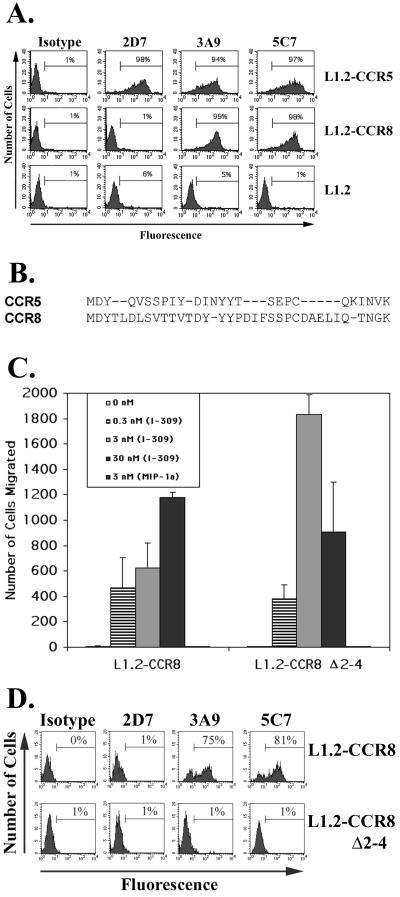
In contrast to the consistent staining of human thymocytes with five anti-CCR5 MAb (2D7, 3A9, 5C7, 182, and 183), we found that on some occasions MAb 3A9 and 5C7 reacted with a much higher percentage of human PBMC than MAb 2D7 (data not shown). To investigate this further, we tested the reactivity of MAb 2D7, 3A9, and 5C7 on a panel of L1.2 cell lines stably expressing CCR4, CCR5, CCR7, CCR8, Bonzo, BOB, or LYGPR. As expected, all three MAb reacted strongly with L1.2-CCR5 cells but not with L1.2 cells expressing CCR4, CCR7, Bonzo, BOB, or LYGPR (not shown). MAb 3A9 and 5C7, but not 2D7, however, reacted strongly with L1.2-CCR8 cells (Fig. 4A). These two MAb reacted with L1.2-CCR8 cells just as strongly as with L1.2-CCR5 cells but did not bind the parental cell line L1.2. Inspection of the predicted amino acid sequences of CCR5 and CCR8 showed that the two receptors have significant homology in their extracellular amino-terminal domains, including the same first three amino acid residues (Fig. 4B). The CCR5 epitopes recognized by 3A9 and 5C7 but not 2D7 have previously been mapped to the amino-terminal domain (36).
FIG. 4.
Cross-reaction of MAb 3A9 and 5C7 with CCR8. (A) L1.2 cells and L1.2 cells stably transfected with either CCR5 or CCR8 were stained with anti-CCR5 MAb. Anti-CCR5 clones 2D7, 3A9, and 5C7 and isotype control MAb were incubated with the three cell lines. Subsequently the cells were washed and incubated with goat anti-mouse IgG-FITC, washed, and analyzed by flow cytometry as described in the legend to Fig. 1. (B) Alignment of the predicted amino-terminal domains of CCR5 and CCR8. (C) Chemotaxis of L1.2-CCR8 and L1.2-CCR8Δ2–4 cells towards I-309 at the indicated concentrations. The assay was performed as described in Materials and Methods. Error bars indicate standard errors of the mean of duplicate wells. (D) L1.2-CCR8 and L1.2-CCR8Δ2–4 cells were stained with anti-CCR5 MAb 2D7, 3A9, and 5C7 and isotype control MAb. Subsequently the cells were washed and incubated with goat anti-mouse IgG-FITC, washed,and analyzed by flow cytometry as described in the legend to Fig. 1.
