Abstract
ALYREF can recognize 5-methylcytosine (m5C) decoration throughout RNAs to regulate RNA metabolism. However, its implications in cancer and precise regulatory mechanisms remain largely elusive. Here, we demonstrated that ALYREF supported colorectal cancer (CRC) growth and migration. Integrated analysis of ALYREF-RIP-Bis-seq and transcriptome profiles identified ribosomal protein S6 kinase B2 (RPS6KB2) and regulatory-associated protein of mTOR (RPTOR) as ALYREF’s possible downstream effectors. Mechanistically, ALYREF formed a complex with ELAV like RNA binding protein 1 (ELAVL1) to cooperatively promote m5C recognition and nuclear export of the two mRNAs. Moreover, ALYREF protein was highly expressed in tumor tissues of CRC patients, which predicted their poor prognosis. E2F transcription factor 6 (E2F6)-mediated transactivation gave a molecular insight into ALYREF overexpression. Collectively, ALYREF recruits ELAVL1 to collaboratively facilitate m5C recognition and nuclear export of RPS6KB2 and RPTOR transcripts for colorectal tumorigenesis, providing RNA m5C methylation as promising therapeutic targets and prognostic biomarkers for CRC.
Subject terms: Colorectal cancer, Predictive markers, Oncogenes, Epigenetics, Cell growth
Introduction
Colorectal cancer (CRC) was the third most common and the second most deadly cancer worldwide in 2020, accounting for 9% of newly diagnosed cancer cases and cancer deaths1. Its global burden aggravates as early-onset CRC incidence rapidly grows2,3. Although novel therapeutic strategies against CRC have brought great survival benefits4, their clinical needs are still far from reached. It is critical to delineate the molecular mechanisms of CRC development, thereby developing novel treatment options and improving CRC patients’ outcomes.
As an RNA modification, RNA m5C deposition uncovers a new layer of posttranscriptional epigenetic reprogramming. It is ubiquitously distributed within every region of mRNAs and many other kinds of RNAs5–8. More importantly, it regulates almost every aspect of RNA metabolism to exert numerous biological functions. Dysregulated m5C deposition is widespread among and indispensable for human diseases especially cancer. But how does it control RNA metabolism to take crucial roles in cancer? RNA m5C modulation is reversibly and dynamically catalyzed, removed and recognized by writers/methyltransferases (NSUN1-7 and DNMT1-3B), erasers/demethylases (TET1-3 and ALKBH1) and readers/RNA binding proteins (ALYREF, YBX1, LIN28B, YTHDF2 and SRSF2), respectively 5–11. These writers and erasers cooperatively regulate RNA m5C levels while m5C readers determine which aspect of RNA metabolic processes is affected after m5C recognition6,8. As the best-characterized m5C regulator, NSUN2 confers oncogenic hallmarks through modulating the m5C level of various transcripts and regulating their metabolism in multiple cancer types6,7,12–19. More and more m5C writers and erasers are confirmed to similarly mediate such posttranscriptional regulation for cancer growth and progression20–22. Nevertheless, how m5C writers and erasers regulate RNA metabolism with the help of m5C readers in cancer is largely unknown. The links between m5C readers and cancer remain far from understood.
Aly/REF export factor (ALYREF) is identified as the first m5C reader that recognizes m5C-methylated RNAs through its K171 residue23. It belongs to the THO complex family and is also called THOC subunit 4 (THOC4). It serves as an essential transcription-export complex (TREX) component24,25 and thus an RNA nuclear export factor. Recent studies have illustrated that ALYREF fine-tunes several RNA metabolic processes in an m5C-dependent manner, facilitating nucleocytoplasmic shuttling, stabilization, splicing and translation of target transcripts12,13,20–22,26–31. ALYREF has been also proved to be a potential predictive biomarker and therapeutic target in some of tumor types25. Nonetheless, the intricate cascades of ALYREF-centered molecular events are still poorly understood, especially its involvement and clinical significance in CRC. Beyond ALYREF, YBX1 and LIN28B can also behave as an m5C reader to maintain mRNA stability for tumorigenesis6,7. More cancer-associated m5C readers and the aspects of RNA metabolic processes they influence remain to be clarified. Most of studies only focus on what downstream effectors of m5C readers are, the precise molecular insights into how m5C readers regulate RNA metabolism is almost unknown. Recent studies have revealed that YBX1 can recruit ELAVL1 and THOC3 to facilitate RNA m5C recognition in cancer6,32. Partners of m5C readers, ALYREF included, are still unclear.
A pan-cancer analysis revealed the mTOR complex 1 (mTORC1) cascade as ALYREF-related pathway33. mTORC1 cascade is a canonical pro-tumorigenic pathway that extensively impacts growth, metastasis, apoptosis, angiogenesis, autophagy, metabolism and immunity34,35. mTORC1 can be activated by PI3K/AKT and MAPK signaling, and then functions through downstream targets including Ribosomal protein S6 kinase (RPS6K) and 4E-binding protein 1 (4E-BP1)34,35. In this cascade, RPS6KB2 and regulatory-associated protein of mTOR (RPTOR) the two components we screened are essential. RPS6KB2 encodes a pivotal substrate of mTORC1 called S6K2, sharing 83% identity in the kinase domain with its homolog S6K136,37. Unlike S6K1 widely-studied and well-recognized, S6K2 was otherwise overlooked as a redundant protein. But the fact is that S6K2 has its own downstream targets different from S6K1 to exerts oncogenic functions, for instance, hnRNPF, B-Raf, PDCD4 and AKT38–41. For another, RPTOR encodes the Raptor protein that is the unique and core component of mTORC1. RPTOR contributes to metastasis, angiogenesis and drug resistance in CRC42–44. It also takes critical parts in growth, autophagy and immunity among other cancers45–48. Nevertheless, whether and how ALYREF regulates the mTOR-related molecules in CRC remains elusive.
In the present study, we unveil ALYREF-endowed oncogenic traits in CRC. Specifically, E2F6 is indispensable to transcriptionally activate ALYREF. In collaboration with ELAVL1, ALYREF binds to and exports m5C-modified RPS6KB2 and RPTOR mRNAs for colorectal tumorigenesis. These findings decipher the epitranscriptomic code of CRC progression and shed light upon novel promising epigenetic therapies against CRC.
Results
ALYREF promotes growth and migration in CRC
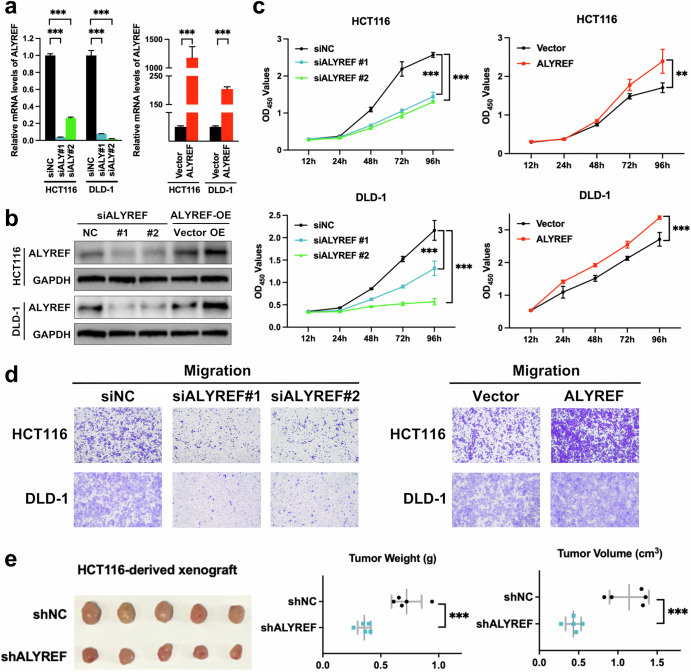
To clarify the biological hallmarks of ALYREF in CRC, we carried out both gain- and loss-of-function experiments. ALYREF was knocked down with two ALYREF-specific siRNAs and forced overexpressed by a plasmid harboring ALYREF, respectively (Fig. 1a, b). CCK-8 and Transwell findings displayed that knockdown of ALYREF suppressed cell proliferation and migration capabilities of CRC cells (Fig. 1c, d, Supplementary Fig. 1a). Consistent with these results, plasmid-mediated ectopic expression of ALYREF enhanced growth and migration abilities of CRC cells (Fig. 1c, d). Moreover, HCT116 cells transduced with lentiviral vectors were subcutaneously inoculated into BALB/c nude mice. In parallel to the in vitro findings, knockdown of ALYREF attenuated CRC tumor growth (Fig. 1e). Hence, these data indicated that ALYREF was responsible for colorectal tumorigenesis and served as a promising therapeutic target for CRC.
