Abstract
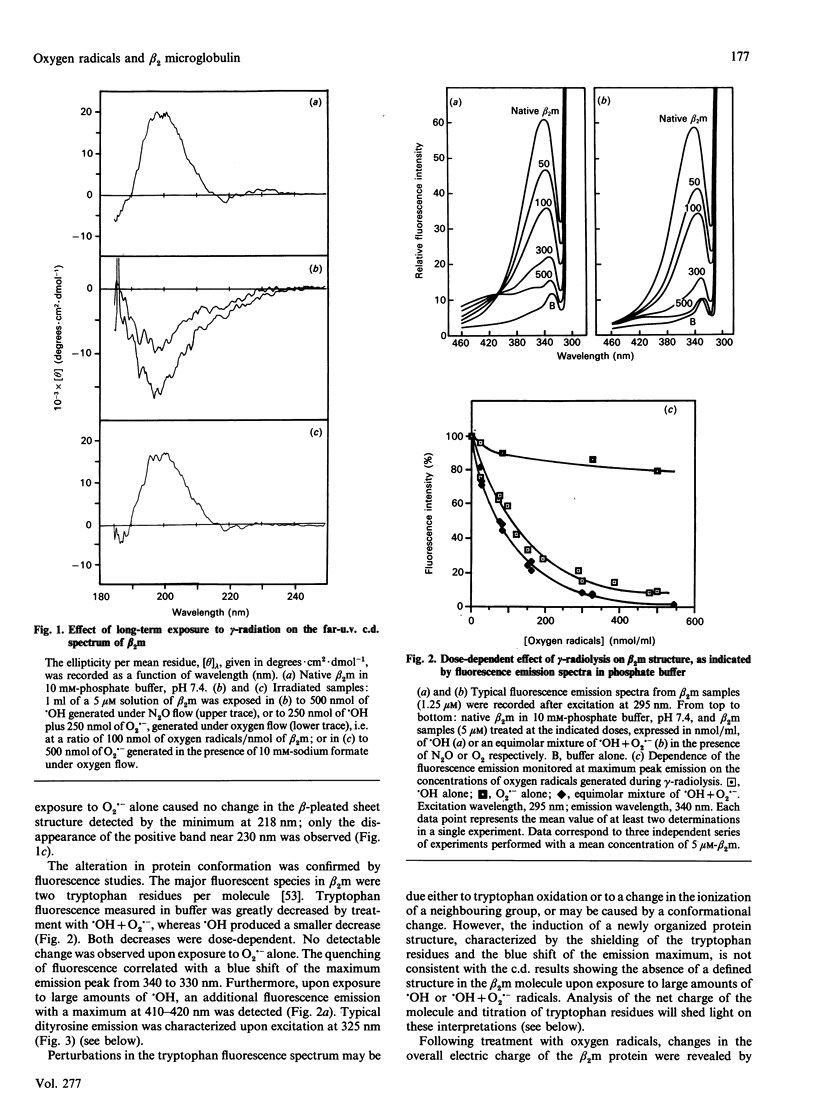
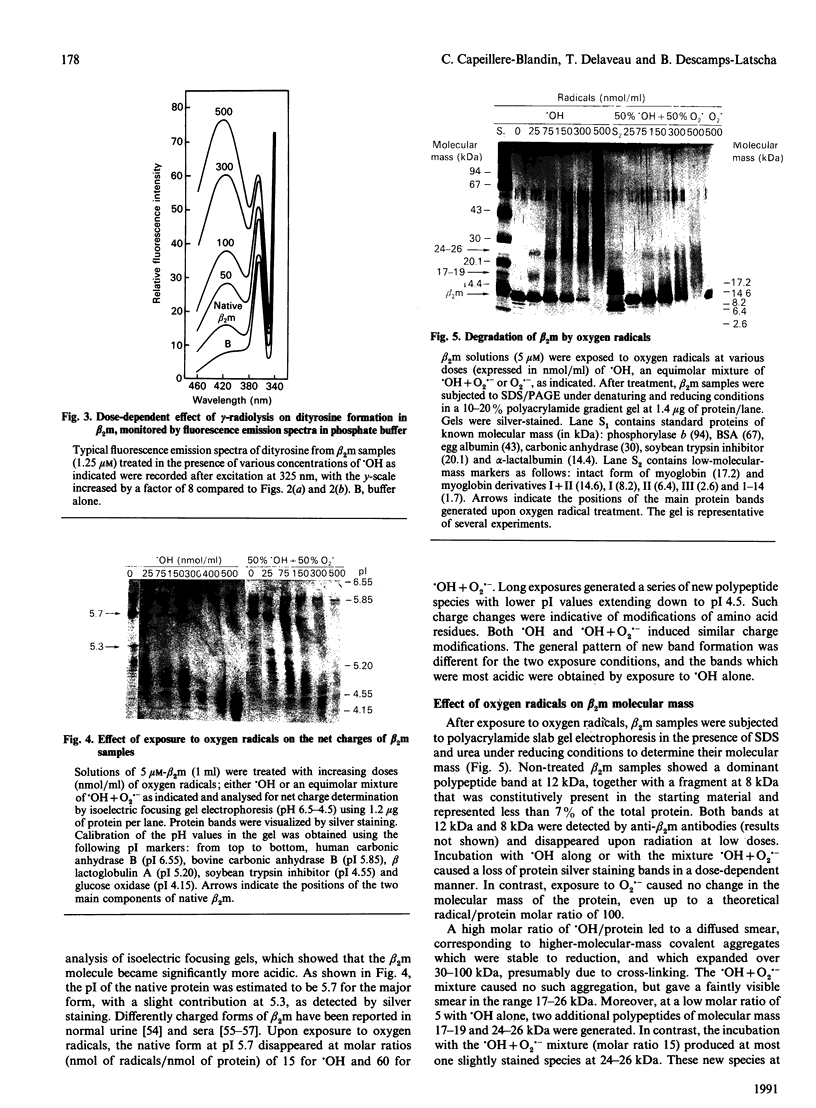
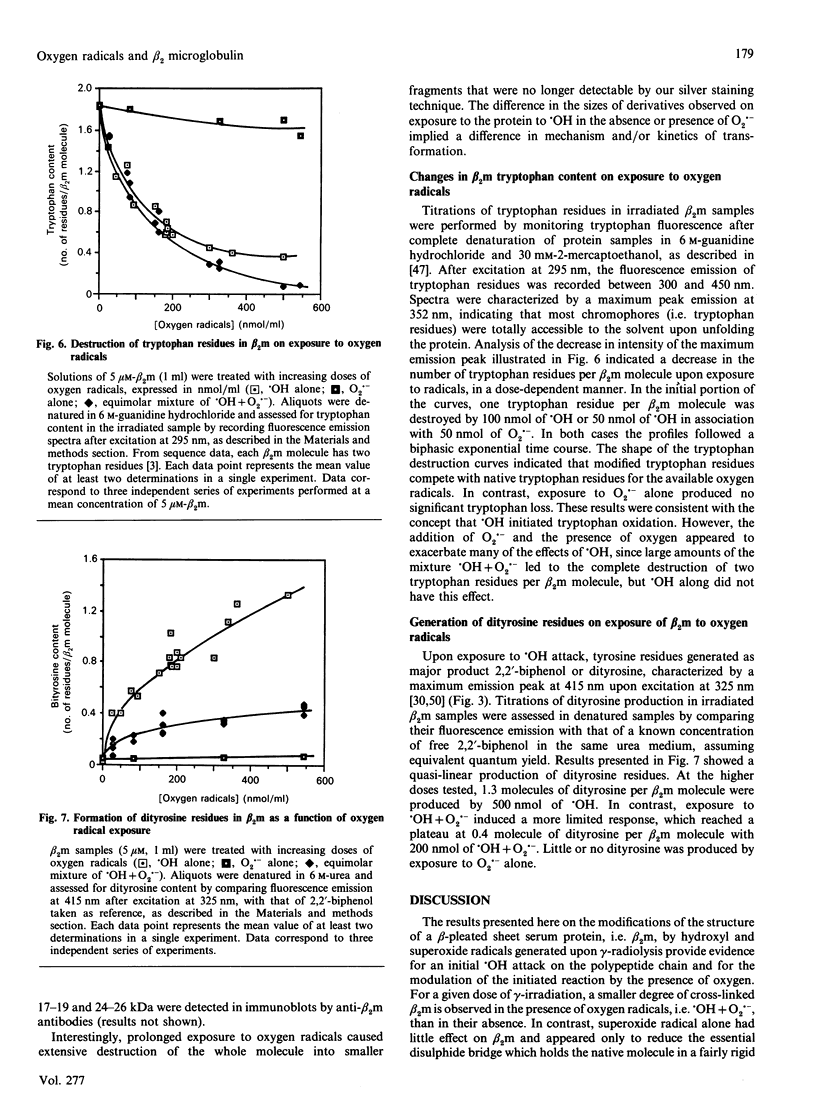
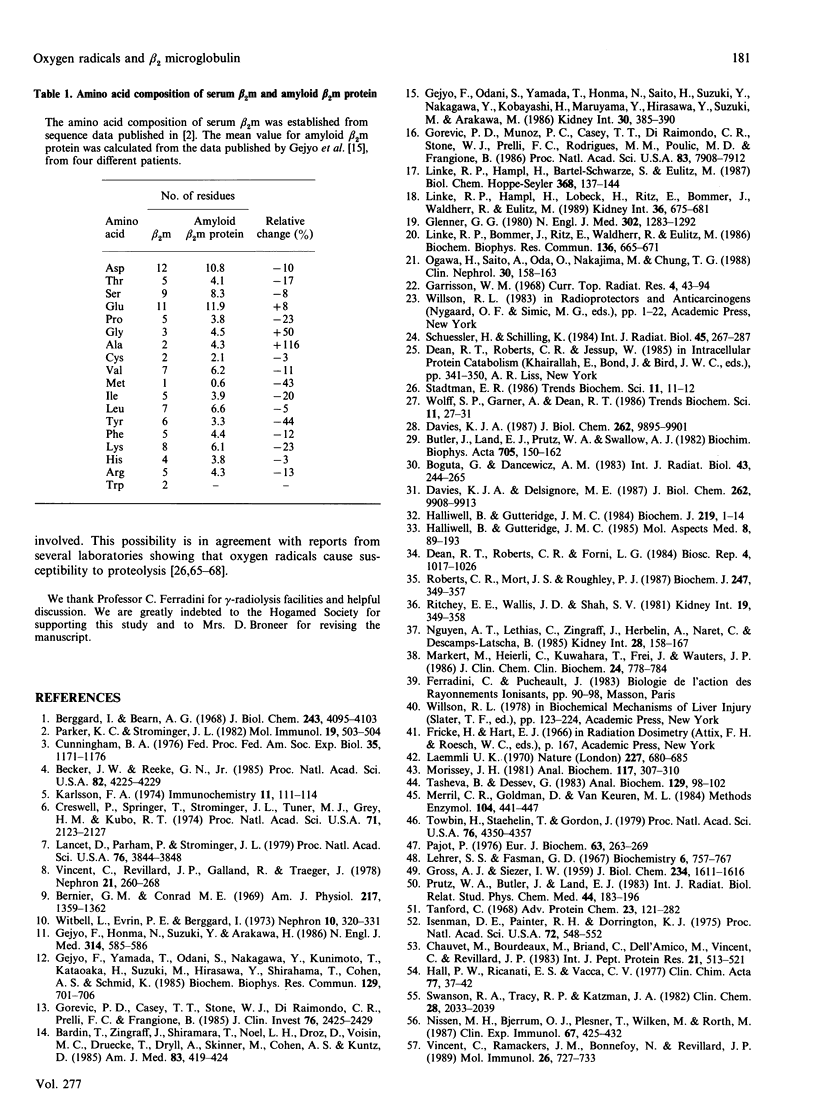
Treatment of human beta 2 microglobulin (beta 2m) with defined oxygen-derived species generated by treatment with gamma-radiation was studied. As assessed by SDS/PAGE, the hydroxyl radicals (.OH) caused the disappearance of the protein band at 12 kDa that represents beta 2m, and cross-linked the protein into protein bands stable to both SDS and reducing conditions. However, when .OH was generated under oxygen in equimolar combination with the superoxide anion radical (O2.-), the high-molecular-mass protein products were less represented, and fragmented derivatives were not obviously detectable. Exposure to .OH alone, or to .OH + O2.- in the presence of O2, induced the formation of beta 2m protein derivatives with a more acidic net electrical charge than the parent molecule. In contrast, O2.- alone had virtually no effect on molecular mass or pI. Changes in u.v. fluorescence during .OH attack indicated changes in conformation, as confirmed by c.d. spectrometry. A high concentration of radicals caused the disappearance of the beta-pleated sheet structure and the formation of a random coil structure. Loss of tryptophan and significant production of dityrosine (2,2'-biphenol type) were noted, exhibiting a clear dose-dependence with .OH alone or with .OH + O2.-. The combination of .OH + O2.- induced a pattern of changes similar to that with .OH alone, but more extensive for c.d. and tryptophan oxidation (2 Trp/beta 2m molecule), and more limited for dityrosine formation. Lower levels of these oxidative agents caused the reproducible formation of species at 18 and 25 kDa which were recognized by antibodies against native beta 2m. These findings provide a model for the protein pattern observed in beta 2m amyloidosis described in the literature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardin T., Zingraff J., Shirahama T., Noel L. H., Droz D., Voisin M. C., Drueke T., Dryll A., Skinner M., Cohen A. S. Hemodialysis-associated amyloidosis and beta-2 microglobulin. Clinical and immunohistochemical study. Am J Med. 1987 Sep;83(3):419–424. doi: 10.1016/0002-9343(87)90750-9. