Abstract
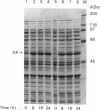
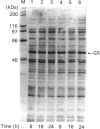
Soluble rat liver glucokinase was expressed at high levels at 22 degrees C in the BL21(DE3)pLysS strain of Escherichia coli. Aspartate-211 of yeast hexokinase has been implicated as a catalytic residue from crystallographic data. The corresponding residue in rat liver glucokinase, aspartate-205, was mutated to alanine and the expressed mutant had 1/500th of the activity of the wild type, with no change in the Km values for glucose or ATP. The results support a role for this residue as a base catalyst in the glucokinase reaction and, most probably, a similar role in the reactions of all members of the hexokinase family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreone T. L., Printz R. L., Pilkis S. J., Magnuson M. A., Granner D. K. The amino acid sequence of rat liver glucokinase deduced from cloned cDNA. J Biol Chem. 1989 Jan 5;264(1):363–369. [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Structure of a complex between yeast hexokinase A and glucose. II. Detailed comparisons of conformation and active site configuration with the native hexokinase B monomer and dimer. J Mol Biol. 1980 Jun 25;140(2):211–230. doi: 10.1016/0022-2836(80)90103-5. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Tauler A., Lange A. J., Chan K., Printz R. L., el-Maghrabi M. R., Granner D. K., Pilkis S. J. Expression of rat hepatic glucokinase in Escherichia coli. Biochem Biophys Res Commun. 1989 Dec 15;165(2):817–825. doi: 10.1016/s0006-291x(89)80039-7. [DOI] [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Mutations in the active site of Escherichia coli phosphofructokinase. Nature. 1987 Jun 4;327(6121):437–439. doi: 10.1038/327437a0. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Ucla C., Mach B. Molecular cloning of glucokinase cDNA. Developmental and dietary regulation of glucokinase mRNA in rat liver. J Biol Chem. 1987 May 5;262(13):6032–6038. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin K., Kurland I., Xu L. Z., Lange A. J., Pilkis J., el-Maghrabi M. R., Pilkis S. J. Expression of mammalian liver glycolytic/gluconeogenic enzymes in Escherichia coli: recovery of active enzyme is strain and temperature dependent. Protein Expr Purif. 1990 Nov;1(2):169–176. doi: 10.1016/1046-5928(90)90012-n. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Andreone T. L., Printz R. L., Koch S., Granner D. K. Rat glucokinase gene: structure and regulation by insulin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4838–4842. doi: 10.1073/pnas.86.13.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Moffatt B. A., Studier F. W. T7 lysozyme inhibits transcription by T7 RNA polymerase. Cell. 1987 Apr 24;49(2):221–227. doi: 10.1016/0092-8674(87)90563-0. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J. Hormonal control of hexokinase activity in animal tissues. Biochim Biophys Acta. 1970 Sep 22;215(3):461–476. doi: 10.1016/0304-4165(70)90097-8. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J. Rat hepatic glucokinase: improved purification and some properties. Arch Biochem Biophys. 1972 Apr;149(2):349–360. doi: 10.1016/0003-9861(72)90333-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab D. A., Wilson J. E. Complete amino acid sequence of rat brain hexokinase, deduced from the cloned cDNA, and proposed structure of a mammalian hexokinase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2563–2567. doi: 10.1073/pnas.86.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibrowski W., Seitz H. J. Rapid action of insulin and cyclic AMP in the regulation of functional messenger RNA coding for glucokinase in rat liver. J Biol Chem. 1984 Jan 10;259(1):343–346. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tauler A., Lange A. J., el-Maghrabi M. R., Pilkis S. J. Expression of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and its kinase domain in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7316–7320. doi: 10.1073/pnas.86.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler A., Rosenberg A. H., Colosia A., Studier F. W., Pilkis S. J. Expression of the bisphosphatase domain of rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6642–6646. doi: 10.1073/pnas.85.18.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. Regulation of glucokinase in liver. Curr Top Cell Regul. 1976;11:1–50. [PubMed] [Google Scholar]
- el-Maghrabi M. R., Pilkis S. J. Expression of rat liver fructose-1,6-bisphosphatase in Escherichia coli. Biochem Biophys Res Commun. 1991 Apr 15;176(1):137–144. doi: 10.1016/0006-291x(91)90900-r. [DOI] [PubMed] [Google Scholar]