Fig. 1. ALYREF is required for the growth and migration capacity of CRC.
a–d CRC cells were exposed to specific siRNAs or plasmids. RNA level, protein level, cell viability and migration capability were detected by RT-qPCR (a), immunoblot (b), CCK-8 (c) and Transwell (d) assays, respectively. e HCT116 cells transduced with lentivirus vectors were subcutaneously inoculated into Balb/c nude mice (n = 5). Tumors were stripped, weighed and measured three weeks after the implantations. Data were presented as the mean ± SD. ALY ALYREF. **P < 0.01; ***P < 0.001; two-sided Student’s t test.
RPS6KB2 and RPTOR serve as downstream targets of ALYREF
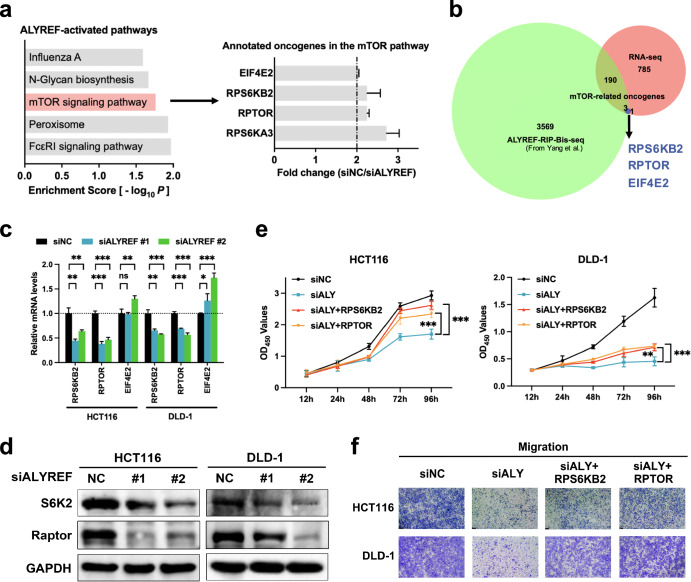
To screen candidate target transcripts and downstream signaling pathways of ALYREF, we performed transcriptome analysis in DLD-1 cells transfected with siALYREF or siNC. Gene expression patterns of the two groups are presented in a heatmap (Supplementary Fig. 2a). Comparing the two gene expression profiles, we identified 2467 differentially expressed genes, of which 979 were positively regulated by ALYREF ( | Log2FC | ≥ 1, P < 0.05, FPKM ≥ 0.1; Supplementary Fig. 2b, Supplementary Data 1). KEGG pathway enrichment analysis listed the mTOR pathway as a most significant ALYREF-activated signaling cascade (Fig. 2a). We then overlapped mTOR-related oncogenes annotated in our RNA-seq data (Fig. 2a) with ALYREF-RIP-Bis-seq data from Yang et al. 23 to identify possible ALYREF-bound mTOR-related transcripts once m5C-methylated. Among the three molecules selected (Fig. 2b), RT-qPCR and immunoblot findings further restricted ALYREF’s downstream targets to RPS6KB2 and RPTOR (Fig. 2c, d). In addition, rescue assays were conducted to investigate the necessity of PRS6KB2 and RPTOR in ALYREF-provoked malignancy. CCK-8 and Transwell results manifested that ALYREF knockdown inhibited proliferation and migration capacity of CRC cells, which could be blocked by overexpression of either RPS6KB2 or RPTOR (Fig. 2e, f). Moreover, the inhibitory effect of ALYREF knockdown on CRC cell proliferation could be further potentiated by the simultaneous knockdown with RPS6KB2 or RPTOR (Supplementary Fig. 3). Altogether, ALYREF promoted colorectal tumorigenesis through regulating RPS6KB2 and RPTOR.
Fig. 2. RPS6KB2 and RPTOR are mTOR-related downstream transcripts of ALYREF.
a Transcriptome analysis was conducted in DLD-1 cells treated with siALYREF or siNC. KEGG pathway enrichment analysis revealed the top five significant ALYREF-activated pathways and oncogenes annotated in the mTOR pathway. b Venn plot showing the overlay of ALYREF-RIP-bis-seq data from Yang et al., ALYREF-upregulated genes and oncogenes annotated in mTOR signaling from our RNA-seq data. c, d Transcripts screened were reverified with RT-qPCR and immunoblot analysis. RPS6KB2 and RPTOR encode S6K2 and Raptor protein, respectively. e, f Cell proliferation and migration capabilities in rescue assays were assessed by CCK8 (e) and Transwell (f) techniques, respectively. Data were presented as the mean ± SD. ALY ALYREF. *P < 0.05; **P < 0.01; ***P < 0.001; ns not significant; two-tailed Student’s t test.
ALYREF acts as an m5C reader to export RPS6KB2 and RPTOR transcripts
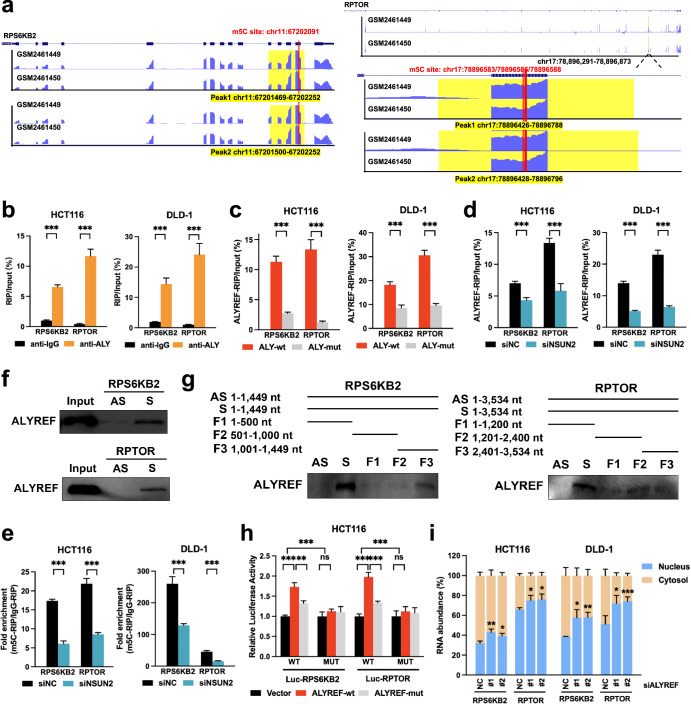
ALYREF has been recently identified as an RNA-interacting protein for m5C-containing transcripts, of which lysine residue K171 is its specific binding site13,23. We thus validated whether ALYREF regulated RPS6KB2 and RPTOR transcripts in an m5C-dependent manner. Putative binding sites were profiled using ALYREF RIP-Bis-seq data from Yang et al. 23, all of which were located on their CDS regions (Fig. 3a, Supplementary Table 1, 2). RIP analysis showed that RPS6KB2 and RPTOR mRNAs were enriched in complexes immunoprecipitated with antibodies against ALYREF compared to control IgG (Fig. 3b). ALYREF K171A mutation defective in its m5C recognition function significantly reduced the binding of ALYREF to RPS6KB2 and RPTOR transcripts (Fig. 3c). Moreover, NSUN2 knockdown repressed binding efficiency of ALYREF to RPS6KB2 and RPTOR mRNAs (Fig. 3d, Supplementary Fig. 4). m5C-RIP assays were performed with designed primers covering the aforementioned ALYREF-bound m5C sites. NSUN2 silencing lowered the m5C levels of RPS6KB2 and RPTOR mRNAs (Fig. 3e, Supplementary Fig. 4). Their direct interactions were then investigated by RNA pull-down assays using in vitro transcribed and biotinylated probes of the two mRNA fragments. ALYREF protein could directly bind to the RPS6KB2 and RPTOR mRNA CDS regions, and ALYREF-interacting regions were specifically mapped to the RPS6KB2 F3 fragment and RPTOR F2 fragment, respectively (Fig. 3f, g). To test whether the m5C sites of RPS6KB2 and RPTOR were required for ALYREF to increase RPS6KB2/RPTOR expression, RPS6KB2 and RPTOR reporters were constructed for dual luciferase reporter assays. The CDS fragments of RPS6KB2 and RPTOR covering ALYREF-recognized m5C sites were cloned into their wild-type forms whereas these m5C sites were defective in their mutant forms. Our results revealed that compared to the control, forced expression of wild-type ALYREF enhanced the luciferase activity of wild-type RPS6KB2/RPTOR but failed to increase that of mutant RPS6KB2/RPTOR (Fig. 3h). It indicated that ALYREF could specifically bind to these m5C sites of RPS6KB2/RPTOR. RPS6KB2/RPTOR mRNA expression required m5C modification modulated by ALYREF. Moreover, ectopic expression of K171A mutant ALYREF (defective in m5C recognition function) has lower luciferase activity of wild-type RPS6KB2/RPTOR than forced expression of wild-type ALYREF (Fig. 3h), suggesting that ALYREF bound to RPS6KB2/RPTOR transcripts in an m5C-dependent manner. These data thus indicated that ALYREF could recognize and bind to m5C-methylated RPS6KB2 and RPTOR transcripts.
