Abstract
Sarcopenia, defined as age-associated loss of skeletal muscle function and muscle mass, is a negative prognostic marker for survival in several tumor entities. However, data evaluating the impact of sarcopenia and fat distribution on penile cancer are rarely described. We performed a retrospective study including 38 patients who were diagnosed with squamous cell carcinoma of the penis. By measuring skeletal muscle mass and fat distribution at axial abdominal computed tomography images at the third lumbar vertebra several body composition parameters including skeletal muscle index (SMI), psoas muscle index (PMI), visceral obesity and visceral-to-subcutaneous fat ratio were determined. Among 38 patients, 26% (n = 10) of the patients with penile cancer were identified as sarcopenic. SMI, age, lymph node metastases, distant metastases and penile cancer of the shaft were identified as significant risk factors for overall survival. PMI and distant metastases were significantly associated with cancer specific survival. None of the analysed adipose tissue parameters could be identified as risk factors for survival in this study. We showed that sarcopenia occurs in a relevant part of patients with penile cancer and is a significant risk factor for overall survival (p = 0.032) and cancer specific survival (p = 0.034) for patients with penile cancer. Regarding fat distribution further studies are needed to evaluate its impact on sarcopenia and survival.
Keywords: Squamous carcinoma, Body composition, Skeletal muscle index, Psoas muscle index, Visceral obesity
Subject terms: Oncology, Urology
Introduction
Penile cancer is a rare disease with a worldwide incidence of 0.8 per 100.000 person-years but shows wide variation across the globe. Although incidence in Europe is low, it has been steadily increasing over the past few decades1. Increased age of the population, decreasing rates of circumcision in children and the increase in human papillomavirus prevalence are discussed as reasons for the increased risk of penile cancer1,2. Along with increasing incidence rates, the survival of patients with penile cancer is simultaneously decreasing3. However, due to the rarity of penile cancer there are only a few studies addressing this effect.
Sarcopenia, defined as age-associated loss of skeletal muscle function and muscle mass, is well recognized as a prognostic marker for survival in cancer patients4. For several tumor entities, including urological malignancies, sarcopenia has been investigated as a negative prognostic factor for postoperative complications and survival5–9. For penile cancer only two studies evaluating the impact of sarcopenia on survival and postoperative complications have been published10,11. The most common parameters to calculate sarcopenia are SMI and PMI12–16. In addition to sarcopenia, several other body composition parameters are being studied, that may have an impact on the prognosis of cancer patients5,17,18. They mostly relate to body fat distribution, such as visceral obesity (VO), visceral fat index (VFI), subcutaneous fat index (SFI) and visceral-to-subcutaneous fat ratio (VSR)19–21. Previous studies showed that fat distribution in favor of visceral fat is associated with several medical disorders and malignancies including prostate, breast, hepatocellular and colorectal cancer and affects survival21,22.
To the best of our knowledge, studies investigating the impact of muscle mass and body fat distribution parameters on the survival of penile cancer are lacking. Therefore, we conducted this study to examine associations of body composition parameters obtained from computed tomography (CT) images of patients with penile cancer.
Patients and methods
Patients
Ethical approval was granted by the institutional ethics committee of the university Regensburg (approval number: 21-2420-104) and the conducted research was performed in accordance with the relevant regulations and guidelines. No informed consent was obtained from the human participants, as the need for informed consent was waived by the ethics committee of the university Regensburg (Art. 27 (4) of the Bavarian Hospital Act).
We retrospectively reviewed patients who underwent surgical treatment due to penile cancer from 01.01.2010 to 31.12.2020 at our institution. In 68 patients squamous cell carcinoma of the penis was histologically verified. Imaging was performed by CT scan of the abdomen in 49 patients and by magnetic resonance imaging (MRI) in 3 patients. Staging wasn’t performed in 16 patients and in 11 patients staging was performed but was not available as digital images. Finally, 38 patients (56%) with available digital CT images and squamous cell carcinoma of the penis were included in the study.
Patient demographic data and comorbidities were collected from in-hospital medical records, including age, body mass index (BMI), American Society of Anesthesiologists physical status classification system (ASA), Charlson comorbidity index (CCI), alcohol abuse, insurance status, tumour localization, the presence of phimosis, smoking status, diabetes mellitus and renal function. Information about surgery, especially the type of resection, was also recorded. Moreover, we collected data about lymph node dissection. In this study collective modified and radical inguinal lymph node dissection and pelvic lymph node dissection were performed. In addition, we reviewed histopathological data including TNM classification.
Follow-up data was collected from our outpatient department by reviewing the medical records. The data collected included follow-up date, date of death, and cause of death. Overall survival (OS) was defined as time of diagnosis to death, irrespective of the cause of death. Cancer-specific survival (CSS) was defined as time from diagnosis to death, in case the event occurred due to the underlying penile cancer.