To further characterize the cross-reactivity of MAb 3A9 and 5C7 with CCR8, we deleted amino acids 2 through 4 of CCR8 to create CCR8Δ2–4. DNA encoding this mutant form of CCR8 was then introduced into L1.2 cells by stable transfection as previously described (35, 37). The resulting L1.2-CCR8Δ2–4 cells were 1.5-fold more active in chemotaxis to I-309, the unique chemokine ligand of CCR8, than L1.2 cells expressing wild-type CCR8 (Fig. 4C). Moreover, maximal chemotaxis of the L1.2-CCR8Δ2–4 cells was observed at a 10-fold-lower concentration of I-309 than for L1.2-CCR8 cells. These results suggest that the L1.2-CCR8Δ2–4 cells expressed an equivalent or slightly greater number of CCR8Δ2–4 molecules on their surface compared to the number of wild-type CCR8 molecules expressed by the L1.2-CCR8 cells. Furthermore, these data show that the CCR8Δ2–4 molecule is a functional chemotactic receptor for I-309. Nevertheless, neither MAb 3A9 nor 5C7 reacted with L1.2-CCR8Δ2–4 cells despite their strong reactivity with L1.2-CCR8 cells (Fig. 4D). Taken together, the data presented in Fig. 4C and 4D show that amino acids 2 through 4 of CCR8, DYT, are necessary for binding by MAb 3A9 and 5C7 but not for binding of the chemokine I-309 or for chemotactic signaling of the receptor.
Chemotaxis of human thymocytes.
Late-stage, AIDS-associated R5 HIV-1 clones (R5-AIDS HIV-1), unlike earlier pre-AIDS R5 HIV-1 isolates from the same patients, replicate to high titer in PHA-stimulated PBMC and in SCID-hu mice (29, 33). Furthermore, R5 AIDS HIV-1 clones are capable of depleting nearly all the CD4+ thymocytes from infected human thymus-liver grafts in SCID-hu mice (29). It is therefore paradoxical that fewer than 5% of thymocytes, on average, reacted with any of the five anti-CCR5 MAb that we used. To explain this apparent paradox, we hypothesized that SCID-hu thymocytes might express low levels of CCR5, below the limit of detection by flow cytometry. To address this hypothesis, we performed chemotaxis assays with SCID-hu-derived thymus-liver graft cells using a variety of chemokines, including MIP-1β, which interacts specifically with CCR5.
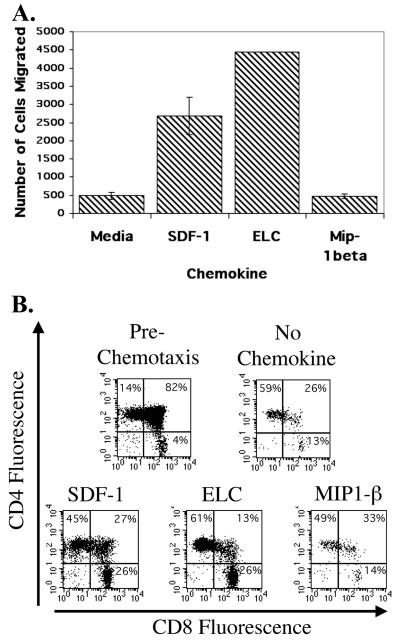
We first determined the optimal concentration of each chemokine in chemotaxis assays with SCID-hu-derived human thymocytes using a range of concentrations of each chemokine. For MIP-1β, RANTES, ELC, and SDF-1, 1 μg/ml gave maximal thymocyte chemotaxis, while for MDC, 0.1 μg/ml elicited the greatest migration (data not shown). These concentrations were used in subsequent experiments to maximize the sensitivity of the assay and to obtain a sufficient number of migrating cells for flow cytometric analysis. The results of a representative chemotaxis assay are shown in Fig. 5A. Significant migration of human thymocytes was seen towards SDF-1 and ELC but not in response to MIP-1β (or RANTES; data not shown). Following chemotaxis, cells were pooled from the bottom chambers of six wells of a 24-well plate, incubated with CD4 and CD8 MAb, and analyzed by flow cytometry. Flow cytometric analysis of the cells pre- and postmigration indicated that mature SP4 and SP8 cells were inherently more mobile than immature CD4-CD8 DP cells (Fig. 5B). This was accentuated by the addition of ELC. In contrast, cells that migrated towards SDF-1 included a greater fraction of immature DP thymocytes than those that migrated towards ELC.
FIG. 5.