Fig. 3. ALYREF serves as an m5C reader to export RPS6KB2 and RPTOR transcripts.
a ALYREF-RIP-Bis-seq from Yang et al. screened ALYREF-recognized m5C sites in the target transcripts. b–d Lysates from naïve or transfected cells were subjected to RIP assays with antibodies against ALYREF or control IgG. The RIP enrichment of a certain sample was normalized to its input. e Lysates from siRNA-transfected cells were subjected to m5C-RIP assays using anti-m5C or control anti-IgG antibodies. Transcripts of interest were detected by specific primers covering m5C sites. The m5C enrichment of each sample was expressed as the fold change in the m5C-RIP group normalized to the IgG-RIP group. f, g Biotin-labeled RNA probes against full-length CDS, their antisense sequences and fragments were subjected to RNA pull-down assays. The protein of interest was detected by anti-ALYREF antibody. h pmirGLO reporter plasmids (RPS6KB2-wt/mut or RPTOR-wt/mut) were co-transfected with plasmids as indicated for dual luciferase reporter assays. The relative luciferase activity was calculated as the ratio of firefly luciferase signal to Renilla luciferase signal and normalized to siNC. i After siRNA silencing, the nucleocytoplasmic distribution of RPS6KB2 and RPTOR mRNAs was measured by RT-qPCR following subcellular fractionation. Data were presented as the mean ± SD. ALY ALYREF; wt wild type; mut mutant. *P < 0.05; **P < 0.01; ***P < 0.001; ns not significant; two-sided Student’s t test.
What happened after ALYREF-mediated m5C recognition? Recent studies have showed that ALYREF can facilitate nuclear export of m5C-decorated RNAs12,21,26–28. Subcellular fractionation results further demonstrated that ALYREF silencing reduced the nucleocytoplasmic shuttling of RPS6KB2 and RPTOR transcripts in CRC cells, manifested by increased nuclear retention (Fig. 3i). Taken together, ALYREF recognized m5C-methylated RPS6KB2 and RPTOR mRNAs to enhance their nuclear export and expression in CRC.
ELAVL1 directly binds to both the ALYREF protein and target transcripts
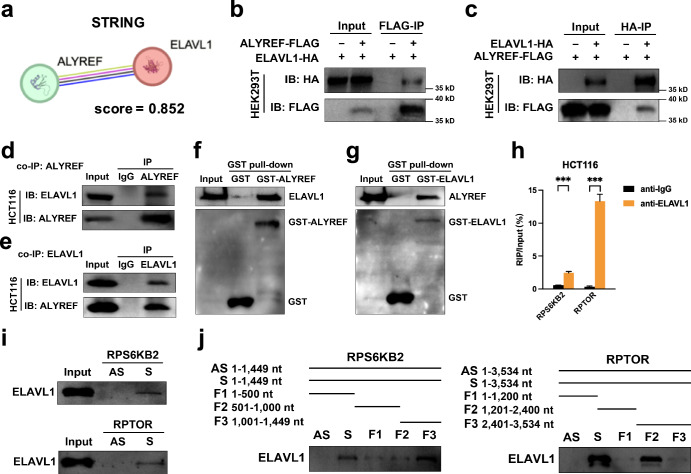
To elaborate how ALYREF control the fates of target mRNAs, we sought its possible partners that can also interact with target transcripts. We focused on ELAVL1 that is a candidate protein interactor of ALYREF predicted by STRING online tool (Fig. 4a), involved in RNA methylation6,49–51 and an mRNA nuclear exporter52,53. We first investigated protein interactions between ALYREF and ELAVL1. This interplay was confirmed by co-IP assays. Exogenous co-IP analysis using ALYREF-Flag and ELAVL1-HA plasmids showed that the ELAVL1 protein was immunoprecipitated with anti-Flag M2 agarose beads (Fig. 4b) and the ALYREF protein was conversely by anti-HA agarose beads (Fig. 4c). Endogenous co-IP analysis with protein A/G magnetic beads reverified that the ELAVL1 protein was present in protein complexes immunoprecipitated with anti-ALYREF antibody (Fig. 4d) and reciprocally the ALYREF protein was enriched in protein immunoprecipitate bound by anti-ELAVL1 antibody (Fig. 4e). The physical protein interaction of ALYREF and ELAVL1 was also tested by GST pull-down assays. His-tagged ELAVL1 bound to GST-ALYREF but not the GST peptide alone (Fig. 4f), and His-tagged ALYREF bound to GST-ELAVL1 but not the GST peptide alone (Fig. 4g). Herein, their direct interplay suggested that ALYREF utilized ELAVL1 as a partner.
Fig. 4. The ELAVL1 protein directly interacts with the ALYREF protein and target mRNAs.
a ALYREF-ELAVL1 protein interaction was predicted by STRING online tool. b, c Exogenous co-IP assays were conducted with anti-Flag M2 (b) or anti-HA (c) agarose beads. d, e Endogenous co-IP assays were conducted with protein A/G magnetic beads to capture primary antibodies against ALYREF (d) or ELAVL1 (e). f, g GST pull-down assays were performed with purified His-tagged protein and spin columns containing GST-tagged proteins or GST alone. h Lysates from naïve cells were subjected to RIP assays with antibodies against ELAVL1 or control IgG. The RIP enrichment of a certain sample was normalized to its input. i, j Biotin-labeled RNA probes against full-length CDS, their antisense sequences and fragments were subjected to RNA pull-down assays. The protein of interest was detected by an anti-ELAVL1 antibody.
We further assessed the interactions between the ELAVL1 protein and target mRNAs. RIP analysis showed an enrichment of RPS6KB2 and RPTOR mRNAs in ELAVL1-immunoprecipitated complexes (Fig. 4h). The RNA pull-down technique was also used to test their direct interaction and delineate their binding regions. ELAVL1 directly coupled to CDS regions of RPS6KB2 and RPTOR mRNAs (Fig. 4i), more specifically the RPS6KB2 F3 fragment and RPTOR F2 fragment (Fig. 4j). In summary, ELAVL1 could directly bind to both the ALYREF protein and target transcripts.