CT image analysis and body composition measurements
Axial abdominal CT images at the level of the third lumbar vertebra (L3) were used to determine body composition parameters as previously described5. Measurements were performed using Osirix DICOM Viewer software (OsiriX MD version 13.0.0, Pixmeo, Geneva, Switzerland). The “Grow Region (2D/3D Segmentation)” tool was used to automatically select the required tissue in one axial image. If necessary, the selected area was corrected manually. The measurements were performed at two continuous axial CT images on which both vertebral spines were visible and the average was calculated.
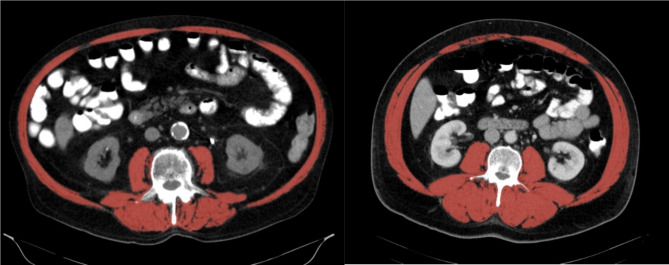
The skeletal muscles in the L3 region include psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques and rectus abdominis (Fig. 1). To differentiate the skeletal muscles from other tissue, a threshold range of Hounsfield units (HU) of -29 to + 150 was used23. The cross-sectional skeletal muscle surface (cm2) was normalized for height in meters squared (m2) to obtain the skeletal muscle index (SMI) (cm2/m2). Sarcopenia was defined as previously described by Martin et al. (males SMI ≤ 43 cm2/m2 if BMI < 25 and ≤ 53 cm2/m2 for all other BMI values)12.
Fig. 1.
Illustration of axial CT images at the third lumbar vertebra with highlighting of the skeletal muscles by selecting the appropriate HU. Left illustration shows a sarcopenic patient (SMI 44,3 cm2/m2), right illustration shows a non-sarcopenic patient (SMI 81,4 cm2/m2).
Psoas muscle index (PMI) (cm2/m2) was computed by normalizing the cross-sectional psoas muscle surface (cm2) for height in meters squared (m2)15. Due to the absence of suitable cut-off-parameters for PMI the median was calculated and the cohort was divided into high and low PMI.
Measurements of subcutaneous and visceral fat tissue were also performed at the level of the third lumbar vertebra as described above. Fat tissue was separated from other tissues by a Hounsfield unit threshold range of − 150 to − 50. Visceral adipose tissue (cm2) and subcutaneous adipose tissue (cm2) were normalized for height in meters squared (m2), resulting in VFI (cm2/m2) and SFI (cm2/m2). VSR was calculated by dividing visceral fat area by subcutaneous fat area23. VO was defined as visceral fat area greater than 163.8 cm220.
Since there are no standardized cut-off parameters for VFI, SFI and VSR the continuous values were used for further calculations. To be able to perform survival analysis for VSR, the cut-off for VSR was used as described by Engelmann et al. (males VSR > 1.421)5.
Statistical analysis
Statistical analysis was performed using SPSS software version 28.0 (IBM, Armonk, NY, USA). Graphs were created using the software GraphPad Prism version 10.2.0 (GraphPad, San Diego, CA, USA) for Windows. Descriptive statistics are reported as median with interquartile range (IQR) or absolute numbers with percentages. Differences between the sarcopenic and non-sarcopenic group were analyzed using the chi-square test for dichotomous parameters and the Wilcoxon-Mann–Whitney U test for non-normally distributed data. Univariable Cox regression analyses were used to identify significant prognostic factors of OS and CSS. Kaplan-Meier curves were used to illustrate OS and CSS. P-values < 0.05 were considered statistically significant. All analyses were considered two-tailed.
Results
Descriptive data
The cohort in this study comprised 38 men with penile cancer with a median age of 64 years (IQR 56–72) at diagnosis. Eighteen (47%) patients had been diagnosed with phimosis. The surgical treatment for penile cancer included biopsy (2.6%), laser ablation (7.9%), circumcision (7.9%), partial penectomy (60.5%) and total penectomy (18.4%). Inguinal lymph node dissection (ILND) was performed in 26 (68.4%) patients. Regarding the site of penile cancer, 23 (60.5%) cases were located at the glans, 8 (21.1%) at the shaft, 2 (5.3%) at the foreskin and 5 (13.2%) cases were located multilocular. All cases of penile cancer histologically corresponded to squamous cell type and were classified as follows: 10.5% Carcinoma in situ, 36.8% T1, 26.3% T2 and 26.3% T3. Grading, based on the world health organization (WHO) classification, was described as follows: G1 8.6%, G2 51.4% and G3 40%. Lymph node metastases were detected in 17 (44.7%) cases, which were classified as follows: N1 15.8%, N2 18.4% and N3 10.5%. Distant metastases were detected in 3 (7.9%) cases. Ten patients underwent systemic chemotherapy (26.3%). The median BMI was 28 kg/m2 (IQR 24.6–31.6), which is classified as overweight. Further descriptive data is shown in Table 1.