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Reeke G. N., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4225–4229. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bernier G. M., Conrad M. E. Catabolsm of human beta-2-microglobulin by the rat kidney. Am J Physiol. 1969 Nov;217(5):1359–1362. doi: 10.1152/ajplegacy.1969.217.5.1359. [DOI] [PubMed] [Google Scholar]
- Boguta G., Dancewicz A. M. Radiolytic and enzymatic dimerization of tyrosyl residues in insulin, ribonuclease, papain and collagen. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Mar;43(3):249–265. doi: 10.1080/09553008314550301. [DOI] [PubMed] [Google Scholar]
- Chauvet M., Bourdeaux M., Briand C., Dell'Amico M., Sarrazin M., Vincent C., Revillard J. P. Fluorescence study of human beta 2 microglobulin. Int J Pept Protein Res. 1983 May;21(5):513–521. doi: 10.1111/j.1399-3011.1983.tb02678.x. [DOI] [PubMed] [Google Scholar]
- Cresswell P., Springer T., Strominger J. L., Turner M. J., Grey H. M., Kubo R. T. Immunological identity of the small subunit of HL-A antigens and beta2-microglobulin and its turnover on the cell membrane. Proc Natl Acad Sci U S A. 1974 May;71(5):2123–2127. doi: 10.1073/pnas.71.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A. Structure and significance of beta2-microglobulin. Fed Proc. 1976 Apr;35(5):1171–1176. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987 Jul 15;262(20):9908–9913. [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220–8226. [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987 Jun 15;262(17):8227–8234. [PubMed] [Google Scholar]
- Davies K. J., Lin S. W., Pacifici R. E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987 Jul 15;262(20):9914–9920. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Forni L. G. Oxygen-centred free radicals can efficiently degrade the polypeptide of proteoglycans in whole cartilage. Biosci Rep. 1984 Dec;4(12):1017–1026. doi: 10.1007/BF01116694. [DOI] [PubMed] [Google Scholar]
- GROSS A. J., SIZER I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959 Jun;234(6):1611–1614. [PubMed] [Google Scholar]
