Abstract
PIK3CA-related overgrowth spectrum (PROS) disorders are caused by somatic mosaic variants that result in constitutive activation of the phosphatidylinositol-3-kinase/AKT/mTOR pathway. Promising responses to molecularly targeted therapy have been reported, although identification of an appropriate agent can be hampered by the mosaic nature and corresponding low variant allele frequency of the causal variant. Moreover, our understanding of the molecular consequences of these variants—for example how they affect gene expression profiles—remains limited. Here we describe in vitro expansion of a human capillary malformation followed by molecular characterization using exome sequencing, single cell gene expression, and targeted long-read single cell RNA-sequencing in a patient with clinical features consistent with Megalencephaly-Capillary Malformation Syndrome (MCAP, a PROS condition). These approaches identified a targetable PIK3CA variant with expression restricted to PAX3+ fibroblast and undifferentiated keratinocyte populations. This study highlights the innovative combination of next-generation single cell sequencing methods to better understand unique transcriptomic profiles and cell types associated with MCAP, revealing molecular intricacies of this genetic syndrome.
Subject terms: Genetics, RNA sequencing
Introduction
Somatic variation occurring within RAS-MAPK, PI3K-AKT, and related pathways are increasingly recognized as a cause of a wide spectrum of vascular malformations and overgrowth syndromes including lymphatic malformations, arteriovenous malformations, cavernous malformations, megalencephaly-capillary malformation syndrome (MCAP), PIK3CA-related overgrowth spectrum (PROS) disorders, megalencephaly-polydactyly-polymicrogyria-hydrocephalus syndrome (MPPH), and others1–10. The clinical spectrum of disease ranges from mild, localized vascular, or lymphatic malformations to debilitating overgrowth syndromes accompanied by developmental delay, seizures, hydrocephalus, symptomatic Chiari I malformations, and body asymmetry1,3–6,9–16. Although clinical improvement has been reported with the use of molecularly targeted therapies such as alpelisib, clinical molecular testing may be non-diagnostic due to the mosaic nature and low variant allele fraction/frequency (VAF) of the variant in available tissues for study17–21. Strategies to overcome challenges in detecting pathogenic variants with low VAF have included in vitro expansion of affected tissues prior to sequencing, high depth sequencing with targeted gene panels, or droplet-based PCR gene panels to study DNA extracted from biofluids such as cerebrospinal fluid, cyst fluid, or venous blood draining affected tissues3,19,22–25.
The introduction of a genetic change during embryogenesis can result in the presence of two or more genetically distinct cell lines in an individual, a phenomenon known as mosaicism. Phenotypic consequences of somatic mosaicism are impacted by the nature and timing of the genetic change including the distribution of affected tissues and extent of disease. Some clinical laboratory genetic tests include PIK3CA among larger constitutional gene panels focused on macrocephaly or overgrowth syndromes for individuals in whom a differential diagnosis may be broad3. PIK3CA testing for the diagnosis of PROS usually requires specimens from an affected region, such as a skin biopsy or a surgical sample from an overgrown tissue, or vascular or lymphatic malformation. Previous reports have shown that postzygotic PIK3CA mutations are generally not detectable in blood samples of affected individuals, though exceptions have occurred in individuals with MCAP syndrome26. Improved detection sensitivity has also been reported with enrichment for suspected causative cell types such as CD31+ endothelial cells; however, sampling of involved tissue can be impractical such as in the setting of a predominantly central nervous system phenotype19,27. Despite the sometimes significant clinical phenotype and morbidities seen with PROS and the patient reported in this study, the VAF of the causal variant is frequently at or below the level of detection for many standard clinical sequencing assays leading to falsely negative clinical testing. The implementation of targeted panel-based sequencing with read depths up to 500× and sensitivity for VAFs as low as 0.15% have permitted the identification of ever lower frequency mosaicism and categorization of the spectrum of phenotypes associated with specific mutations3,4,19. Additionally, while cell-free DNA techniques are emerging for detection of mosaic variation, generally access of disease-involved tissue requires invasive sampling through biopsy or skin punch. The need for invasive testing can create barriers to care due to the desire of families to avoid invasive testing and the need for involving the desire of families to participate in an invasive procedure and the need for prior authorization from payors.
Despite these genomic insights, it remains unclear how low frequency mosaicism involving a small fraction of cells leads to the dramatic spectrum of PROS phenotypes. Techniques to analyze diseased tissues and probe the impact of somatic mosaic variation have evolved in recent years. Single cell RNA sequencing (scRNA-seq) is a powerful tool to analyze transcriptional signatures in individual cells, but current technology, by design, produces short-read sequencing biased towards the 3ʹ end of transcripts, which has limited value for genotyping variants distal from the polyA tail28–30. Integration of long-read sequencing data can be helpful to capture full-length transcripts, however, current long-read technologies have insufficient read output, scalability, and limited depth of coverage for the confident assignment of genotypes to thousands of transcriptionally profiled single cells. MAS-ISO-seq (Multiplexed ArrayS ISOform SEQuencing) was recently developed to permit high-throughput long-read transcriptome sequencing, helping to overcome this barrier31. This approach uses PCR to combine up to 15 cDNA molecules into concatenated molecules, which are then sequenced using the Pacific Biosciences (PacBio) circular consensus sequencing approach. Subsequent “de-concatenation” is achieved in silico by leveraging established primer sequences and known 10× single cell barcodes to facilitate the assignment of transcripts to single cells. A comparable library preparation kit called ‘Kinnex’ corresponding to this method was commercially released by PacBio.