Table 1.
Patient characteristics of the entire cohort, sarcopenic patients and non-sarcopenic patients.
| Characteristics | Entire Cohort | Sarcopenic | Non-sarcopenic | p-Value |
|---|---|---|---|---|
| n = 38 (%) | n = 10 (26.3%) | n = 28 (73.7%) | ||
| Median age at diagnosis (IQR) | 64.5 (56.3–72.3) | 76.5 (70.3–80.5) | 59.5 (52.3–70) | < 0.001 |
| Median SMI (IQR) | 56.4 (49.2–65.6) | 48 (42.9–50.2) | 60.7 (55.3–67) | < 0.001 |
| Median PMI (IQR) | 7.4 (6.2–8.4) | 6.9 (6.1–7.6) | 7.7 (6.6-9) | 0.127 |
| Low PMI, n (%) | 20 (52.6%) | 7 (70%) | 13 (46.4%) | 0.200 |
| Median VSR (IQR) | 1.1 (0.7–1.6) | 1.5 (1.2–2.1) | 1 (0.6–1.4) | 0.022 |
| High VSR, n (%) | 12 (31.6%) | 6 (60%) | 6 (21.4%) | 0.024 |
| Median VFI (IQR) | 76 (42.6–97.4) | 86.6 (66.6–99) | 70.6 (39.6–96.3) | 0.220 |
| Median SFI (IQR) | 59.4 (41.2–76.3) | 60.6 (40.4–82.8) | 55.4 (40.5–76.7) | 0.791 |
| High VO, n (%) | 27 (71.1%) | 9 (90%) | 18 (64.3%) | 0.124 |
| pT-stage, n (%) | ||||
| pCis | 4 (10.5%) | 0 (0%) | 4 (14.3%) | 0.513 |
| pT1 | 14 (36.8%) | 3 (30%) | 10 (35.7%) | |
| pT2 | 10 (26.3%) | 3 (30%) | 7 (24.9%) | |
| pT3 | 10 (26.3%) | 4 (40%) | 6 (21.4%) | |
| pN-stage, n (%) | ||||
| pN0 | 21 (55.3%) | 4 (40%) | 17 (60.7%) | 0.593 |
| pN1 | 6 (15.8%) | 2 (20%) | 4 (14.3%) | |
| pN2 | 7 (18.4%) | 2 (20%) | 5 (17.9%) | |
| pN3 | 4 (10.5%) | 2 (20%) | 2 (7.1%) | |
| cM-stage, n (%) | 3 (7.9%) | 0 (0%) | 3 (10.7%) | 0.281 |
| Median BMI (IQR) | 27.9 (24.6–31.6) | 27.8 (25.2–30.3) | 28.6 (24.5–31.7) | 0.740 |
| BMI, n (%) | ||||
| Underweight (< 18.5) | 0 (0%) | 0 (0%) | 0 (0%) | 0.181 |
| Normal (18.5–24.9) | 11 (28.9%) | 2 (20%) | 9 (32.1%) | |
| Overweight (25–29.9) | 14 (36.8%) | 6 (60%) | 8 (28.6%) | |
| Obese (30–34.9) | 13 (43.2%) | 2 (20%) | 11 (39.3%) | |
| Phimosis, n (%) | 18 (47.3%) | 6 (60%) | 12 (42.8%) | 0.351 |
| ASA-Score, n (%) | ||||
| 1 | 6 (15.8%) | 2 (20%) | 4 (14.3%) | 0.277 |
| 2 | 19 (49.9%) | 3 (30%) | 16 (57.1%) | |
| 3 | 13 (34.2%) | 5 (50%) | 8 (28.6%) | |
| Smoker, n (%) | 12 (31.6%) | 3 (30%) | 9 (32.1%) | 0.900 |
| Cancer localization, n (%) | ||||
| Foreskin | 2 (5.3%) | 0 (0%) | 2 (7.14%) | 0.156 |
| Glans | 23 (60.5%) | 4 (40%) | 19 (67.9%) | |
| Shaft | 8 (21.1%) | 4 (40%) | 4 (14.3%) | |
| Multilocular | 5 (13.2%) | 2 (20%) | 3 (10.7%) | |
| Diabetes mellitus, n (%) | 7 (18.4%) | 2 (20%) | 5 (17.9%) | 0.881 |
| Private insurance | 11 (28.9%) | 3 (30%) | 8 (28.6%) | 0.932 |
| Alcohol abuse | 4 (10.4%) | 2 (20%) | 2 (7.1%) | 0.255 |
| Chemotherapy, n (%) | 10 (26.3%) | 8 (80%) | 2 (7.14%) | 0.882 |
| CCI | ||||
| 0 | 4 (10.5%) | 0 (0%) | 4 (14.3%) | 0.206 |
| 1–2 | 13 (34.2%) | 1 (10%) | 12 (42.8%) | 0.060 |
| 3–4 | 14 (36.8%) | 5 (50%) | 9 (32.1%) | 0.315 |
| ≥ 5 | 7 (18.4%) | 4 (40%) | 3 (10.7%) | 0.040 |
| LND | ||||
| None | 12 (13.6%) | 2 (20%) | 10 (35.7%) | 0.359 |
| Modified | 11 (28.9%) | 2 (20%) | 9 (32.1%) | 0.467 |
| Radical | 5 (13.2%) | 1 (10%) | 4 (14.3%) | 0.731 |
| Pelvin | 10 (26.3%) | 5 (50%) | 5 (17.9%) | 0.048 |
n = count of patients (percentage); IQR, interquartile range; SMI, skeletal muscle index; PMI, psoas muscle index; VSR, visceral-to-subcutaneous fat ratio; VFI, visceral fat index; SFI, subcutaneous fat index; VO, visceral obesity; pT-stage, pathological Tumor stage; pN-stage, pathological nodal classification; cM-stage, clinical metastases classification; BMI, body mass index; ASA, American Society of Anesthesiologists. CCI, Charlson comorbidity index; LND, lymph node dissection; p < 0.05 is considered statistically significant.