(A) Results of a representative chemotaxis assay performed in duplicate with SCID-hu thymus-liver graft cells and the indicated chemokines or medium control. The number of cells which migrated to the bottom chamber of the Transwells is shown. Six separate wells were combined for each group and incubated with CD4-FITC and CD8-PerCP, and the light scatter-gated cells were quantified by flow cytometry. Error bars indicate standard errors of the mean of duplicate groups of six wells. The ELC assay shown is for a single group of six wells due to a technical problem. (B) Two-color dot plots of CD4 and CD8 expression on the cells quantified in panel A which migrated to the indicated chemokines. Prechemotaxis cells were kept on ice and stained at the same time as the cells subjected to the chemotaxis assay.
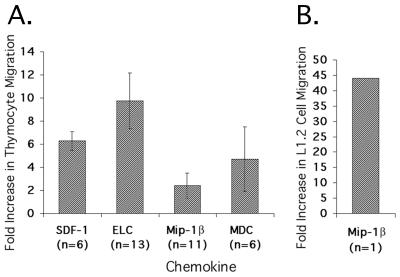
We performed 20 chemotaxis assays with one or more of the chemokines MIP-1β, SDF-1, MDC, and ELC using thymocytes derived from SCID-hu mice created with tissue from nine donors. Figure 6A shows the average fold increase in the number of cells that migrated towards each chemokine compared to cells incubated with medium alone. We saw significant chemotaxis to SDF-1 (P < 0.008), ELC (P < 0.02), and MDC (P < 0.03) but not to MIP-1β (P < 0.4). Concurrent L1.2-CCR5 cell chemotaxis assays with MIP-1β showed positive chemotaxis, indicating that the MIP-1β we used was active and that CCR5 could mediate chemotaxis under the assay conditions used (Fig. 6B). On average, ELC gave the greatest increase in chemotaxis compared to medium alone despite the fact that more thymocytes express CXCR4 than CCR7. In several assays, however, more thymocytes exhibited chemotaxis towards SDF-1 than towards ELC.
FIG. 6.
(A) Average fold increase in migrated SCID-hu thymus-liver graft cells observed in chemotaxis assays with the listed chemokine compared to medium alone. The number of cells that migrated towards each chemokine in each experiment was compared to the number of cells that migrated in the presence of medium alone. The number of chemotaxis assays performed with each chemokine is indicated. Error bars designate the standard error of the mean, and asterisks designate statistically significant results (SDF-1, P < 0.008; ELC, P < 0.02; and MDC, P < 0.03). (B) Fold increase in migrated L1.2-CCR5 cells observed in a chemotaxis assay done in parallel with one of the thymocyte chemotaxis assays shown in A. The number of cells that migrated towards MIP-1β was compared to the number of cells that migrated in the presence of medium alone.
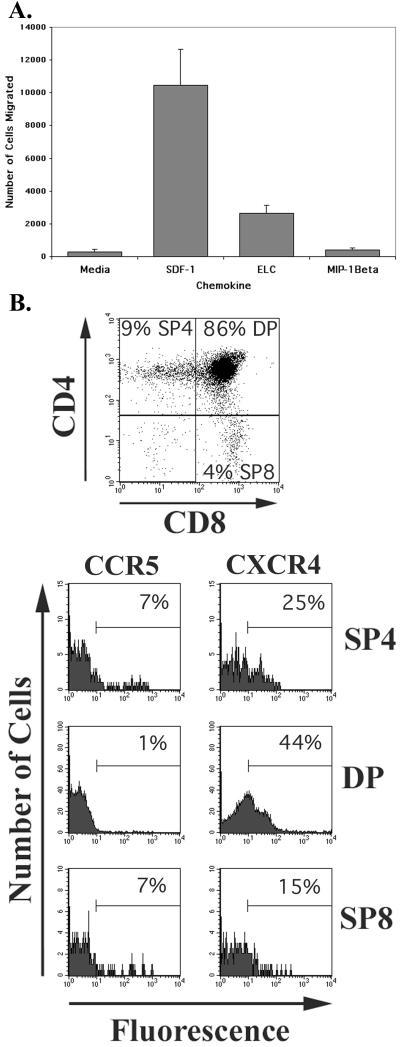
To test the generality of the data that we obtained with thymocytes derived from SCID mice bearing human thymus-liver grafts, we repeated several of the assays described above with thymus tissue obtained from pediatric patients undergoing cardiac surgery at the University of Virginia Medical Center. We found that pediatric thymocytes behaved very similarly in chemotaxis assays with SDF-1, ELC, and MIP-1β (Fig. 7A). Furthermore, the pediatric thymocytes exhibited nearly identical surface expression of CD4, CD8, CCR5, and CXCR4 as SCID-hu mouse-derived thymocytes (Fig. 7B). Moreover, the distribution of CCR5 and CXCR4 expression on the major subsets of thymocytes defined by CD4 and CD8 expression was nearly identical. These results validate our work with SCID-hu-derived thymocytes by showing that they accurately replicate data obtained with pediatric thymocytes.