- Gejyo F., Homma N., Suzuki Y., Arakawa M. Serum levels of beta 2-microglobulin as a new form of amyloid protein in patients undergoing long-term hemodialysis. N Engl J Med. 1986 Feb 27;314(9):585–586. doi: 10.1056/NEJM198602273140920. [DOI] [PubMed] [Google Scholar]
- Gejyo F., Odani S., Yamada T., Honma N., Saito H., Suzuki Y., Nakagawa Y., Kobayashi H., Maruyama Y., Hirasawa Y. Beta 2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986 Sep;30(3):385–390. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985 Jun 28;129(3):701–706. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Casey T. T., Stone W. J., DiRaimondo C. R., Prelli F. C., Frangione B. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985 Dec;76(6):2425–2429. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Munoz P. C., Casey T. T., DiRaimondo C. R., Stone W. J., Prelli F. C., Rodrigues M. M., Poulik M. D., Frangione B. Polymerization of intact beta 2-microglobulin in tissue causes amyloidosis in patients on chronic hemodialysis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7908–7912. doi: 10.1073/pnas.83.20.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Hall P. W., Ricanati E. S., Vacca C. V. Characterization of human beta2-microglobulin by isoelectric focusing. Clin Chim Acta. 1977 May 16;77(1):37–42. doi: 10.1016/0009-8981(77)90399-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Painter R. H., Dorrington K. J. The structure and function of immunoglobulin domains: studies with beta-2-microglobulin on the role of the intrachain disulfide bond. Proc Natl Acad Sci U S A. 1975 Feb;72(2):548–552. doi: 10.1073/pnas.72.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A. Physical-chemical properties of beta 2-microglobulin. Immunochemistry. 1974 Mar;11(3):111–114. doi: 10.1016/0019-2791(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S., Fasman G. D. Ultraviolet irradiation effects in poly-L-tyrosine and model compounds. Identification of bityrosine as a photoproduct. Biochemistry. 1967 Mar;6(3):757–767. doi: 10.1021/bi00855a017. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Bommer J., Ritz E., Waldherr R., Eulitz M. Amyloid kidney stones of uremic patients consist of beta 2-microglobulin fragments. Biochem Biophys Res Commun. 