Here, we report the use of MAS-ISO-seq to study the transcriptome in PIK3CA-altered fibroblasts from a capillary malformation in a patient with suspected MCAP. Despite initially negative results obtained from sequencing DNA extracted from peripheral blood mononuclear cells to identify an underlying MCAP-associated variant, high-depth whole exome sequencing of cultured cells identified a constitutively activating single nucleotide variant (SNV) in PIK3CA: NM_006218.4:c.3139C>T;p.His1047Tyr; this variant is known to confer susceptibility to the PIK3CA inhibitor alpelisib and to mTOR inhibitors and confirmed the diagnosis of MCAP. Subsequent single cell RNA-sequencing of cultured cells using two next-generation sequencing (NGS) approaches (short-read sequencing of the 3ʹ ends of transcripts and long-read targeted sequencing of PIK3CA) identified a PAX3+/SOX11+ fibroblast-like population enriched for the variant. Cell clusters enriched for PIK3CA-mutant cells (PIK3CAmut) exhibited a distinct gene expression profile compared to cell clusters with fewer PIK3CAmut cells, providing insight into how a variant restricted to a small population of cells might lead to the dramatic clinical phenotype seen in patients with MCAP. These results illustrate the rapidly advancing potential for the application of single cell sequencing technologies to probe the mechanisms by which previously undetectable somatic mosaicism may lead to debilitating clinical syndromes.
Results
Case description
A male of Northern European ancestry born at 36 weeks was noted at birth to have right facial asymmetry and widespread port wine stains. He was otherwise well-appearing and did not exhibit seizure-like activity. Birth history was significant for in utero exposure to methamphetamines and hepatitis C. Although decreased facial movement was noted on the enlarged right side, the remainder of his neurologic exam was within normal limits. At birth, widespread port wine stains, a form of capillary malformation, were noted throughout his trunk and bilateral lower extremities.
Clinical diagnostic testing
Given concern for a PROS disorder such as Klippel–Trenaunay syndrome or Megalencephaly-Capillary Malformation syndrome (MCAP), the patient was referred to clinical geneticist in the Division of Genetic & Genomic Medicine. An MRI of the brain revealed asymmetric enlargement of the right cerebral hemisphere and cortical dysplasia (Fig. 1a,b). Lower extremity radiographs showed a leg length discrepancy of > 1 cm (Fig. 1c). Due to the constellation of clinical findings, blood was sent for PIK3CA sequence analysis and deletion/duplication testing on day of life 5, but no pathogenic variants were identified. An abdominal ultrasound was negative for embryonal tumors. Formal language testing at 2 years 4 months of age identified a mixed receptive-expressive language disorder with an overall language ability at the 7th percentile for his age group. Subsequent developmental testing found him to be below average for his age group in the domains of physical, social-emotional, cognitive, communication, and general development with average adaptive skills.
Fig. 1.
Clinical presentation and diagnostic approach. (a,b) T2 coronal (a) and T1 axial (b) MRI obtained age 2 shows enlarged right cerebral cortex with effacement of the right ventricle and cortical dysplasia. (c) AP lower extremity radiograph obtained at age 2 shows right greater than left leg length discrepancy. (d) Diagnostic testing included whole exome sequencing from skin cell culture. (e) Single cell gene expression was performed, in parallel with long-read sequencing, targeted for PIK3CA transcripts, to enable genotyping of single cells for the pathogenic PIK3CA variant.
High-depth exome sequencing identifies a pathogenic PIK3CA variant
Given continued clinical suspicion for a PROS disorder, a skin biopsy of the right trunk was obtained at 2 years 2 months of age for in vitro expansion of skin affected by port wine stains. Targeted sequencing of DNA from cultured fibroblasts performed at an outside clinical lab for PIK3CA copy number and sequence variation (mean depth 144×) was negative. The patient was subsequently enrolled in a translational research protocol and high-depth exome sequencing was performed on DNA extracted from cultured cells (Fig. 1d) (mean depth of 244× coverage) which identified a missense variant in PIK3CA (NM_006218.4:c.3139C>T;p.His1047Tyr). Allelic depth at the variant position was 386× with a variant allele frequency/fraction (VAF) of 11.9% for the PIK3CA:p.His1047Tyr alteration. Clinical Sanger sequencing of PCR products obtained from the same DNA extract confirmed the presence of this activating variant (Fig. S1), which is known to confer susceptibility to the PIK3CA inhibitor alpelisib and mTOR inhibitors (tacrolimus, everolimus, sirolimus). This variant was classified as a Tier I (Level A) variant indicating confirmed pathogenicity32.
PIK3CA mutation status is associated with increased fraction of G1 phase cells
To better understand the effect of PIK3CA mutational status on the transcriptome of individual cells, we performed short- and long-read single cell sequencing of cultured cells (Fig. 1d,e). Single cell short-read RNA-sequencing yielded 12,378 cells that passed QC filtering and were included in downstream analysis. A total of 10 clusters were identified from the single cell data (Fig. 2a). Although short-read RNA-sequencing techniques are capable of identifying variants biased towards the 3ʹ end, few reads will be of sufficient length to capture variants at longer distances from the 3ʹ end. Overall, 1.2% (173/14,092) of single cells profiled by short-read sequencing had coverage to capture the PIK3CA:c.3139C>T locus, permitting genotyping of these cells using short-read methodology alone, although the small number of cells does not permit differential gene expression. By incorporating long-read sequencing of the transcripts via MAS-ISO-seq long-read RNA-sequencing of the PIK3CA transcript to the barcoded single cell transcriptomes, we captured the locus of the known PIK3CA:c.3139C>T variant in 15.3% (1894/12,378) of cells profiled. Thus, the use of the multiplexed MAS-ISO-seq methodology permitted > 12-fold improvement over short-read technologies in the capacity to genotype single cells (Fig. S2).