FIG. 7.
(A) Results of a representative chemotaxis assay performed in duplicate with cells derived from pediatric thymus tissue and the indicated chemokines or medium control. The number of cells that migrated to the bottom chamber of the Transwells is shown. The contents of six separate wells were combined for each group and incubated with CD4-FITC and CD8-PerCP, and the light scatter-gated cells were quantified by flow cytometry. Error bars indicate standard errors of the mean of duplicate groups of six cells. (B) Two-color dot plots of CD4 and CD8 expression and single-color histograms of CCR5 and CXCR4 expression on the cells used in the chemotaxis assay shown in A. The CD4 and CD8 stains were used to differentiate the anti-CCR5 and anti-CXCR4 immunofluorescence of the major thymocyte subsets as in Fig. 1. Staining was performed with cells not used in the chemotaxis assay.
DISCUSSION
Our results show that the chemokine receptors CCR5, CCR4, CCR7, and CXCR4 are expressed on various fractions of human thymocytes. We found that fewer than 5% of the cells expressed CCR5, based on staining with five anti-CCR5 MAb. Furthermore, we did not see evidence for the induction of CCR5 expression by R5 HIV-1 infection. A moderate fraction of SCID-hu-derived human thymocytes expressed CCR4 (16%) and CCR7 (11%), while a large fraction expressed CXCR4 (53%). The fraction of cells expressing CCR7 was the most variable among the receptors tested, ranging from 3 to 28%. Immunohistochemical analysis of frozen thymus-liver graft sections using anti-CCR5 MAb 2D7 and anti-CXCR4 MAb 12G5 gave results which were consistent with the percentage of positive cells that we measured by flow cytometry.
We found that two widely used CCR5 MAb, 3A9 and 5C7, cross-react with CCR8. This may explain discrepancies among previous reports regarding the fraction of thymocytes that expressed CCR5. In a recent report, CCR8 mRNA was detected nonquantitatively in all thymocyte subsets delineated by CD4 and CD8, while CCR8 protein was detected by the binding of [125I]-I-309 on DP, SP4, and doubly negative cells (20). Moreover, we showed that amino acids 2 to 4 of CCR8 were necessary for binding 3A9 and 5C7. We found, however, that the five anti-CCR5 MAb tested (2D7, 3A9, 5C7, 182, and 183) gave consistent results in assays on thymus-liver grafts derived from multiple donors. Nevertheless, we occasionally found that 3A9 and 5C7 reacted with a much greater proportion of PBMC than did 2D7. This may be explained by variable sulfation of the amino termini of CCR5 and CCR8, which has been described (10). Both 3A9 and 5C7 consistently reacted with CCR5 and CCR8 on transfected cells. Hill and colleagues have previously reported similar discrepancies between the reactivity of several anti-CCR5 MAb on primary and transfected cells (14). Taken together, these results suggest that reactivity with the anti-CCR5 MAb 2D7 is the most reliable indicator of CCR5 expression on primary cells. Furthermore, these data suggest that the reactivity of MAb directed to the amino-terminal domain of CCR5 may be an unreliable indicator of CCR5 expression on primary cells for two reasons: they may cross-react with CCR8, and their reaction with both CCR5 and CCR8 may be affected by sulfation of tyrosine residues in the amino-terminal domains of both proteins.