1986 Apr 29;136(2):665–671. doi: 10.1016/0006-291x(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Hampl H., Bartel-Schwarze S., Eulitz M. Beta 2-microglobulin, different fragments and polymers thereof in synovial amyloid in long-term hemodialysis. Biol Chem Hoppe Seyler. 1987 Feb;368(2):137–144. doi: 10.1515/bchm3.1987.368.1.137. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Hampl H., Lobeck H., Ritz E., Bommer J., Waldherr R., Eulitz M. Lysine-specific cleavage of beta 2-microglobulin in amyloid deposits associated with hemodialysis. Kidney Int. 1989 Oct;36(4):675–681. doi: 10.1038/ki.1989.245. [DOI] [PubMed] [Google Scholar]
- Marx G., Chevion M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem J. 1986 Jun 1;236(2):397–400. doi: 10.1042/bj2360397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nguyen A. T., Lethias C., Zingraff J., Herbelin A., Naret C., Descamps-Latscha B. Hemodialysis membrane-induced activation of phagocyte oxidative metabolism detected in vivo and in vitro within microamounts of whole blood. Kidney Int. 1985 Aug;28(2):158–167. doi: 10.1038/ki.1985.136. [DOI] [PubMed] [Google Scholar]
- Nissen M. H., Bjerrum O. J., Plesner T., Wilken M., Rørth M. Modification of beta-2-microglobulin in sera from patients with small cell lung cancer: evidence for involvement of a serine protease. Clin Exp Immunol. 1987 Feb;67(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Saito A., Oda O., Nakajima M., Chung T. G. Detection of novel beta 2-microglobulin in the serum of hemodialysis patients and its amyloidogenic predisposition. Clin Nephrol. 1988 Sep;30(3):158–163. [PubMed] [Google Scholar]
- Pajot P. Fluroescence of proteins in 6-M guanidine hydrochloride. A method for the quantitative determination of tryptophan. Eur J Biochem. 1976 Mar 16;63(1):263–269. doi: 10.1111/j.1432-1033.1976.tb10228.x. [DOI] [PubMed] [Google Scholar]
- Parker K. C., Strominger J. L. Sequence of human beta 2-microglobulin: a correction. Mol Immunol. 1982 Mar;19(3):503–504. doi: 10.1016/0161-5890(82)90217-6. [DOI] [PubMed] [Google Scholar]