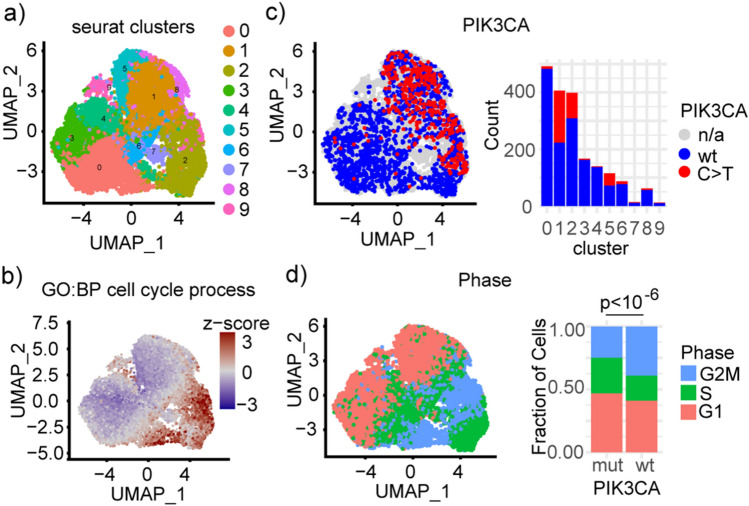
Fig. 2.
PIK3CAmut cells are transcriptionally distinct and mitotically active. (a) Unsupervised clustering using principal component analysis followed by dimensionality reduction using uniform manifold approximation and projection (UMAP) yielded 10 clusters. (b) A UMAP plot of enrichment for the GO:BP cell cycle pathway shows a significant contribution to both the first and second principal components. (c) Cells enriched for PIK3CAmut cells cluster together on a UMAP plot. Barplot (right) showing count of genotyped cells in each cluster. (d) UMAP (left) plot shows cells clustering by phase and barplot (right) shows an increased fraction of PIK3CAmut cells in the G1 growth phase of the cell cycle.
Gene expression quantification through short- and long-read single cell sequencing revealed variation driven by PIK3CA mutational status and cell cycle status in the first principal component, while the second principal component was driven primarily by cell cycle status (Fig. 2b–d). Despite cell cycle regression, the top five biological processes contributing to the PCA space were all associated with the cell cycle including Cell Cycle Process, Mitotic Cell Cycle, Mitotic Cell cycle process, Chromosome Organization, and Cell Division (Table S1). PIK3CA mutation status was assigned to clusters with > 100 genotyped cells. Clusters with PIK3CA mutations in > 10% of genotyped cells were designated PIK3CAmut while those with < 10% mutational burden were designated PIK3CAwt (Fig. 2c). A greater proportion of PIK3CAmut cells were assigned to the G1 growth phase of the cell cycle (Fig. 2d).
PIK3CAmut cells express neural crest lineage markers
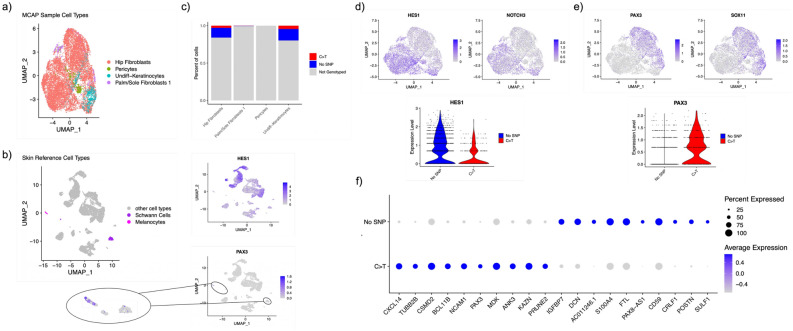
Although significant homogeneity was noted overall, clusters enriched for PIK3CAmut cells clustered together along the first principal component (Fig. 2c). Using automated mapping to a publicly available reference skin set33, our clusters were classified as follows: hip fibroblasts (10,747/12,378; 86.8% of all cells), undifferentiated keratinocytes (882/12,378; 7.1%), palm/sole fibroblasts 1 (372/12,378; 4%), and pericytes (377/12,378; 3%) (Fig. 3a). The reference skin set showed that compared to the bHLH transcription factor HES1, which is expressed across multiple cell type clusters, PAX3 is normally expressed only in Schwann cells and melanocytes (Fig. 3b). However, genotyping of the cultured skin cells (Fig. 3c) revealed PIK3CAwt clusters were highly enriched for the NOTCH3 receptor and HES1 (Fig. 3d), while PIK3CAmut clusters (fibroblasts and undifferentiated keratinocytes) were highly enriched for the neural crest lineage transcription factors PAX3 and SOX11 (Fig. 3e). This finding shows that PIK3CAmut fibroblasts and undifferentiated keratinocytes express typically melanocyte and neura crest fate specific markers.
Fig. 3.