Impact of sarcopenia on demographic and tumor characteristics
The median SMI was 56.4 cm2/m2 (IQR 49.2–65.6 cm2/m2). Ten patients (26.3%) were classified as sarcopenic. With a median age of 76.5 years (IQR 70.3–80.5) vs. 59.5 years (IQR 52.3–70.0) (p < 0.001), those patients were significantly older. Furthermore, significantly more sarcopenic patients were classified with a high VSR (60% vs. 21%, p = 0.024). No significant differences were found between both groups regarding BMI, ASA, VFI, SFI, VO, tumour localization, TNM classification, lymph node metastases, distant metastases, presence of phimosis, smoking status, diabetes mellitus and renal function (Table 1).
There was no difference in terms of t-stage between patients who received staging and those who did not and therefore had to be excluded from the study (p = 0.731).
Survival analysis
The median observation time was 47 months (IQR 14.3–73.4). At the time of analysis 27 (71%) patients were alive and 11 (29%) patients had died. In univariate cox regression analysis age, sarcopenia (SMI), lymph node metastases, distant metastases and penile cancer of the shaft were significantly associated with decreased OS (Table 2). The continuous parameters SMI (cm2/m2) and PMI (cm2/m2) showed a tendency towards statistical significance (p = 0.065 and p = 0.067, respectively) concerning OS.
Table 2.
Univariate Cox Regression analysis for overall survival in patients with penile cancer.
| Characteristics | HR | 95%-CI | p-Value |
|---|---|---|---|
| Age at diagnosis | 1.09 | 1.02–1.17 | 0.011 |
| Presence of sarcopenia (SMI) | 3.54 | 1.02–12.3 | 0.046 |
| Presence of sarcopenia (PMI) | 2.3 | 0.59–8.93 | 0.227 |
| SMI (continous) | 0.93 | 0.86-1.00 | 0.065 |
| PMI (continous) | 0.67 | 0.43–1.03 | 0.067 |
| VSR (continous) | 1.83 | 0.51–6.48 | 0.350 |
| High VSR | 2.43 | 0.70–8.39 | 0.161 |
| VFI | 0.99 | 0.98–1.01 | 0.386 |
| SFI | 0.98 | 0.95–1.01 | 0.109 |
| VO | 0.95 | 0.25–3.67 | 0.938 |
| pT-stage | |||
| pTis | 0.41 | 0-18.12 | 0.455 |
| pT1 | 1.05 | 0.30–3.74 | 0.935 |
| pT2 | 0.67 | 0.14–3.14 | 0.608 |
| pT3 | 2.36 | 0.66–8.37 | 0.186 |
| pN-stage | 6.55 | 1.38-31 | 0.018 |
| cM-stage | 7.41 | 1.83–29.92 | 0.005 |
| BMI | 1.11 | 0.97–1.26 | 0.132 |
| BMI categorized | |||
| Normal | 0.58 | 0.12–2.73 | 0.490 |
| Overweight | 0.65 | 0.17–2.52 | 0.533 |
| Obese | 2.23 | 0.67–8.03 | 0.184 |
| Phimosis | 1.84 | 0.52–6.52 | 0.347 |
| ASA-Score | |||
| 1 | 1.20 | 0.26–5.65 | 0.818 |
| 2 | 0.41 | 0.11–1.59 | 0.197 |
| 3 | 2.16 | 0.63–7.48 | 0.224 |
| Smoker | 0.80 | 0.21–3.10 | 0.749 |
| Localization | |||
| Foreskin | 0.45 | 0.00-4173.15 | 0.595 |
| Glans | 0.53 | 0.15–1.84 | 0.320 |
| Shaft | 3.63 | 1.02-13.00 | 0.047 |
| Multilocular | 0.75 | 0.10–5.90 | 0.781 |
| Diabetes mellitus | 1.13 | 0.24–5.33 | 0.877 |
| Private insurance | 0.57 | 0.11–2.67 | 0.472 |
| Alcohol abuse | 0.79 | 0.10–6.28 | 0.827 |
| Median Creatinine (mg/dl) (IQR) | 0.80 | 0.21–3.10 | 0.749 |
| Chemotherapy | 1.89 | 0.48–7.34 | 0.380 |
| CCI | |||
| 0 | 0.89 | 0.11–7.03 | 0.911 |
| 1–2 | 0.46 | 0.1–2.17 | 0.328 |
| 3–4 | 2.94 | 0.82–10.42 | 0.096 |
| ≥ 5 | 0.45 | 0.06–3.53 | 0.445 |
| LND | |||
| None | 0.5 | 0.11–2.37 | 0.385 |
| Modified | 0.6 | 0.13–2.83 | 0.518 |
| Radical | 0.81 | 0.1–6.36 | 0.837 |