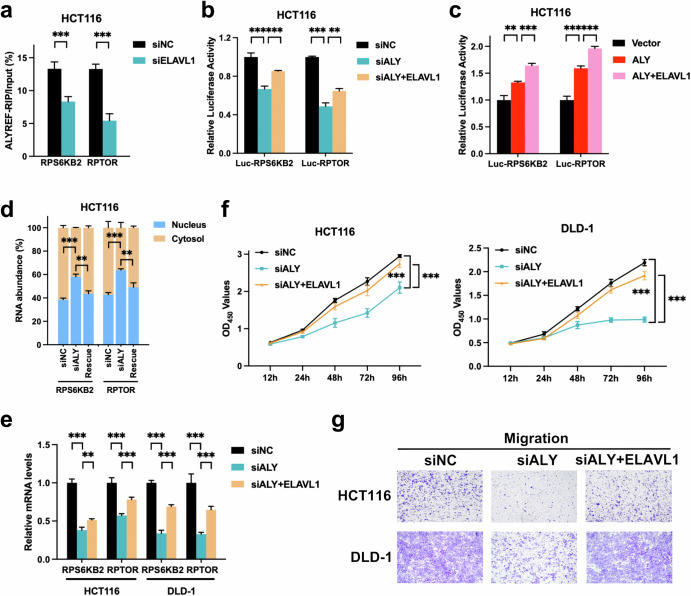
ALYREF and ELAVL1 cooperatively increased m5C recognition and nuclear export of target mRNAs
As ELAVL1 directly bound to the ALYREF protein as well as target transcripts, we reasoned that ALYREF recruited ELAVL1 for cooperative posttranscriptional regulation of target mRNAs. RIP assays were first conducted with the anti-ALYREF antibody to test the impact of ELAVL1 on ALYREF’s binding to target transcripts. RIP data revealed that ALYREF’s binding to the two mRNAs was both attenuated by ELAVL1 knockdown (Fig. 5a). To investigate whether ELAVL1 involved in ALYREF’s binding to m5C sites of target transcripts, dual luciferase reporter assays were conducted using the same wild-type RPS6KB2/RPTOR reporters as above (Fig. 3h). ALYREF knockdown expectedly decreased the luciferase activity of wild-type RPS6KB2 and RPTOR, which could be abrogated by ectopic expression of ELAVL1 (Fig. 5b). Besides, forced expression of ALYREF actually increased the luciferase activity of wild-type RPS6KB2 and RPTOR, which could be further strengthened by ectopic expression of ELAVL1 (Fig. 5c). These data indicated that ALYREF and ELAVL1 cooperatively bound to m5C sites of RPS6KB2 and RPTOR transcripts. To conclude, ALYREF and ELAVL1 coordinated to increase m5C recognition of RPS6KB2 and RPTOR transcripts.
Fig. 5. ALYREF recruits ELAVL1 to cooperatively facilitate m5C recognition and export target transcripts for colorectal tumorigenesis.
a control and treated CRC cells were harvested for RIP assays with anti-ALYREF or control anti-IgG antibodies. RIP enrichment of certain samples was quantified and normalized to the input. b, c pmirGLO reporter plasmids (RPS6KB2-wt/mut or RPTOR-wt/mut) were co-transfected with plasmids/siRNAs as indicated for dual luciferase reporter assays. The relative luciferase activity of each sample was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity and normalized to siNC. d–g RNA nucleocytoplasmic shuttling, RNA expression level, growth ability and migration capability were quantified by subcellular fractionation (d), RT-qPCR (e), CCK-8 (f) and Transwell (g) techniques in rescue experiments, respectively. Data were presented as the mean ± SD. ALY ALYREF. Rescue, siALYREF + ELAVL1. **P < 0.01; ***P < 0.001; two-sided Student’s t test.
We then examined whether ELAVL1 recruitment participated in downstream RNA metabolism and biological actions of ALYREF-mediated m5C recognition. As expected, subcellular fractionation results showed that ectopic expression of ELAVL1 rescued the inhibitory effects of ALYREF knockdown on nuclear export of the two transcripts in CRC (Fig. 5d). RT-qPCR analysis demonstrated that forced expression of ELAVL1 also blocked the inhibitory effects of ALYREF silencing on the expression levels of the two target mRNAs in CRC (Fig. 5e). Moreover, CCK8 and Transwell observations exhibited that ALYREF knockdown reduced the growth and migration activities of CRC cells, which could be mitigated by ELAVL1 overexpression (Fig. 5f, g). In summary, ALYREF formed a complex with ELAVL1 to collaboratively increase m5C recognition, nuclear export and expressions of RPS6KB2 and RPTOR mRNAs for colorectal tumorigenesis.
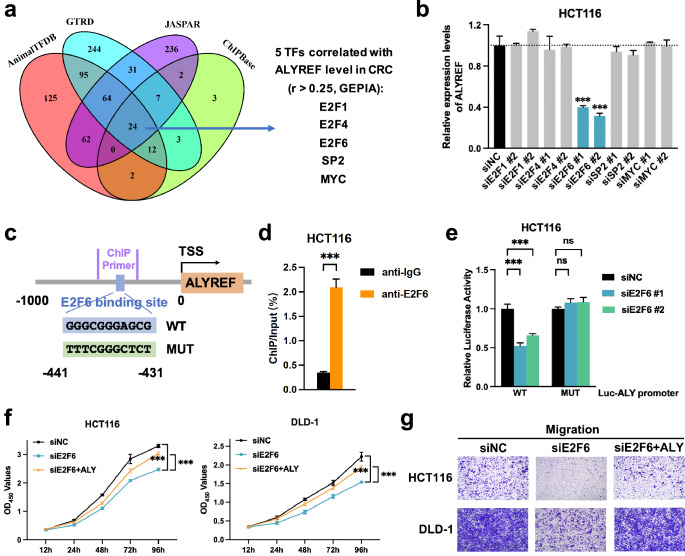
E2F6 serves as an upstream transcriptional activator of ALYREF
To explicate ALYREF’s possible upstream regulator, four common online databases were integrated (JASPAR, AnimalTFDB 3.0, GTRD and ChIPBase V2.0) to prescreen 24 candidate transcriptional factors binding to the cis-element of the ALYREF promoter (within 1000 bp upstream of the transcription start site, Fig. 6a). Five of the candidates positively correlated with the ALYREF level (r > 0.25, GEPIA) were validated. Only E2F6-specific siRNAs remarkably downregulated the ALYREF mRNA and protein level in HCT116 cells (Fig. 6b, Supplementary Figs. 5 and 6). We then used the ChIP-qPCR experiment to verify E2F6’s binding to ALYREF promoter. The transcriptional factor E2F6 bound to the most likely cis-element of the ALYREF promoter predicted by JASPAR database (Fig. 6c, d). Whether E2F6 coupled to the predicted specific site of the ALYREF promoter was further confirmed by dual luciferase reporter assay. The data showed that E2F6-specific siRNAs significantly decreased the luciferase activity of the ALYREF wild-type promoter reporter but not its mutant (Fig. 6e). To confirm whether the above binding influenced biological activities, rescue experiments were performed. CCK8 and Transwell results manifested that growth and migration capabilities were reduced in CRC cells exposed to the E2F6-specific siRNA, both of which could be mitigated by the plasmid harboring ALYREF (Fig. 6f, g), suggesting that E2F6-mediated proliferation and migration of CRC cells in an ALYREF-dependent manner. Taken together, E2F6 served as an upstream transcriptional activator of ALYREF in CRC.
Fig. 6. E2F6 serves as an upstream transcriptional activator of ALYREF.
a, b Candidate transcriptional factors bound to the ALYREF promoter were predicted in silico (a) and verified with RT-qPCR (b). c Schematic plot of the putative E2F6 binding site in the ALYREF promoter. d Naïve cells were harvested for ChIP assays with anti-E2F6 or anti-IgG antibodies. ChIP enrichment was quantified by RT-qPCR with specific primers for the ALYREF promoter and normalized to the input. e pGL3 reporter plasmid carrying the ALYREF-wt/mut promoter was co-transfected with Renilla reporter plasmid and siRNAs as indicated for dual luciferase reporter assays. Relative luciferase activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity and normalized to siNC. f, g Growth and migration capabilities were quantified by CCK-8 (f) and Transwell (g) techniques in rescue experiments, respectively. Data were presented as the mean ± SD. ALY ALYREF. ***P < 0.001; ns not significant; two-sided Student’s t test.