Overall, migration of thymocytes to the chemokines that we tested correlated with detection of the corresponding chemokine receptor by flow cytometry. Thymocytes were responsive to SDF-1, MDC, and ELC but not to MIP-1β in chemotaxis assays. Moreover, these results are consistent with other reports in the literature of thymocyte chemotaxis in response to SDF-1, MDC, and ELC and their lack of statistically significant chemotaxis to MIP-1β (1, 5, 39). Our results, however, are not consistent with one report of significant thymocyte chemotaxis towards MIP-1β (7). The explanation for this discrepancy is not obvious; however, many factors may be involved, including the thymocyte isolation procedure used. This group used CD14 MAb and paramagnetic beads to remove monocytes-macrophages and multiple Ficoll-Hypaque step gradient centrifugations to remove erythrocytes. One or both of these steps, which were not used by us or other groups, may have contributed to thymocyte activation, which in turn may have potentiated MIP-1β-mediated chemotaxis.
CCR5, which is the only known MIP-1β receptor, was detected on far fewer thymocytes than receptors for the other chemokines (11). The fraction of cells expressing CXCR4, CCR4, and CCR7, the unique receptors for SDF-1, MDC, and ELC, respectively, however, did not correlate strictly with the extent of the thymocyte chemotactic response to each chemokine. This indicates that other factors, such as the nature of the signals transmitted by each receptor, may contribute to chemotaxis. Mature SP thymocytes were more mobile than immature DP thymocytes in these assays, with or without added chemokine. We conclude that CCR5 is likely expressed on fewer than 5% of human thymocytes and is not likely to be involved in the migration of a large subpopulation of thymocytes. In contrast, the chemokine receptors CXCR4, CCR4, and CCR7 are expressed on larger subsets of thymocytes and can mediate significant chemotaxis. These receptors and their corresponding chemokine ligands may therefore be involved in the movement of thymocytes during their development.
Our data do not, however, readily explain our previous finding that R5-AIDS HIV-1 clones are capable of depleting nearly all CD4+ thymocytes from infected SCID-hu thymus-liver grafts (30). These results could be reconciled if the cytopathic effects of R5 HIV-1 infection in the thymus resulted from indirect killing of CCR5− cells following infection of CCR5+ cells. Alternatively, the restriction of R5-AIDS HIV-1 clones to infect only cells expressing CCR5 documented in tissue culture may not be absolute in thymus-liver graft tissue. Finally, it is possible that all thymocytes go through a stage of development during which they express CCR5. If this were true, then all thymocytes would be susceptible to the cytopathic effects of R5-AIDS HIV-1 clones despite the fact that at any given time fewer than 5% of human thymocytes express CCR5.
ACKNOWLEDGMENTS
The first two authors, James R. Taylor, Jr., and Katherine Kimbrell, contributed equally to this work.
We thank Erin Tobias for cutting frozen tissue sections, Colin de Bakker for advice on immunohistochemistry, and David Chantry of ICOS Corporation for instruction in the chemotaxis assay and helpful discussions. We thank Irving Kron, G. Randall Green, and Jeffrey Cope of the University of Virginia Department of Surgery for providing pediatric thymus tissue. We also thank Victoria Camerini for help with immunohistochemistry and for helpful discussions and review of the manuscript.
This work was supported by NIH grants R29AI39943 and R01AI47729 to D.C. and by Millennium Pharmaceuticals. R.S. was supported by University of Virginia Infectious Diseases training grant T32AI07046, and K.C.K. was supported by an Elizabeth Glaser Pediatric AIDS Foundation Student Intern Award.