| Pelvin | 2.96 | 0.86–10.26 | 0.086 |
HR, hazard ratio; CI, confidence interval; SMI, skeletal muscle index; PMI, psoas muscle index; VSR, visceral-to-subcutaneous fat ratio; VFI, visceral fat index; SFI, subcutaneous fat index; VO, visceral obesity; pT-stage, pathological Tumor stage; pN-stage, pathological nodal classification; cM-stage, clinical metastases classification; BMI, body mass index; ASA, American Society of Anesthesiologists. CCI, Charlson comorbidity index; LND, lymph node dissection; p < 0.05 is considered statistically significant.
Moreover, continuous PMI (cm2/m2) and distant metastases were significantly associated with CSS. For low PMI, age and penile cancer of the shaft univariate analysis revealed a trend towards statistical significance (p = 0.069 and p = 0.096, respectively) concerning CSS (Table 3).
Table 3.
Univariate Cox Regression analysis for cancer specific survival in patients with penile cancer.
| Characteristics | HR | 95%-CI | p-Value |
|---|---|---|---|
| Age at diagnosis | 1.07 | 0.997–1.15 | 0.061 |
| Presence of sarcopenia (SMI) | 2.21 | 0.52–9.28 | 0.278 |
| Presence of sarcopenia (PMI) | 7 | 0.86–57.09 | 0.069 |
| SMI (continous) | 0.95 | 0.88–1.03 | 0.220 |
| PMI (continous) | 0.57 | 0.34–0.96 | 0.037 |
| VSR (continous) | 1.23 | 0.31–4.91 | 0.773 |
| High VSR | 1.48 | 0.35–6.19 | 0.593 |
| VFI | 0.99 | 0.97–1.01 | 0.443 |
| SFI | 0.98 | 0.96-1.0 | 0.249 |
| VO | 0.67 | 0.16–2.82 | 0.589 |
| pT-stage | |||
| pTis | 0.04 | 0.00-453.3 | 0.500 |
| pT1 | 0.95 | 0.23–3.98 | 0.945 |
| pT2 | 0.88 | 0.18–4.36 | 0.875 |
| pT3 | 2.16 | 0.51 − 0.06 | 0.293 |
| pN-stage | 125.39 | 0.29-53405.57 | 0.118 |
| cM-stage | 11.47 | 2.53–52.05 | 0.002 |
| BMI | 1.1 | 0.94–1.27 | 0.228 |
| BMI cat. | |||
| Normal | 0.76 | 0.15–3.79 | 0.742 |
| Overweight | 0.50 | 0.10–2.49 | 0.399 |
| Obese | 2.37 | 0.59–9.47 | 0.224 |
| Phimosis | 2.06 | 0.49–8.46 | 0.324 |
| ASA-Score | |||
| 1 | 1.59 | 0.32–7.86 | 0.573 |
| 2 | 0.32 | 0.06–1.57 | 0.148 |
| 3 | 2.20 | 0.55–8.83 | 0.266 |
| Smoker | 1.11 | 0.26–4.63 | 0.891 |
| Localization | |||
| Foreskin | 0.05 | 0.00-13975.82 | 0.631 |
| Glans | 0.53 | 0.13–2.11 | 0.365 |
| Shaft | 3.4 | 0.80-14.37 | 0.096 |
| Multilocular | 0.95 | 0.12–7.72 | 0.961 |
| DM | 1.51 | 0.30–7.47 | 0.617 |
| Private insurance | 0.742 | 0.15–3.68 | 0.715 |
| Alcohol abuse | 0.99 | 0.12–8.11 | 0.996 |
| Creatinine | 1.106 | 0.26–4.63 | 0.891 |
| Chemotherapy | 2.70 | 0.64–11.35 | 0.201 |
| CCI | |||
| 0 | 1.14 | 0.14–9.32 | 0.900 |
| 1–2 | 0.26 | 0.03–2.13 | 0.211 |
| 3–4 | 3.29 | 0.79–13.78 | 0.103 |
| ≥ 5 | 0.57 | 0.07–4.65 | 0.600 |
| LND | |||
| None | 0.66 | 0.13–3.28 | 0.613 |
| Modified | 0.34 | 0.04–2.78 | 0.315 |
| Radical | 0.04 | 0.00-453.31 | 0.501 |
| Pelvin | 5.02 | 1.2-21.07 | 0.027 |
HR, hazard ratio; CI, confidence interval; SMI, skeletal muscle index; PMI, psoas muscle index; VSR, visceral-to-subcutaneous fat ratio; VFI, visceral fat index; SFI, subcutaneous fat index; VO, visceral obesity; pT-stage, pathological Tumor stage; pN-stage, pathological nodal classification; cM-stage, clinical metastases classification; BMI, body mass index; ASA, American Society of Anesthesiologists. CCI, Charlson comorbidity index; LND, lymph node dissection; p < 0.05 is considered statistically significant.