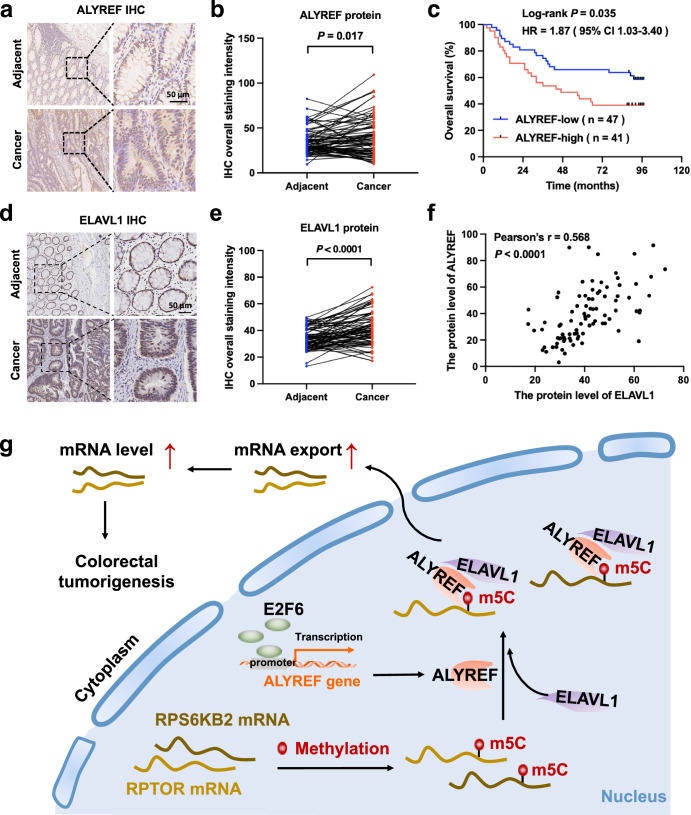
ALYREF protein is elevated in CRC patients and predicts their poor outcomes
To analyze the clinical relevance of ALYREF expression in CRC, ALYREF protein levels were detected in a tissue microarray with IHC. Compared to paired normal tissues, ALYREF protein was highly expressed in CRC tissues (Fig. 7a, b). The Kaplan-Meier survival plot illustrated that the elevated ALYREF protein level shortened the overall survival of CRC patients (log-rank P = 0.035; Fig. 7c). Moreover, ELAVL1 expression pattern of the same tissue microarray was also analyzed. IHC results revealed that ELAVL1 protein level was higher in CRC tissues than that of adjacent normal tissues (Fig. 7d, e). The protein expression level of ELAVL1 was positively correlated to that of ALYREF in CRC patients (Pearson’s r = 0.568, P < 0.0001; Fig. 7f). These data indicated the prognostic value of ALYREF expression and its positive correlation with ELAVL1 expression in CRC patients.
Fig. 7. ALYREF is highly expressed in CRC patients with poor outcomes.
a–f ALYREF and ELAVL1 protein expression in CRC tissues and matched normal tissues from a tissue microarray was assessed using IHC. Typical images (a, d) are shown, together with expression (b, e), survival (c) and correlation results (f). Scale bar, 50 µm. Paired t test in (b, e), Log-rank test in (c) and Pearson’s correlation test in (f). g Schematic presentation of the ALYREF/ELAVL1/m5C axis in RNA export and colorectal tumorigenesis. E2F6-transactivated ALYREF interacted with ELAVL1 to cooperatively facilitate m5C recognition and nuclear export of RPS6KB2 and RPTOR transcripts, thereby increasing their expressions and tumor growth in CRC.
Discussion
CRC is a life-threatening gastrointestinal disorder and its outcomes await improvement, which relies on every effort to unmask its molecular events as deeply and thoroughly as possible. Growing evidence has revealed that aberrant RNA m5C methylation plays crucial parts in cancer initiation and progression. Most of studies focus on oncogenic features of the m5C writer NSUN2 in various cancer types6,7,12–19. But what roles other m5C regulators play in cancer and their precise regulatory networks are largely unknown. RNA binding proteins are particularly underestimated cancer culprits54. We initially verified that ALYREF served as an m5C reader to promote colorectal tumorigenesis (Figs. 1–3, 7g). Besides, epitranscriptomic-targeted antitumor therapies are being developed5. ALYREF’s oncogenic behaviors assure a promising therapeutic target. The elevated ALYREF protein level of prognostic value in CRC tissues further warrants a possible clinical targeting population and a compelling prognostic biomarker (Fig. 7a–c). Molecular insights into its dysregulation, otherwise, remain elusive. A possible reason lies in ALYREF’s high amplification frequency from TCGA and CCLE CRC cohorts33. Transcriptional regulation can give another explanation, which remains uninvestigated among all m5C regulators except for E2F1-transactivated NSUN2 in esophageal cancer7. We initially identified E2F6 as an upstream transcriptional activator of ALYREF (Fig. 6). Moreover, there are other potential mechanisms behind ALYREF elevation to be discovered in future studies. Collectively, ALYREF embraces a bright future as a novel therapeutic target and prognostic biomarker for CRC.
As we know, m5C readers interpret m5C signals of target transcripts to dictate RNA metabolic and biological processes6,8,55. ALYREF works as an m5C reader to facilitate several aspects of RNA metabolism, RNA nuclear export in particular12,21,26–28. Nevertheless, how ALYREF-promoted colorectal tumorigenesis with its m5C recognition functions remains unclear. Integrating our transcriptome data with the ALYREF-RIP-Bis-seq profile published23, we restricted ALYREF’s downstream transcripts to RPS6KB2 and RPTOR (Fig. 2) that separately encode the well-known mTORC1 substrate S6K2 and unique component Raptor37,56. It was confirmed that ALYREF recognized RPS6KB2 and RPTOR transcripts m5C-methylated by NSUN2 to enhance their nuclear export and expression for colorectal tumorigenesis (Figs. 3, 7g). The findings decoded the indispensable role of ALYREF-mediated m5C recognition and downstream RNA export in colorectal oncogenic features.
Some investigators have recently tried to gain a molecular insight into how m5C readers regulate RNA metabolism in terms of their interacting proteins. YBX1 can form a complex with its partners, ELAVL1 or THOC3, to facilitate RNA m5C recognition and stabilization in cancer6,32. In the light of the studies, the present work initially sought ALYREF’s potential partner. Our attention was paid to ELAVL1 that is an m5C reader YBX1-interacting protein, a predicted ALYREF’s partner from the STRING website (Fig. 4a) and an mRNA export adaptor6,52,53. We for the first time unveiled that ALYREF formed a complex with ELAVL1 to cooperatively facilitate m5C recognition and nuclear export of RPS6KB2 and RPTOR transcripts, thereby promoting colorectal tumorigenesis (Figs. 4, 5, 7g). It should be noticed that whether ELAVL1 is exactly indispensable for ALYREF’s RNA export function and oncogenic hallmark needs to be further confirmed using the CRISPR-Cas9 system. Another question is how ELAVL1 facilitates export of m5C-decorated target mRNAs after being recruited by ALYREF? As we know, ELAVL1 can export RNAs from nucleus to cytoplasm via several routes. The best-characterized way is used by RNA cargos harboring AU-rich elements (AREs) that are typically defined as the core motif AUUUA in 3’UTR regions and widely extended to AU/GU/U-rich elements57. ELAVL1 initially interacts with ARE-containing mRNAs in the nucleus, and then exports them with the help of nuclear export signals from other adaptors or itself HNS domain53,58–64. Beyond AREs, another cis-acting element is the posttranscriptional regulatory element where ELAVL1 can binds for exporting CD83 mRNA65,66. Moreover, increasing evidence has shown interplays between ELAVL1 and m5C/m6A-methylated transcripts with its roles in RNA export obscure6,49–51,67. How to enhance the m5C-methylated RNA export upon ELAVL1 recruitment of ALYREF deserves deeper investigations in the future. Which way ELAVL1 employs and if another way exits should be further determined. One more thing to note, there are two canonical pathways to access RNA export: the NXF1- and CRM1-dependent pathways that may separately involve ALYREF and ELAVL1 adaptors53. Their crosstalk from the perspective of ALYREF-ELAVL1 protein interaction is unclarified. Intriguingly, our data found that ALYREF and ELAVL1 could recruit each other to increase their bindings to RPS6KB2 and RPTOR transcripts (Fig. 5a–c, Supplementary Fig. 7). ALYREF and ELAVL1 were required and coordinated to export the two mRNAs (Figs. 3i, 5d, Supplementary Fig. 8). However, the precise action mode of how they export mRNAs in a cooperative manner needs to be further deciphered in future studies, especially to determine its connection with the canonical RNA export pathways.
In summary, this study reveals the involvement of E2F6-transactivated ALYREF in colorectal tumorigenesis. Mechanistically, ALYREF coordinates with ELAVL1 to fine-tune nuclear export and expressions of RPS6KB2 and RPTOR transcripts m5C-methylated by NSUN2. Our data elaborate the implications of the ALYREF/ELAVL1/m5C axis in CRC and provide targeting RNA m5C modification as a promising anticancer therapeutic strategy.
Methods
Antibodies
Anti-ALYREF (#ab202894), anti-ELAVL1 (#ab200342), anti-NSUN2 (#ab259941), anti-HA (#ab9110) and anti-rabbit IgG (#ab172730) antibodies were purchased from Abcam (Cambridge, UK). Anti-E2F6 antibody for immunofluorescence and the other assays were obtained from Santa Cruz (#sc-390022) and Abcam (#ab53061), respectively. Anti-GST (#10000-0-AP) antibody was purchased from Proteintech (Wuhan, CHN). The remaining antibodies were commercially available from Cell Signal Technology (CST, Danvers, MA), including primary antibodies against S6K2 (#14130), Raptor (#2280), GAPDH (#5174) and Flag (#2368), as well as secondary anti-rabbit (#7074) and anti-mouse (#7076) antibodies.