PIK3CAmut clusters express neural crest markers. (a) UMAP plot of cultured skin cells mapped to a normal human skin cell reference. (b) UMAP plot of normal human skin cell reference with melanocytes (pink) and Schwann cells (purple) highlighted. Feature plots (right) showing the wide expression of the bHLH transcription factor HES1 across multiple cell clusters and the limited expression of neural crest transcription factor PAX3 in melanocytes and Schwann cells. (c) Barplot displaying percent of genotyped cells in each cell type. (d) Feature plot (top) showing expression of the Notch-regulated bHLH transcription factor HES1 and the NOTCH3 receptor in the PIK3CAwt enriched clusters and violin plot (lower) showing expression of HES1 transcript in PIK3CAmut versus PIK3CAwt clusters. (e) Feature plot (top) showing expression of the neural crest transcription factors PAX3 and SOX11 in the PIK3CAmut enriched clusters and violin plot (lower) showing expression of HES1 transcript in PIK3CAmut versus PIK3CAwt clusters. (f) Dotplot showing expression of top 10 expressed markers in PI3KCAwt versus PIK3CAmut cells.
PIK3CAwt cells express notch pathway signaling genes
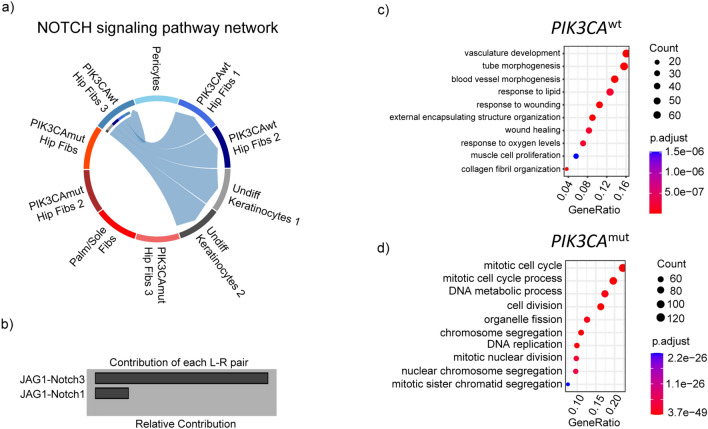
The most highly differentially expressed genes in PIK3CAwt cells (versus mutated cells) included genes involved in the insulin-like growth factor pathway (IGFBP7, IGFBP4), chondroitin sulfate metabolism (DCN), non-coding RNAs (AC011246.1, PAX8-AS1), effectors of canonical notch signaling (HES1) and WNT signaling (WNT5A). Conversely, the mostly highly expressed genes in PIK3CAmut cells were involved in inflammation (CXCL14), cytoskeleton (TUBB2B), chromatin remodeling (BC11B), cell–cell interactions (NCAM1), and regulation of transcription (PAX3, NFIB) (Fig. 3f, Table S2). An analysis of ligand receptor signaling patterns using CellChat revealed a PIK3CAwt fibroblast population as an effector of Notch signaling via JAG1-NOTCH3 and JAG1-NOTC1 signaling (Fig. 4a,b). Gene set enrichment analysis of PIK3CAwt cells revealed a predominance of genes involved in blood vessel formation and wound healing while PIK3CAmut cells were enriched for genes involved in cell division (Fig. 4c,d).
Fig. 4.
Notch signaling is absent in PIK3CAmut populations. (a) Chord diagram showing Notch-mediated signaling between PIK3CAwt but not PIK3CAmut fibroblasts and undifferentiated keratinocytes. (b) Barplot showing the relative contribution of the JAG1-NOTCH3 versus JAG1-NOTCH1 receptor signaling to overall Notch pathway signaling. (c,d) Dotplot of the top 10 enriched Gene Ontology Biological Processes in PIK3CAwt (c) and PIK3CAmut (d) cells.
PIK3CA mutation status drives chemokine signaling
Analysis of the communication probabilities of 1939 receptor-ligand signaling pairs in 223 pathways using CellChat identified three outgoing signaling (sender) patterns representing undifferentiated keratinocytes (Pattern 1, black), PIK3CAwt fibroblasts 1–3 and pericytes (pattern 2, blue), and PIK3CAmut fibroblasts 1–3 and palm/sole fibroblasts (pattern 3, red) (Fig. 4a). While sender pattern 1 was dominated by epidermal growth factor (EGF), immune (CD226, IL6), and endothelin (EDN) pathways, sender pattern 2 was dominated by ephrin (EPHA), periostin, activin, and BMP pathways. The most significant pathway contribution to the relatively silent PIK3CAmut sender pattern 3 was the cell adhesion molecule pathway (CADM). Incoming receiver patterns were not associated with PIK3CAmut status (Figs. 4c, 5a,b).
Fig. 5.
PIK3CAmut clusters exhibit distinct receptor/ligand signaling behavior. (a) Heatmaps of sender patterns of clusters show three predominant cell patterns (left) corresponding to ligand-mediated communications (right) from keratinocytes, PIK3CAwt, and PIK3CAmut clusters respectively. (b) Heatmaps of receiver cell patterns (left) and receptor-mediated communication patterns (right) showing the relative contribution of cell clusters to the microenvironment.
Discussion
The detection of disease-associated somatic mosaic variants in patients with clinical signs suggestive of PROS and related conditions is challenging due to the low VAF. These variants can escape detection in clinical sequencing when limited tissue samples are tested or if assay sensitivity is inadequate. Consequently, implementing molecularly targeted therapy to address potentially life-threatening symptoms becomes hindered. In our study, we addressed this challenge by employing clinical cell culture expansion of a skin punch biopsy to facilitate the diagnosis of a molecularly targetable PIK3CA variant. Bulk exome sequencing of DNA derived from cultured cells enabled enrichment for a dividing cell population and the subsequent identification of this variant via standard clinical whole exome sequencing followed by confirmatory Sanger sequencing1. The use of cell culture to selectively amplify dividing cells from affected tissues is a useful adjunct to direct sequencing of affected tissues and may be readily translated into the workflow of many clinical laboratories. Additionally, we utilized a novel single-cell assay to perform gene expression profiling coupled with single cell genotyping via targeted long-read RNA-sequencing from the same cell, facilitating mechanistic insights into disease pathogenesis.