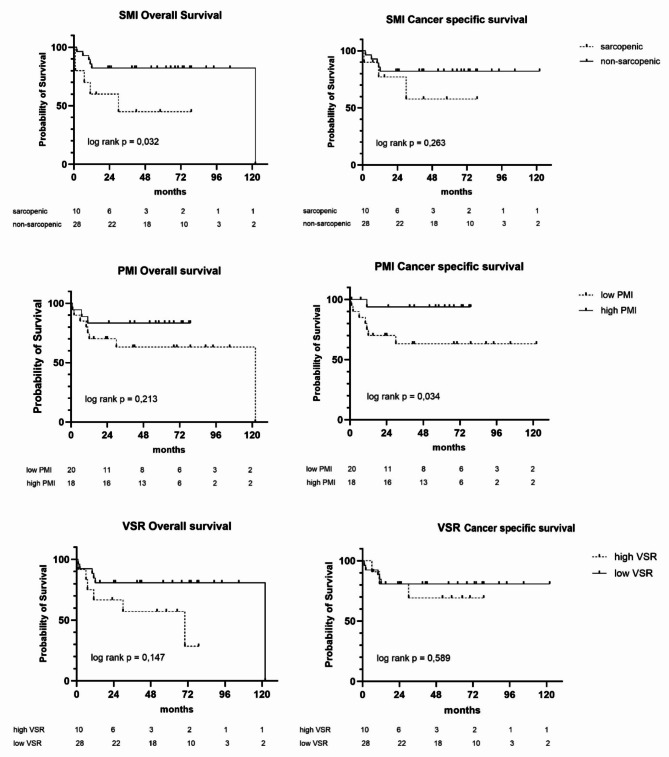
Kaplan-Meier curves for patients stratified by sarcopenia (SMI) showed a significantly lower OS for sarcopenic patients (41 vs. 101 months, p = 0.032). Kaplan-Meier curves for patients stratified by PMI showed a significantly lower CSS for patients with low PMI (74 and 81 months, respectively (p = 0.034). Further survival curves for SMI, PMI and VSR are illustrated in Fig. 2.
Fig. 2.
Kaplan–Meier survival curves for overall survival and cancer specific survival stratified by different body composition parameters (SMI, PMI, VSR). SMI, skeletal muscle index; PMI, psoas muscle index; VSR, visceral-to-subcutaneous fat ratio.
None of the analysed adipose tissue parameters (VFI, SFI, VSR, and VO) could be identified as risk factor for OS or CSS in this study cohort.
Discussion
Sarcopenia was described in several studies as predictor for poorer survival, especially in patients with malignancies7,24,25. To date, only two studies have been published that investigated the prevalence and influence of sarcopenia in patients with penile cancer10,11. Therefore, we conducted this study and analyzed low skeletal muscle mass in patients with penile cancer retrospectively. Our results show a prevalence of sarcopenia of 26% among patients with penile cancer. Takkamoto et al. investigated sarcopenia in patients with penile carcinoma using the PMI and found that the prevalence of sarcopenia was comparable at 32%11. Sharma et al. even showed a prevalence of 51% for patients with penile carcinoma, who underwent lymph node dissection10. However, it should be noted that each study used different diagnostic criteria for sarcopenia. The Martin criteria, which we used in our study, were determined from 1473 cancer patients and represent a fairly accurate classification for sarcopenia12. Sharma et al. used cut-off values validated by the European working group on sarcopenia in older people in 201026. Takkamoto et al. instead determined their own threshold values for PMI using receiver operating characteristic analysis (ROC)11. As there are several options to define and measure sarcopenia, it is quite difficult to compare results of different studies. Although the methods in these studies differ, their results are in line with our findings and confirm, that sarcopenia is found in a relevant proportion of patients with penile cancer and should be considered in treatment alongside targeted tumor therapy.