- Prütz W. A., Butler J., Land E. J. Phenol coupling initiated by one-electron oxidation of tyrosine units in peptides and histone. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Aug;44(2):183–196. doi: 10.1080/09553008314550981. [DOI] [PubMed] [Google Scholar]
- Ritchey E. E., Wallin J. D., Shah S. V. Chemiluminescence and superoxide anion production by leukocytes from chronic hemodialysis patients. Kidney Int. 1981 Feb;19(2):349–358. doi: 10.1038/ki.1981.26. [DOI] [PubMed] [Google Scholar]
- Roach P. D., Noël S. P. Biotinylation of low density lipoproteins via free amino groups without loss of receptor binding activity. J Lipid Res. 1987 Dec;28(12):1508–1514. [PubMed] [Google Scholar]
- Roberts C. R., Mort J. S., Roughley P. J. Treatment of cartilage proteoglycan aggregate with hydrogen peroxide. Relationship between observed degradation products and those that occur naturally during aging. Biochem J. 1987 Oct 15;247(2):349–357. doi: 10.1042/bj2470349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler H., Schilling K. Oxygen effect in the radiolysis of proteins. Part 2. Bovine serum albumin. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Mar;45(3):267–281. doi: 10.1080/09553008414550381. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Starke-Reed P. E., Oliver C. N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989 Dec;275(2):559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Swanson R. A., Tracy R. P., Katzmann J. A., Wilson D. M., Young D. S. Beta 2-microglobulin determined by radioimmunoassay with monoclonal antibody. Clin Chem. 1982 Oct;28(10):2033–2039. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C., Ramackers J. M., Bonnefoy N., Revillard J. P. Charge heterogeneity of beta 2-microglobulin in lymphoid cells. Mol Immunol. 1989 Aug;26(8):727–733. doi: 10.1016/0161-5890(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Vincent C., Revillard J. P., Galland M., Traeger J. Serum beta2-microglobulin in hemodialyzed patients. Nephron. 1978;21(5):260–268. doi: 10.1159/000181402. [DOI] [PubMed] [Google Scholar]
- Wibell L., Evrin P. E., Berggård I. Serum 2 -microglobulin in renal disease. Nephron. 1973;10(5):320–331. doi: 10.1159/000180203. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]