REFERENCES
- 1.Annunziato F, Romagnani P, Cosmi L, Beltrame C, Steiner B H, Lazzeri E, Raport C J, Galli G, Manetti R, Mavilia C, Vanini V, Chantry D, Maggi E, Romagnani S. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J Immunol. 2000;165:238–246. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camerini D, Su H P, Gamez-Torre G, Johnson M L, Zack J A, Chen I S. Human immunodeficiency virus type 1 pathogenesis in SCID-hu mice correlates with syncytium-inducing phenotype and viral replication. J Virol. 2000;74:3196–3204. doi: 10.1128/jvi.74.7.3196-3204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell J J, Pan J, Butcher E C. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163:2353–2357. [PubMed] [Google Scholar]
- 6.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dairaghi D J, Franz-Bacon K, Callas E, Cupp J, Schall T J, Tamraz S A, Boehme S A, Taylor N, Bacon K B. Macrophage inflammatory protein-1β induces migration and activation of human thymocytes. Blood. 1998;91:2905–2913. [PubMed] [Google Scholar]
- 8.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 9.Eugen-Olsen J, Iversen A K, Garred P, Koppelhus U, Pedersen C, Benfield T L, Sorensen A M, Katzenstein T, Dickmeiss E, Gerstoft J, Skinhoj P, Svejgaard A, Nielsen J O, Hofmann B. Heterozygosity for a deletion in the CKR-5 gene leads to prolonged AIDS-free survival and slower CD4 T-cell decline in a cohort of HIV-seropositive individuals. AIDS. 1997;11:305–310. doi: 10.1097/00002030-199703110-00007. [DOI] [PubMed] [Google Scholar]
- 10.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 11.Garlisi C G, Xiao H, Tian F, Hedrick J A, Billah M M, Egan R W, Umland S P. The assignment of chemokine-chemokine receptor pairs: TARC and MIP-1 beta are not ligands for human CC-chemokine receptor 8. Eur J Immunol. 1999;29:3210–3215. doi: 10.1002/(SICI)1521-4141(199910)29:10<3210::AID-IMMU3210>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Glushakova S, Grivel J C, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis L B. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa H, Nomura T, Kohno M, Tateishi N, Suzuki Y, Maeda N, Fujisawa R, Yoshie O, Fujita S. Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood. 2000;95:30–38. [PubMed] [Google Scholar]
- 14.Hill C M, Kwon D, Jones M, Davis C B, Marmon S, Daugherty B L, DeMartino J A, Springer M S, Unutmaz D, Littman D R. The amino terminus of human CCR5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology. 1998;248:357–371. doi: 10.1006/viro.1998.9283. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, Jackson R, Ostrowski M, Hoxie J, Offord R, Fauci A S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4+ T cells: role of signal transduction. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchen S G, Zack J A. Distribution of the human immunodeficiency virus coreceptors CXCR4 and CCR5 in fetal lymphoid organs: implications for pathogenesis in utero. AIDS Res Hum Retroviruses. 1999;15:143–148. doi: 10.1089/088922299311565. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Tiffany H L, King L, Murphy P M, Golding H, Zaitseva M B. CCR8 on human thymocytes functions as a human immunodeficiency virus type 1 coreceptor. J Virol. 2000;74:6946–6952. doi: 10.1128/jvi.74.15.6946-6952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locati M, Murphy P M. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–440. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 22.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 23.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski M A, Justement S J, Catanzaro A, Hallahan C A, Ehler L A, Mizell S B, Kumar P N, Mican J A, Chun T W, Fauci A S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 25.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picchio G R, Gulizia R J, Wehrly K, Chesebro B, Mosier D E. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappaport J, Cho Y Y, Hendel H, Schwartz E J, Schachter F, Zagury J F. 32 bp CCR-5 gene deletion and resistance to fast progression in HIV-1 infected heterozygotes. Lancet. 1997;349:922–923. doi: 10.1016/S0140-6736(05)62697-9. [DOI] [PubMed] [Google Scholar]
- 28.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 29.Schramm B, Penn M L, Speck R F, Chan S Y, De Clercq E, Schols D, Connor R I, Goldsmith M A. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J Virol. 2000;74:184–192. doi: 10.1128/jvi.74.1.184-192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scoggins R M, Taylor J R, Jr, Patrie J, van't Wout A B, Schuitemaker H, Camerini D. Pathogenesis of primary R5 human immunodeficiency virus type 1 clones in SCID-hu mice. J Virol. 2000;74:3205–3216. doi: 10.1128/jvi.74.7.3205-3216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su L, Kaneshima H, Bonyhadi M L, Lee R, Auten J, Wolf A, Du B, Rabin L, Hahn B H, Terwilliger E, McCune J M. Identification of HIV-1 determinants for replication in vivo. Virology. 1997;227:45–52. doi: 10.1006/viro.1996.8338. [DOI] [PubMed] [Google Scholar]
- 32.Tersmette M, Goede R E Y D, Al B J M B, Winkel I M, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 34.van't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vicenzi E, Bordignon P P, Biswas P, Brambilla A, Bovolenta C, Cota M, Sinigaglia F, Poli G. Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4+ T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation. J Virol. 1999;73:7515–7523. doi: 10.1128/jvi.73.9.7515-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, Mackay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1 in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]