Furthermore, our data show that sarcopenia is a negative predictive factor for survival of patients with penile cancer. Univariate Cox regression analysis showed a significantly reduced OS for sarcopenia, calculated by SMI, and a significantly reduced CSS for sarcopenia, calculated by PMI. This is in line with several other studies that investigated the influence of sarcopenia on the survival of patients with solid tumor diseases5–7,15,15,22,24.
Beside sarcopenia, we showed, that also advanced age and distant metastases are significant risk factors for OS and CSS, respectively.
Due to the small number of patients, we were unable to perform a multivariate analysis that could identify independent risk factors. Nevertheless, we think that every published data contributes significantly to the existing state of knowledge, particularly in the context of rare diseases like penile cancer. For more reliable results we suggest performing further investigations within multicenter studies to create bigger patient cohorts as penile cancer is a relatively rare disease.
To the best of our knowledge, no comparable study has yet investigated the influence of fat distribution on the outcome of penile cancer. Especially visceral obesity is associated with medical disorders such as cardiovascular disease and several malignancies21. Moreover, sarcopenic obesity, defined by the co-existence of obesity and sarcopenia, was mentioned as risk factor for frailty, comorbidities and mortality27. Our data show that sarcopenic patients have a significantly higher VSR than non-sarcopenic patients. 60% of patients with sarcopenia were classified as high VSR, however only 21% of non-sarcopenic patients were classified as high VSR. These results show that sarcopenia should not only be treated as a single symptom, but as a complex of physical changes in elderly people. Although our study did not show any significant influence for OS and CSS regarding body fat distribution parameters, the graphical representation of the Kaplan Meier curves for VSR suggests a difference in survival between patients with high and low VSR.
In literature, there are conflicting results in terms of fat distribution and survival regarding other cancer entities. High visceral fat seems to affect survival among patients with colorectal, pancreatic and prostate cancer negatively28,29. Performing a systematic review, Lopez et al. found that high levels of subcutaneous fat and low levels of VAT/SAT were associated with a longer survival, in patients at advanced stages of prostate cancer29. On the other hand, high visceral fat was mentioned as protective factor for survival in patients with renal cell carcinoma and urothelial cancer of the upper urinary tract6,29,30. Lee HW et al. determined fat distribution of 2178 patients with renal cell carcinoma and showed that high visceral fat was associated with longer cancer-specific survival (p = 0.01) and overall survival (p = 0.03). Further research is required to assess the role of visceral and sarcopenic obesity in patients with penile cancer.
Beside the relatively small size of patients in this study, another limitation of our study is that due to the presented method only patients with available CT could be included in the study. Although we didn’t find any significant differences regarding t-stage of patients with and without staging, this diagnostic technique could influence the results by selecting patients in advance in retrospective studies. A conceivable method to assess skeletal muscle mass would be to determine psoas muscle mass using ultrasound. This portable, cheap and non-invasive method could be used especially for prospective studies to monitor skeletal muscle mass and sarcopenia, respectively, without any radiation exposure31.
Due to the retrospective design several diagnostic parameters relating to sarcopenia, such as muscle force measurement by hand-grip-strength and functional tests like chair-stand-test, as well as information about dietary habits, nutritional status and physical activity could not be recorded. There are several studies, that show that exercise and nutritional support program has the potential to reduce sarcopenia and improve outcome in elderly sarcopenic patients with cancer disease32. We believe that patients with penile cancer should also be specifically screened for secondary diseases such as sarcopenia. Also with regard to the shrinking budget of the healthcare system, this personalized approach not only improves patient outcomes but also optimizes the use of limited funds, ensuring that support is provided to those who will benefit most.
As we showed, sarcopenia is present in a relevant part of patients with penile cancer. Therefore, we suggest prospective studies to collect named data and develop nutritional- and exercise programs to improve live quality and survival alongside targeted tumor therapy.
Conclusions
In conclusion, sarcopenia occurs in a relevant part of patients with penile cancer and is significantly associated with a reduced OS and CSS. Regarding fat distribution parameters we showed that VSR is associated with sarcopenia. Thus, beside low muscle mass also fat distribution, particularly high visceral fat, should be taken into focus for further studies. Additional prospective research is needed to evaluate whether early intervention of muscle mass maintaining and fat reduction, including exercise and specific nutritional optimization, may achieve better cancer management and thus, better outcome results.