This innovative single cell approach revealed the PIK3CA variant was enriched in a fibroblast-like cell population characterized by the expression of neural crest markers PAX3 and SOX11 and an absence of NOTCH signaling regulation, which is known to maintain epithelial self-renewal and promote establishment of the keratinocyte lineage34,35. In addition to their contribution to the PROS overgrowth spectrum diseases, activating mutations in PIK3CA are among the three most commonly identified genetic alterations in cancers36,37. PIK3CA codes for the catalytic subunit of the enzyme phosphatidylinositol 3-kinase (PI3K) which activates migration, survival, cell cycle, and growth pathways38. Mutations are clustered in either the helical domain (E545K, E542K) or the kinase domain (H1047R), as seen in our case37,39,40. Several lines of evidence indicate that activating mutations in the kinase domain, including the H1047R mutation, lead to cell growth and hypertrophy via p70S6K and mTOR-dependent mechanisms without activating cell cycle pathways38–43. Our observation that PIK3CAmut clusters are enriched for cells in the G1 growth phase of the cell cycle is consistent with the clinical finding in this patient of hemibody hypertrophy without development of cancer. Although acquisition of a PIK3CA mutation is typically a late event in cancer, clinical trials in multiple cancers have noted that PIK3CA kinase domain mutations confer increased sensitivity to treatment with mTOR inbitors compared to helicase mutations suggesting that the activating role of kinase mutations primarily affects growth pathways37,39. Overall, these findings support obervations that the role of PIK3CA in cell division and migration is decoupled from the role of PIK3CA in cell growth and hypertrophy39,40,42,43.
Expression of PAX3 in a healthy human skin reference appeared to be restricted to neural crest lineages such as melanocytes and schwann cells44. Although PAX3 is an established marker for skin melanocytes, normal nevi, and malignant melanoma, murine studies have identified PAX3 as a direct regulator of Notch effectors such as HES145,46. As the PIK3CAmut PAX3+ populations identified in these in vitro studies do not express other melanocyte markers such as MLANA, their in vivo correlate remains to be identified. The finding that clusters enriched for the PIK3CA mutation are not subject to NOTCH1/3 regulation is of particular relevance to this patient’s clinical condition. NOTCH3, and to a lesser extent NOTCH1, have a well established role in the promotion of angiogenic remodeling of the fetal primary capillary plexus to form arteries47–52. Furthermore, NOTCH1 and NOTCH3 deficient mouse models exhibit a loss of pericyte-induced stabilization of developing blood vessels that leads to arteriovenous malformations and NOTCH3 mutations lead to the clinical stroke syndrome CADASIL48,53,54. Given the known role of Notch signaling in the maturation of blood vessels, specifically in the involution of small capillaries during the formation of larger vessels, the absence of Notch signaling in cell clusters enriched for activating PIK3CA mutations reflects the raises the possibility that an expanding PIK3CAmut population leads to widespread disruption of NOTCH1/3-dependent maturation of the fetal capillary plexus into mature arteries and persistence of fetal capillary networks after birth.
Our findings support two potential hypotheses regarding the etiology of the PROS disorders. In one, specific progenitor cell populations are prone to prolonged expansion upon exposure to mutant PIK3CA while in another, the presence of mutant PIK3CA induces a blockade to differentiation. Arteriovenous malformations, a related somatically driven vascular overgrowth anomaly, appear to be driven by activating KRAS mutations that are restricted to CD31+ endothelial cells, lending support to the hypothesis that a mutation in a single cell type may lead to an overgrowth syndrome affecting the surrounding tissue27. Similarly, our finding that the PIK3CAmut cell population exhibits a distinct outgoing signaling profile supports the hypothesis that these cells may exert a unique trophic effect on the surrounding tissue. Indeed, mutant clusters exhibited increased outgoing PDGF signaling which has long been recognized as a driver of angiogenesis55. Although the low VAF of pathogenic variants identified in disease-involved tissue specimens from patients is suggestive of a unique molecular event restricted to a specific population of cells, further research is warranted to determine whether the distinct transcriptional signature is a direct result of fate decisions influenced by the constitutively activated PIK3CA or rather, related to a post-zygotic mutation event restricted to a select population of fate-committed cells1,4,15,19,22.
Our research not only enhances our biological understanding of vascular malformations but also introduces a powerful long-read NGS workflow. This innovative approach facilitates the precise assignment of genomic alterations to individual cells within mosaic diseased tissue, opening up new avenues for comprehensive molecular analyses. Although our study utilized a hybridization capture approach to specifically enrich PIK3CA transcripts, the described method is suitable for detecting other variants of interest by enriching for different gene set(s). Moving forward, application of multiplexed single cell RNA-sequencing with targeted long-read based genotyping to diseased tissues such as skin, resected vascular malformations, and tonsils resected at the time of surgery for Chiari malformations may be expected to provide further insights into the mechanisms by which small populations of mutated cells influence the surrounding microenvironment to produce human disease.