Cell culture, transfection and infection
These experiments were conducted as described previously68. All cell lines and culture reagents were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, CHN) and Biological Industries (Israel), respectively. Cell lines were incubated at 37 °C with 5% CO2 in standard media supplemented with 10% FBS and 1% penicillin-streptomycin solution. The basic media of HCT116, DLD-1 and HEK293T cells were McCoy’s 5 A, RPMI 1640 and DMEM, respectively. Plasmids/siRNAs were transiently transfected into cells with Lipofectamine 3000 (Invitrogen, Carlsbad, CA) for in vitro experiments. Cells were transduced with lentivirus vectors screened by puromycin for animal studies.
Cell proliferation
Cell proliferation ability was detected using a CCK-8 commercial kit (Dojindo laboratories, Tokyo, JPN) according to the manufacturer’s protocol. Briefly, control and treated CRC cells were seeded into 96-well plates at a density of 2000–4000 cells per well. CCK-8 (10 μl/well) was added into cells at the indicated time points and then incubated with cells for 2 h. Absorbance was measured at 450 nm using a spectrophotometer.
Cell migration
Transwell® Permeable Supports (Corning #3422, NY) were employed to assess cell migration capability. Briefly, 200 μl serum-free medium and 600 μl complete medium with 10% FBS were added into the upper chambers and the lower compartment, respectively. Control and treated cells (about 20,000–40,000 cells/well) were resuspended with serum-free medium and seeded into the upper chamber. After incubating for 36 h, migrated cells in the lower compartment were fixed by 4% paraformaldehyde, stained with crystal violet, captured under a microscope and quantified using ImageJ software.
Vector construction
Plasmids, siRNAs and lentivirus vectors were commercially provided by Tsingke Biotech (Beijing, CHN), RIOBIO (Guangzhou, CHN) and Genechem (Shanghai, CHN), respectively. Sequences of interest were cloned into the pcDNA3.1 eukaryotic vector for gene overexpression. GST-tagged and His-tagged sequences were cloned into BamHI/EcoRI-digested pGEX-4T-1 and BamHI/XhoI-digested pET-28a (+) prokaryotic vectors for GST pull-down assays, respectively. Wild-type and mutant RPS6KB2/RPTOR reporter plasmids were established with the Sacl/Sall-digested pmirGLO vector. The Kpnl/BglII-digested pGL3 vector co-transfected with the Renilla reporter plasmid was utilized to construct wild-type and mutant ALYREF promoter reporter plasmids. All vector sequence information and binding sites involved are presented in Supplementary Table 3, 4.
Subcellular fractionation
Subcellular fractionation assays were performed with a PARIS™ kit (Invitrogen #AM1921, USA) in accordance with the vender’s instructions. Control and treated cells (about 5 × 106 cells/group) collected were washed in cold PBS and lysed with 400 µl ice-cold Cell Fractionation Buffer for 10 mins on ice. After centrifuging at 500 × g for 5 mins, the supernatant was carefully aspirated and transferred into a new RNase-free EP tube as the cytoplasmic lysate. The pellet was then lysed in 400 µl Cell Disruption Buffer (keep the volume of Cell Fractionation and Disruption Buffer equal) and homogenized with repetitive vigorous vortexes (1 vortex/10 min, 15 secs/vortex) for 40 mins on ice. Following centrifuging at 12,000 × g for 5 mins, the supernatant was carefully aspirated and transferred into a new RNase-free EP tube as the nuclear lysate. Both cytoplasmic and nuclear RNAs were extracted by RNAiso Blood (Takara #9113, JPN) and subjected to RT-qPCR.
RT-qPCR and western blot
These assays were carried out with previously described standard protocols68. For RT-qPCR, total RNAs were extracted with RNAiso Plus (Takara #9109, JPN) and utilized to synthesize the first-strand cDNA with a commercial reverse transcription kit (Vazyme #R222-01, CHN). The synthesized cDNA was then subjected to the qPCR procedure with a commercial kit (Yeasen #11202ES08, CHN). The amplified transcript level of each specific gene was normalized to ACTB. Primer sequence information is shown in Supplementary Table 5. For immunoblots, protein lysates were obtained by CytoBuster protein extraction reagent (Merck Millipore) in the presence of a protease inhibitor cocktail (Roche, Basel, Switzerland) and quantified by BCA Protein Assay Kit (Applygen, Beijing, CHN). The equal amount of protein lysates was subjected to SDS-PAGE and transferred to PVDF membranes (Merck Millipore). Membranes were probed with 5% BSA at room temperature for 3 h, primary antibodies (1:1000) at 4° C overnight and secondary antibodies (1:2000) at room temperature for 1 h. Every step was followed by sufficient washing throughout. Signals were visualized after exposure to Clarity Western ECL substrate (Bio-Rad, Hercules, CA) and captured with the ChampGel® 5000 gel imaging system (SinSage Tech, Beijing, CHN).
RNA-seq
The sequencing procedure and data analysis were executed by CloudSeq Biotech (Shanghai, CHN). Briefly, total RNAs from control and treated cells were isolated by Trizol with genomic DNA removal. Their integrity was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies). RNAs of good quality were then subjected to next-generation sequencing on an Illumina NovaSeq 6000 (Illumina, San Diego, CA).
Dual luciferase reporter assay
Dual luciferase reporter assays (Promega) were executed based on the manufacturer’s protocol. HCT116 cells were seeded onto 24-well plates (6000–8000 cells/well) and allowed to adhere overnight. The cells were then co-transfected with a mixture of one reporter plasmid and certain siRNA/plasmid (without luciferase) for 36 h. Luciferase activity of collected cells was then detected by the Dual-Luciferase Assay System (Promega #E1910). Briefly, cells were lysed in Passive Lysis Buffer (200 μl/well) at room temperature for 30 mins. The lysates (50 μl/well) were transferred to Optiplate-96 plate. After an exposure to LAR II buffer (100 μl/well) and immediately back-to-back STOP buffer (100 μl/well), Firefly and Renilla luciferase values were sequentially read by Spark® Multimode Microplate Reader (Tecan, Switzerland). Renilla luciferase served as an internal control. The relative luciferase activity was calculated as the ratio of firefly luciferase signal to Renilla luciferase signal and normalized to the control group.
m5C-RIP-qPCR
The assay was conducted with an m5C MeRIP kit (CloudSeq #GS-ET-003, CHN) following the vender’s instructions. Briefly, total RNAs were extracted with RNAiso Plus (Takara) and then fragmented with fragmentation buffer, of which 3 μg was kept as input. PGM magnetic beads prewashed were rotationally probed with 2 µl anti-m5C antibody or rabbit IgG for 1 h at room temperature. Fragmented RNAs were rotationally incubated with these antibody-bound beads for 1 h at 4 °C and then purified by an RNA clean and concentrantor-5 kit. Purified RNAs and inputs were then subjected to RT-qPCR with specific primers for RPS6KB2 and RPTOR (Supplementary Table 5). Nonspecific signals were removed by washing throughout. m5C enrichment of certain RNAs for each sample was denoted as the methylated RNA level of the m5C-RIP group compared to that of the IgG-RIP group.
RIP-qPCR
The assay was performed using a Magna RIPTM kit (Millipore #17-700) in accordance with the vendor’s manual. Briefly, 5 μg of certain antibody (anti-ALYREF, anti-ELAVL1 or anti-rabbit IgG) was coated onto 50 μl of protein A/G magnetic beads for 30 mins at room temperature. About 2 × 107 cells were lysed with 100 μl RIP Lysis Buffer for 5 mins on ice. With one-tenth reserved as inputs, the rest of RIP lysate was rotationally incubated with 900 μl RIP immunoprecipitation buffer containing the above antibody-conjugated bead at 4 °C overnight. RNAs immunoprecipitated were purified by digestion with 150 μl proteinase K buffer and consecutive extraction with 400 μl phenol-chloroform-isoamyl alcohol (PCI) mixture. Nonspecific signals were removed by washing throughout. Binding of RNA-protein complex was compared by relative expression levels of purified RNAs that were quantified by RT-qPCR and normalized to their inputs.