Author contributions
Valerie Hartmann: data collection, management, literature search, analysis and interpretation, manuscript writing. Roman Mayr: substantial contributions to the conception and design of the work; analysis and interpretation. Maximilian Burger: coordination, interpretation, manuscript review, manuscript editing. Simon Udo Engelmann, Christoph Pickl, Maximilian Haas, Sebastian Kälble, Aybike Hofmann, Christopher Goßler, Christoph Eckl and Renate Pichler: manuscript review and manuscript editing. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fu, L. et al. Global pattern and trends in penile cancer incidence: Population-based study. JMIR Public Health Surveill.8, e34874. 10.2196/34874 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierzbicka, M., Klussmann, J. P., San Giorgi, M. R., Wuerdemann, N. & Dikkers, F. G. Oral and laryngeal HPV infection: Incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine39, 2344–2350. 10.1016/j.vaccine.2021.03.047 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven, R. H. A. et al. Population-based survival of penile cancer patients in Europe and the United States of America: No improvement since 1990. Eur. J. cancer (Oxf. Engl. 1990)49, 1414–1421. 10.1016/j.ejca.2012.10.029 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Dent, E. et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging22, 1148–1161. 10.1007/s12603-018-1139-9 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Engelmann, S. U. et al. Body composition of patients undergoing radical cystectomy for bladder Cancer: Sarcopenia, low psoas muscle index, and myosteatosis are independent risk factors for mortality. Cancers15. 10.3390/cancers15061778 (2023). [DOI] [PMC free article] [PubMed]
- 6.Pickl, C. et al. Body composition as a comorbidity-independent predictor of survival following nephroureterectomy for urothelial cancer of the upper urinary tract. Cancers15. 10.3390/cancers15020450 (2023). [DOI] [PMC free article] [PubMed]
- 7.Mayr, R. et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J. Cachex. Sarcopenia Muscle9, 505–513. 10.1002/jcsm.12279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, K. et al. Sarcopenia defined by skeletal muscle mass index at the third lumbar vertebra is a prognostic factor for extensive-stage small cell lung cancer patients: A retrospective study. J. Thorac. Dis.14, 2645–2651. 10.21037/jtd-22-782 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang, C. L. et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: Analysis from a large-scale cohort. Medicine95, e3164. 10.1097/MD.0000000000003164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma, P. et al. Sarcopenia as a predictor of complications in penile cancer patients undergoing inguinal lymph node dissection. World J. Urol.33, 1585–1592. 10.1007/s00345-014-1471-6 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Takkamoto, D. et al. A low psoas muscle volume is associated with a poor prognosis in penile cancer. Oncotarget11, 3526–3530. 10.18632/oncotarget.27719 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin, L. et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol.31, 1539–1547. 10.1200/JCO.2012.45.2722 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Derstine, B. A. et al. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep.8, 11369. 10.1038/s41598-018-29825-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagliafico, A. S., Bignotti, B., Torri, L. & Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med.127, 228–237. 10.1007/s11547-022-01450-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasahara, R. et al. A low Psoas muscle index before treatment can predict a poorer prognosis in advanced bladder cancer patients who receive gemcitabine and nedaplatin therapy. Biomed. Res. Int.2017, 7981549. 10.1155/2017/7981549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong, M. et al. Age-specific reference values for low psoas muscle index at the L3 vertebra level in healthy populations: A multicenter study. Front. Nutr.9, 1033831. 10.3389/fnut.2022.1033831 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan, G. T. et al. Association between systemic inflammation and malnutrition with survival in patients with cancer sarcopenia—a prospective multicenter study. Front. Nutr.8, 811288. 10.3389/fnut.2021.811288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi, Y. et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 101, 565–574. 10.1097/TP.0000000000001587 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Grillot, J. et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin. Nutr.39, 3024–3030. 10.1016/j.clnu.2020.01.001 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Doyle, S. L. et al. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr. Res. (New York N. Y.)33, 171–179. 10.1016/j.nutres.2012.12.007 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Shuster, A., Patlas, M., Pinthus, J. H. & Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol.85, 1–10. 10.1259/bjr/38447238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara, N. et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol.63, 131–140. 10.1016/j.jhep.2015.02.031 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Bamba, S. et al. Assessment of body composition from CT images at the level of the third lumbar vertebra in inflammatory bowel disease. Inflamm. Bowel Dis.27, 1435–1442. 10.1093/ibd/izaa306 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Feliciano, E. M. C. et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C SCANS study. JAMA Oncol.3, e172319. 10.1001/jamaoncol.2017.2319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, E. Y. et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J. Thorac. Oncol.10, 1795–1799. 10.1097/JTO.0000000000000690 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older people. Age Ageing39, 412–423. 10.1093/ageing/afq034 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donini, L. M. et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes. Facts15, 321–335. 10.1159/000521241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao, J., Mazurak, V. C., Olobatuyi, T. A., Caan, B. J. & Prado, C. M. Visceral adiposity and cancer survival: A review of imaging studies. Eur. J. Cancer Care27, e12611. 10.1111/ecc.12611 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Lopez, P. et al. Associations of fat and muscle mass with overall survival in men with prostate cancer: A systematic review with meta-analysis. Prostate Cancer Prostatic Dis.25, 615–626. 10.1038/s41391-021-00442-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, H. W. et al. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int. J. Urol.22, 455–461. 10.1111/iju.12716 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Chianca, V. et al. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. (N.Y.)47, 3205–3216. 10.1007/s00261-021-03294-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto, K. et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer20, 913–918. 10.1007/s10120-016-0683-4 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.