Methods
Human subjects
Written informed consent was obtained from the patient’s parents in this study under a research protocol approved by the Institutional Review Board (IRB) at Nationwide Children’s Hospital (IRB17-00206). All research presented in this study was performed in accordance with relevant guidelines and regulations as set forth by the IRB at Nationwide Children’s Hospital. The patient is a male of Northern European ancestry who was born at term and presented on the first day of life. He was 3 years old at the time of biopsy.
Cell culture
Skin biopsy tissue from the right trunk was morcellated, digested in collagenase at 37 °C for 1 h then pelleted in a centrifuge at 900 RPM × 10 min. The pellet was resuspended in alpha-MEM with 20% FBS, 1.5% l-glutamine, 1% penicillin streptomycin, and 1 ml fungizone, plated in a T25, and incubated T25 at 37 °C and 5% CO2. Flasks were passaged with trypsin–EDTA.
Exome sequencing
Exome sequencing was performed as a clinical test. Briefly, libraries for enhanced exome sequencing were prepared using 100 ng of DNA isolated from cultured cells using the NEB Ultra II FS Kit (New England Biolabs) followed by target enrichment with IDT xGen Lockdown v2.0 human exome reagent (catalog number 10005153) with xGenCNV Backbone Panel and Cancer spike-in (Integrated DNA Technologies, Coralville, IA). Libraries were sequenced on an Illumina NovaSeq6000 (Illumina, Inc., San Diego, CA) to generate 150 bp paired-end reads. Output data were aligned and analyzed using the Churchill workflow which uses a balanced regional parallelization strategy to perform variant discovery56.
Clinical sanger sequencing
To validate the PIK3CA finding, Sanger sequencing was performed on PCR products amplified from the extracted genomic DNA originally used for exome sequencing. Forward and reverse sequencing reactions were performed with the Big Dye v3.1 terminator mix (ThermoFisher Scientific, Waltham, MA). Sequencing was performed on the Applied Biosystems 3730 instrument. Primer sequences are as follows: PIK3CA_Ex21_F (3ʹ-GTAAAACGACGGCCAGCTGAGCAAGAGGCTTTGGAG-5ʹ); PIK3CA_Ex21_R (3ʹ-CAGGAAACAGCTATGACCAGAGTGAGCTTTCATTTTCTCA-5ʹ).
10× genomics 3ʹ-based single cell RNA-sequencing library preparation
To generate libraries for a 3’-based single cell RNA-sequencing, a single confluent T25 flask of cells cultured from a skin biopsy of affected tissue at passage three were harvested using tryspin-EDTA. Purified cells were then processed for library preparation according to the manufacturer protocol for Chromium Next GEM Single-Cell 3’-Reagent Kit v.3.1. Libraries were sequenced on an Illumina NovaSeq 6000 instrument to generate paired-end sequencing data with a minimum of 50,000 reads per cell.
Long-read single cell RNA-sequencing library preparation
To identify coding variants from single cells prepared using the 10× Genomics 3ʹ kit, 75 ng of pre-fragmented cDNA from the 10× Genomics workflow was used as input into the PacBio Kinnex single-cell RNA kit (PN 102-166-600) with the following modifications: cDNA remaining post-TSO artifact removal was enriched for PIK3CA transcripts using a custom probe panel and the xGen Hybridization and Wash Kit protocol (Integrated DNA Technologies, #1080577). Enriched cDNA was then used as input into Kinnex PCR for subsequent array formation according to the manufacturer’s recommendations. Sequencing of the final SMRTbell library was performed using the Sequel II Binding Kit 3.2, the Sequel II Sequencing Plate 2.0, and a single 8 M SMRT Cell with an on plate loading concentration of 100 pM. Data collection included a 2-h pre-extension followed by a 30 h movie.
Analysis of long-read single cell RNA-sequencing data
Preliminary analysis included the use of the PacBio application “Read Segmentation and Single-Cell Iso-Seq” to perform SKERA (https://skera.how/) read splitting or de-concatenation of the array into original 10× Genomic cDNA molecules based on MAS barcodes. A total of 14,999,323 segmented reads were generated with an average length of 637 bp. The analysis application provides aligned BAMs, which were then split using samtools, to generate a new BAM per single cell. Bcftools mpileup was then used to extract reads for our PIK3CA variant of interest. Genotyping calls for the variant were then added as a metadata column to the single cell Seurat object.
Analysis of 3ʹ-based single cell RNA-sequencing
Data preprocessing, including read alignment to the GRCh38 reference transcriptome, filtering, barcode counting, and unique molecular identifier counting, were performed using 10× Genomics CellRanger v.6.0 software following the default parameters for the ‘count’ pipeline. The resulting count files were input into Seurat version 4 using R version 4.2.1 and analyzed as follows57,58. Only cells with mitochondrial RNA < 10%; features > 500; RNA counts > 1000 and < 100,000 were included for downstream analysis. Initial analysis revealed a prominent role of cell cycle genes in the first principal component, so cell cycle phase assignment was performed using the CellCycleScoring function and the cell-cycle difference was calculated as the difference between the S-phase and the G2M phase scores. Normalization, identification of variable features, and data scaling were performed using SCTransform. To eliminate biased variance, cell cycle phases and mitochondrial reads were regressed out. Principal component analysis and dimensionality reduction using RunPCA and RunUMAP. Both reverse and forward PCA were performed to permit mapping to a cell reference. Clustering and differential gene expression were performed using FindNeighbors and FindAllMarkers. A publicly available skin reference dataset generated from the dermis and epidermis of human hip, palm, and sole (GSE20232) was downloaded and processed as above to generate a reference datasest of normal human skin. An anchor dataset was generated using FindTransferAnchors and cultured cells were mapped to the reference using TransferData. The R Bioconductor packages msigdbr, clusterProfiler, and fgsea were utilized to perform gene set enrichment analysis of the clusters and PCA component spaces to identified enriched Biological Processes (GO:BP) from the GeneOntology database. The R Bioconductor package CellChat was utilized to analyze receptor-ligand interactions between clusters59.