RNA pull-down
RNAs were first transcribed by the MEGAscript T7 Transcription Kit (Thermo Fisher Scientific # AM1334). The amplified RNAs were then end-labeled with desthiobiotin using Pierce RNA 3′ End Desthiobiotinylation Kit (Thermo Fisher Scientific #20163). The labeled RNAs were finally pulled down with the Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific #20164). Briefly, up to 50 pmol of biotinylated RNAs was incubated with 50 μl of streptavidin magnetic beads for 30 mins at room temperature. RNA-bound magnetic beads were then rotationally probed with 2 mg of protein lysates (prepared by Pierce™ IP Lysis Buffer, Thermo Fisher Scientific #87787) at 4 °C for 1 h. Nonspecific signals were removed by washing throughout. Protein samples were eluted by boiling in SDS buffer and subjected to western blot using anti-ALYREF and anti-ELAVL1 antibodies.
Co-IP
Lysates from naïve or transfected cells were prepared by lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitor cocktail), with one-tenth retained as inputs. For exogenous co-IP assays, protein lysates were directly rotated with 50 μl of anti-Flag M2 (Sigma #A2220) or anti-HA (Roche #11815016001) agarose beads at 4 °C overnight. For endogenous co-IP assays, supernatants were initially probed with 5 μg of primary antibodies (anti-ALYREF, anti-ELAVL1 or anti-rabbit IgG) and then with 50 μl of SureBeads™ Protein G Magnetic Beads (Bio-Rad #1614023). Protein samples were eluted by boiling in SDS buffer and subjected to western blot using anti-ALYREF and anti-ELAVL1 antibodies.
GST pull-down
The assay was performed using a Pierce™ GST Protein Interaction Pull-Down Kit (Thermo Fisher Scientific #21516). His-tagged and GST-tagged fusion proteins were mass-produced by the IPTG-induced E. coli strain BL21 (DE3) expression system. Briefly, plasmids harboring certain recombinant protein or GST were transformed into E. coli strain BL21 (DE3). The protein expressions were induced by 1 mM ITPG and confirmed with Coomassie blue staining. His-fused proteins were isolated by B-PERTM with Enzymes Bacterial Protein Extraction Reagent (Thermo Fisher Scientific #90078) and then purified with a HisPur™ Ni-NTA Purification Kit (Thermo Fisher Scientific #88229). GST-fused proteins/GST were lysed by Pull-Down Lysis Buffer and then immobilized to Pierce spin columns containing pre-equilibrated glutathione agarose resin. With inputs retained, 150 μg of His-fused proteins purified were incubated in spin columns containing GST-fused proteins/GST at 4 °C overnight. Protein immunoprecipitates were eluted in glutathione elution buffer, boiled and subjected to immunoblotting.
ChIP-qPCR
ChIP procedures were conducted using a Magna ChIP™ A/G ChIP Kit (Millipore #17-10085). Briefly, chromatins were sequentially cross-linked, isolated and sheared as follows: HCT116 cells (approximately 1 × 107 cells from a 10 cm dish/sample) were fixed with 5 ml 1% formaldehyde (10 mins, 37 °C), quenched by 0.125 M glycine (5 mins, 37 °C), lysed in 200 μl SDS lysis buffer (10 mins, 4 °C) and sonicated using a Bioraptor® Plus sonication device (high-frequency, 20–30 cycles that shear DNA into 100–500 bp fragments, 4 °C). With one-tenth saved as an input, the rest of sheared chromatin lysates was rotated with 5 μg of certain antibody (anti-E2F6 or anti-rabbit IgG) at 4 °C overnight and consecutively 60 μg of protein A/G magnetic beads at 4 °C for 1 h. The immunoprecipitated DNA-protein complexes were then incubated in 500 μl ChIP elution buffer. DNAs were de-crosslinked by 5 M NaCl at 65 °C for 4 h and then incubated with a mixture (10 µl of 0.5 M EDTA, 20 µl of 1 M pH 6.5 Tris-HCl, and 2 µl of 10 mg/mL Proteinase K) at 45 °C for 1 h. The free DNAs were purified with a PCR product purification kit (QIAGEN #28006) and analyzed by RT-qPCR with specific primers for the ALYREF promoter (Supplementary Table 5).
Animal experiments
BALB/c nude mice (Vital River Laboratories, Beijing, CHN) were housed under specific pathogen-free conditions in the Xiamen University Laboratory Animal Center. Mice were randomly divided into two groups (n = 5 for each group) by drawing lots: shNC and shALYREF. HCT116 cells transduced with shALYREF lentivirus vector or its control were subcutaneously inoculated into BALB/c nude mice. Three weeks later, tumors were stripped and weighed following mice euthanasia by cervical dislocation. In accordance to ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments), the study protocol was approved by the Xiamen Animal Care and Use Committee.
IHC
A tissue microarray of CRC tissues and matched normal tissues was commercially obtained from OUTDO BIOTECH (Shanghai, CHN) for IHC staining. The 4-μm thick FFPE tumor section underwent deparaffinization in a multifunctional automatic dyeing machine (LEICA, #ST5020) and EDTA antigen retrieval in a DAKO PT Link instrument. The pretreated slide was incubated with primary anti-ALYREF antibody overnight at 4 °C. Endogenous peroxidase removal, EnVision/HRP incubation and DAB+ Chromogen staining were then performed with a DAKO EnVisionTM FLEX visualization system (#K8002) on a DAKO Autostainer Link 48 instrument. The slide was restained with hematoxylin, mounted and visualized by an Aperio scanner (Aperio XT, LEICA). The overall staining intensity of every sample was calculated using the Cytoplasmic v2 module of AperioESM software. Complying with the provisions of the Declaration of Helsinki, the study protocol was approved by the ethics committee of Shanghai Outdo Biotech Company (SHYJS-CP-1707002) and the informed consents were obtained from all participants.
Statistical analysis
All data are representative of three independent experiments and were analyzed by GraphPad Prism Version 8.0.1 or IBM SPSS Version 25 software. Data are expressed as the mean ± SD. Differences between two groups were compared using two-tailed Student’s t-test or paired t-test. Survival analysis was performed by the Kaplan–Meier method with the log-rank test. The correlation of ALYREF and ELAVL1 protein expression was analyzed with Pearson’s correlation test. P < 0.05 indicated statistical significance.
Supplementary information
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (82002496), Xiamen Municipal Bureau of Science and Technology (3502Z20244ZD1003, 3502Z20214ZD3005 and 3502Z20204003), the Natural Science Foundation of Fujian Province (2020J05300) and Startup Fund for scientific research of Fujian Medical University (2023QH1317).
Author contributions
Conception and supervision, X.T.L., F.Y. and X.C.W.; Methodology, X.T.L. and L.H.Z.; Experiment performance, L.H.Z., J.X.W. and B.Q.Z; Data analysis, X.T.L., L.H.Z., J.X.W., B.Q.Z. and J.P.K.; Manuscript writing, X.T.L. and L.H.Z.; Manuscript reviewing, X.T.L., F.Y. and X.C.W.; Funding acquisition, X.T.L., F.Y. and J.P.K. All authors have read and approved the final manuscript.
Data availability
The protein interplays of ALYREF and ELAVL1 predicted by STRING database is publicly available (https://string-db.org/). Information of transcriptional factors predicted is publicly available from online databases (JASPAR, GTRD, ChIPBase V2.0 and AnimalTFDB 3.0). The other data are provided within the article and supplementary information, otherwise available from the corresponding author upon reasonable request. Source data are provided with the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xicheng Wang, Email: xicheng_wang@hotmail.com.
Feng Ye, Email: yefengdoctor@xmu.edu.cn.
Xiaoting Lin, Email: xiaotinglin@xmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-024-00737-0.