Statistics and reproducibility
The FindAllMarkers feature in Seurat was used to perform differential gene expression with the default Wilcoxon Rank Sum Test. A min.pct of 0.25 and a log fold change threshold of 0.25 was used. An adjusted p-value of < 0.05 was used to identify significantly enriched Biological Processes (GO:BP) from the GeneOntology database. Pearson’s Chi-squared test was performed to determine significance between cell clusters and cell cycle phases of PIK3CAwt and PIK3CAmut. Enrichment scores of receptor-ligand interactions between clusters were calculated using the CellChat R Bioconductor package which combines cell–cell communication analysis with differential gene expression analysis. Due to the singular nature of the clinical skin biopsy sample, exome, Sanger, 3ʹ single cell, and long-read single cell sequencing was performed as an N of 1. Additionally, since each cell is assumed unique in single cell sequencing, it is not conventional to have biological replicates.
Supplementary Information
Acknowledgements
We are grateful for the patient and family who participated in this study. The schematics in Fig. 1d,e were generated using biorender.com.
Author contributions
Conceptualization: CEC, ARM, KEM. Methodology: CEC, ARM, KEM. Software: MAW, JJW, KEM. Formal analysis: MAW, JJW. Investigation: MAW, JJW, JBN, MEHG. Resources: EAV, PW, ERM, CEC, PW. Data curation: MAW, JJW, TD, EARG, PW. Visualization: MAW, EARG. Writing—Original draft: MAW. Writing—Review & editing: All authors. Supervision: ARM, KEM. Project administration: CEC, ARM, KEM. Funding acquisition: CEC, ERM.
Funding
This work was supported by the Nationwide Foundation Pediatric Innovation Fund.
Data availability
Single cell data is available for browsing at https://www.nationwidechildrens.org/specialties/institute-for-genomic-medicine/ped-cell-browser. Raw and processed data for single cell RNA-sequencing has been deposited to NCBI Gene Expression Omnibus (GSE253153) and for exome sequencing has been deposited to dbGaP under accession number phs001820.v1.p1.
Code availability
All R code is provided as a supplemental file ‘PIK3CA_R_code.doc’.
Competing interests
The authors declare no competing interests.
Informed consent
Written informed consent was obtained from the patient’s parents under a research protocol approved by the Institutional Review Board at Nationwide Children’s Hospital (IRB17-00206).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72167-8.
References
- 1.Cottrell, C. E. et al. Somatic PIK3R1 variation as a cause of vascular malformations and overgrowth. Genet. Med.23(10), 1882–1888 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis, S. et al. Growth hormone deficiency in megalencephaly-capillary malformation syndrome: An association with activating mutations in PIK3CA. Am. J. Med. Genet. A182(1), 162–168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNulty, S. N. et al. Diagnostic utility of next-generation sequencing for disorders of somatic mosaicism: A five-year cumulative cohort. Am. J. Hum. Genet.105(4), 734–746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park, H. J. et al. Detailed analysis of phenotypes and genotypes in megalencephaly-capillary malformation-polymicrogyria syndrome caused by somatic mosaicism of PIK3CA mutations. Orphanet. J. Rare Dis.15(1), 205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riviere, J. B. et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet.44(8), 934–940 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zenner, K. et al. Somatic activating BRAF variants cause isolated lymphatic malformations. HGG Adv.3(2), 100101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyre, M. et al. Somatic PIK3CA mutations in sporadic cerebral cavernous malformations. N. Engl. J. Med.385(11), 996–1004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng, J. et al. Somatic MAP3K3 mutation defines a subclass of cerebral cavernous malformation. Am. J. Hum. Genet.108(5), 942–950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolaev, S. I., Fish, J. E. & Radovanovic, I. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N. Engl. J. Med.378(16), 1561–1562 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Priemer, D. S. et al. Activating KRAS mutations in arteriovenous malformations of the brain: Frequency and clinicopathologic correlation. Hum. Pathol.89, 33–39 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Deleu, T., Jansen, K. & Calenbergh, F. V. Brain overgrowth associated with megalencephaly-capillary malformation syndrome causing progressive Chiari and syringomyelia. Surg. Neurol. Int.13, 211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loughan, A. R., Harrell, M., Perna, R., Allen, A. & Suddarth, B. Megalencephaly-capillary malformation polymicrogyria: A review and complex pediatric case report. Appl. Neuropsychol. Child.6(4), 369–377 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Mirzaa, G. M. et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: Two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am. J. Med. Genet. A158A(2), 269–291 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Mirzaa, G. M. & Dobyns, W. B. The “megalencephaly-capillary malformation” (MCAP) syndrome: The nomenclature of a highly recognizable multiple congenital anomaly syndrome. Am. J. Med. Genet. A161A(8), 2115–2116 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Sarma, K., Nayak, M. K., Mishra, B. & Gaikwad, S. B. Megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP): A rare dynamic genetic disorder. Cureus14(5), e25123 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St John, L. J. & Rao, N. Autism spectrum disorder in a child with megalencephaly-capillary malformation-polymicrogyria syndrome (MCAP). BMJ Case Rep.14(12), e247034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards, E. A. et al. Monitoring arteriovenous malformation response to genotype-targeted therapy. Pediatrics146, 3 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Lekwuttikarn, R., Lim, Y. H., Admani, S., Choate, K. A. & Teng, J. M. C. Genotype-guided medical treatment of an arteriovenous malformation in a child. JAMA Dermatol.155(2), 256–257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, D. et al. Genomic profiling informs diagnoses and treatment in vascular anomalies. Nat. Med.29(6), 1530–1539 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venot, Q. et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature558(7711), 540–546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venot, Q. et al. Author Correction: Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature568(7752), E6 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Chen, W. L. et al. The utility of cerebrospinal fluid-derived cell-free DNA in molecular diagnostics for the PIK3CA-related megalencephaly-capillary malformation (MCAP) syndrome: A case report. Cold Spring Harb. Mol. Case Stud.8, 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zenner, K. et al. Cell-free DNA as a diagnostic analyte for molecular diagnosis of vascular malformations. Genet. Med.23(1), 123–130 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmieri, M. et al. Cell-free DNA next-generation sequencing liquid biopsy as a new revolutionary approach for arteriovenous malformation. JVS Vasc. Sci.1, 176–180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang, F. et al. Molecular diagnosis of mosaic overgrowth syndromes using a custom-designed next-generation sequencing panel. J. Mol. Diagn.19(4), 613–624 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Kuentz, P. et al. Molecular diagnosis of PIK3CA-related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet. Med.19(9), 989–997 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Morita, H. & Komuro, I. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N. Engl. J. Med.378(16), 1561 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Nam, A. S. et al. Somatic mutations and cell identity linked by genotyping of transcriptomes. Nature571(7765), 355–360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giustacchini, A. et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med.23(6), 692–702 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Cheow, L. F. et al. Single-cell multimodal profiling reveals cellular epigenetic heterogeneity. Nat. Methods13(10), 833–836 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Al’Khafaji, A. M. et al. High-throughput RNA isoform sequencing using programmed cDNA concatenation. Nat. Biotechnol.42, 582 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, M. M. et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn.19(1), 4–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, S. et al. Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun.11(1), 4239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen, B. C. et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev.20(8), 1028–1042 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou, B. et al. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target Ther.7(1), 95 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science304(5670), 554 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Janku, F. et al. PIK3CA mutations in advanced cancers: Characteristics and outcomes. Oncotarget3(12), 1566–1575 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia, Z., Kumar, A., Marques, M., Cortes, I. & Carrera, A. C. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J.25(4), 655–661 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao, L. & Vogt, P. K. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc. Natl. Acad. Sci. U.S.A.105(7), 2652–2657 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao, L. & Vogt, P. K. Hot-spot mutations in p110alpha of phosphatidylinositol 3-kinase (pI3K): Differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle9(3), 596–600 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shioi, T. et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J.19(11), 2537–2548 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fingar, D. C., Salama, S., Tsou, C., Harlow, E. & Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev.16(12), 1472–1487 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saucedo, L. J. & Edgar, B. A. Why size matters: Altering cell size. Curr. Opin. Genet. Dev.12(5), 565–571 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Wiedemann, J. et al. Differential cell composition and split epidermal differentiation in human palm, sole, and hip skin. Cell Rep.42(1), 111994 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medic, S. & Ziman, M. PAX3 expression in normal skin melanocytes and melanocytic lesions (naevi and melanomas). PLoS ONE5(4), e9977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakazaki, H. et al. Key basic helix-loop-helix transcription factor genes Hes1 and Ngn2 are regulated by Pax3 during mouse embryonic development. Dev. Biol.316(2), 510–523 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Luo, W. et al. Arterialization requires the timely suppression of cell growth. Nature589(7842), 437–441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kofler, N. M., Cuervo, H., Uh, M. K., Murtomaki, A. & Kitajewski, J. Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Sci. Rep.5, 16449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasan, S. S. et al. Endothelial Notch signalling limits angiogenesis via control of artery formation. Nat. Cell Biol.19(8), 928–940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conway, E. M., Collen, D. & Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res.49(3), 507–521 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Adams, R. H. & Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol.8(6), 464–478 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Kofler, N. M. et al. Notch signaling in developmental and tumor angiogenesis. Genes Cancer2(12), 1106–1116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tefft, J. B. et al. Notch1 and Notch3 coordinate for pericyte-induced stabilization of vasculature. Am. J. Physiol. Cell Physiol.322(2), C185–C196 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domenga, V. et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev.18(22), 2730–2735 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battegay, E. J., Rupp, J., Iruela-Arispe, L., Sage, E. H. & Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol.125(4), 917–928 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly, B. J. et al. Churchill: An ultra-fast, deterministic, highly scalable and balanced parallelization strategy for the discovery of human genetic variation in clinical and population-scale genomics. Genome Biol.16(1), 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol.36(5), 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuart, T. et al. Comprehensive integration of single-cell data. Cell177(7), 1888–1902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin, S. et al. Inference and analysis of cell–cell communication using cell chat. Nat. Commun.12(1), 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single cell data is available for browsing at https://www.nationwidechildrens.org/specialties/institute-for-genomic-medicine/ped-cell-browser. Raw and processed data for single cell RNA-sequencing has been deposited to NCBI Gene Expression Omnibus (GSE253153) and for exome sequencing has been deposited to dbGaP under accession number phs001820.v1.p1.
All R code is provided as a supplemental file ‘PIK3CA_R_code.doc’.