References
- 1.Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Siegel, R. L. et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut68, 2179–2185 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Kehm, R. D. et al. Age-specific trends in colorectal cancer incidence for women and men, 1935–2017. Gastroenterology161, 1060–1062.e1063 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet394, 1467–1480 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Han, X., Wang, M., Zhao, Y. L., Yang, Y. & Yang, Y. G. RNA methylations in human cancers. Semin. Cancer Biol.75, 97–115 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Chen, X. et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol.21, 978–990 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Su, J. et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene40, 5814–5828 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y. S., Yang, W. L., Zhao, Y. L. & Yang, Y. G. Dynamic transcriptomic m(5) C and its regulatory role in RNA processing. Wiley Interdiscip. Rev. RNA12, e1639 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Dai, X. et al. YTHDF2 binds to 5-methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal. Chem.92, 1346–1354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, B. et al. m5C regulator-mediated modification patterns and tumor microenvironment infiltration characterization in colorectal cancer: one step closer to precision medicine. Front. Immunol.13, 1049435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, H. L. et al. SRSF2 plays an unexpected role as reader of m(5)C on mRNA, linking epitranscriptomics to cancer. Mol. Cell83, 4239–4254.e4210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu, X., Zhang, Y., Zhang, J. & Zhang, X. NSun2 promotes cell migration through methylating autotaxin mRNA. J. Biol. Chem.295, 18134–18147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, J. Z. et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun.41, 560–575 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun, Z. et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene39, 6906–6919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei, L. et al. RNA methyltransferase NSUN2 promotes gastric cancer cell proliferation by repressing p57(Kip2) by an m(5)C-dependent manner. Cell Death Dis.11, 270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Y. et al. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett.430, 57–66 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Khan, M., Hou, S., Azam, S. & Lei, H. Sequence-dependent recruitment of SRSF1 and SRSF7 to intronless lncRNA NKILA promotes nuclear export via the TREX/TAP pathway. Nucleic Acids Res.49, 6420–6436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, D. et al. YB-1 is a positive regulator of KLF5 transcription factor in basal-like breast cancer. Cell Death Differ.29, 1283–1295 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu, W. et al. Positive epigenetic regulation loop between AR and NSUN2 promotes prostate cancer progression. Clin. Transl. Med.12, e1028 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, Z. et al. NSUN4 mediated RNA 5-methylcytosine promotes the malignant progression of glioma through improving the CDC42 mRNA stabilization. Cancer Lett.597, 217059 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Zhang, Y. et al. CDK13 promotes lipid deposition and prostate cancer progression by stimulating NSUN5-mediated m5C modification of ACC1 mRNA. Cell Death Differ.30, 2462–2476 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, M. et al. NSUN6-mediated 5-methylcytosine modification of NDRG1 mRNA promotes radioresistance in cervical cancer. Mol. Cancer23, 139 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang, X. et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res.27, 606–625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacheco-Fiallos, B. et al. mRNA recognition and packaging by the human transcription-export complex. Nature616, 828–835 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, Y., Xing, C. & Peng, H. ALYREF (Aly/REF export factor): a potential biomarker for predicting cancer occurrence and therapeutic efficacy. Life Sci.338, 122372 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y. et al. mRNA m(5)C inhibits adipogenesis and promotes myogenesis by respectively facilitating YBX2 and SMO mRNA export in ALYREF-m(5)C manner. Cell Mol. Life Sci.79, 481 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Y. et al. mRNA m5C controls adipogenesis by promoting CDKN1A mRNA export and translation. RNA Biol.18, 711–721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu, Z. et al. Suppression of DDX39B sensitizes ovarian cancer cells to DNA-damaging chemotherapeutic agents via destabilizing BRCA1 mRNA. Oncogene39, 7051–7062 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Wang, N. et al. m(5)C-dependent cross-regulation between nuclear reader ALYREF and writer NSUN2 promotes urothelial bladder cancer malignancy through facilitating RABL6/TK1 mRNAs splicing and stabilization. Cell Death Dis.14, 139 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding, S. et al. Epigenetic addition of m(5)C to HBV transcripts promotes viral replication and evasion of innate antiviral responses. Cell Death Dis.15, 39 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo, S. et al. NSUN2-mediated m(5) C RNA methylation dictates retinoblastoma progression through promoting PFAS mRNA stability and expression. Clin. Transl. Med.13, e1273 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, T. et al. THOC3 interacts with YBX1 to promote lung squamous cell carcinoma progression through PFKFB4 mRNA modification. Cell Death Dis.14, 475 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du, E. et al. A pan-cancer analysis reveals genetic alterations, molecular mechanisms, and clinical relevance of m(5) C regulators. Clin. Transl. Med.10, e180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murugan, A. K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol.59, 92–111 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Hua, H. et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol.12, 71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavares, M. R. et al. The S6K protein family in health and disease. Life Sci.131, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Sever, N. & Cengiz Şahin, S. S6K2 promises an important therapeutic potential for cancer. Future Oncol.15, 95–102 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Goh, E. T. et al. Involvement of heterogeneous ribonucleoprotein F in the regulation of cell proliferation via the mammalian target of rapamycin/S6 kinase 2 pathway. J. Biol. Chem.285, 17065–17076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sridharan, S. & Basu, A. S6 kinase 2 promotes breast cancer cell survival via Akt. Cancer Res.71, 2590–2599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardo, O. E. et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. EMBO J.25, 3078–3088 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liwak, U. et al. Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol. Cell. Biol.32, 1818–1829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulhati, P. et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.71, 3246–3256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shuhua, W. et al. Autophagy-related genes Raptor, Rictor, and Beclin1 expression and relationship with multidrug resistance in colorectal carcinoma. Hum. Pathol.46, 1752–1759 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Wen, F. F. et al. Expression of Raptor and Rictor and their relationships with angiogenesis in colorectal cancer. Neoplasma67, 501–508 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Hoshii, T. et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J. Clin. Invest.122, 2114–2129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwata, T. N. et al. Conditional disruption of raptor reveals an essential role for mTORC1 in B cell development, survival, and metabolism. J. Immunol.197, 2250–2260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venugopal, S. V., Caggia, S., Gambrell-Sanders, D. & Khan, S. A. Differential roles and activation of mammalian target of rapamycin complexes 1 and 2 during cell migration in prostate cancer cells. Prostate80, 412–423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie, C. M. & Sun, Y. The MTORC1-mediated autophagy is regulated by the FBXW7-SHOC2-RPTOR axis. Autophagy15, 1470–1472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature485, 201–206 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Visvanathan, A. et al. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene37, 522–533 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Yue, B. et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol. Cancer18, 142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi, J. et al. Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence. Nucleic Acids Res.38, 1547–1558 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui, N. & Borden, K. L. mRNA export and cancer. Wiley Interdiscip. Rev. RNA3, 13–25 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Zhao, Y. et al. RNA-binding proteins: underestimated contributors in tumorigenesis. Semin. Cancer Biol.86, 431–444 (2022). [DOI] [PubMed] [Google Scholar]
- 55.Roundtree, I. A. & He, C. RNA epigenetics-chemical messages for posttranscriptional gene regulation. Curr. Opin. Chem. Biol.30, 46–51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell149, 274–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fallmann, J., Sedlyarov, V., Tanzer, A., Kovarik, P. & Hofacker, I. L. AREsite2: an enhanced database for the comprehensive investigation of AU/GU/U-rich elements. Nucleic Acids Res.44, D90–D95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallouzi, I. E. & Steitz, J. A. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science294, 1895–1901 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Fan, X. C. & Steitz, J. A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl Acad. Sci. USA95, 15293–15298 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doller, A. et al. Posttranslational modification of the AU-rich element binding protein HuR by protein kinase Cdelta elicits angiotensin II-induced stabilization and nuclear export of cyclooxygenase 2 mRNA. Mol. Cell. Biol.28, 2608–2625 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vreeland, A. C., Levi, L., Zhang, W., Berry, D. C. & Noy, N. Cellular retinoic acid-binding protein 2 inhibits tumor growth by two distinct mechanisms. J. Biol. Chem.289, 34065–34073 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim, I., Hur, J. & Jeong, S. HuR represses Wnt/β-catenin-mediated transcriptional activity by promoting cytoplasmic localization of β-catenin. Biochem. Biophys. Res. Commun.457, 65–70 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Noh, J. H. et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev.30, 1224–1239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poganik, J. R. et al. Post-transcriptional regulation of Nrf2-mRNA by the mRNA-binding proteins HuR and AUF1. FASEB J.33, 14636–14652 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prechtel, A. T. et al. Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element. J. Biol. Chem.281, 10912–10925 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Fries, B. et al. Analysis of nucleocytoplasmic trafficking of the HuR ligand APRIL and its influence on CD83 expression. J. Biol. Chem.282, 4504–4515 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Chang, Y. Z. et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-κB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett.511, 36–46 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Lin, X. et al. lncRNA ITGB8-AS1 functions as a ceRNA to promote colorectal cancer growth and migration through integrin-mediated focal adhesion signaling. Mol. Ther.30, 688–702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protein interplays of ALYREF and ELAVL1 predicted by STRING database is publicly available (https://string-db.org/). Information of transcriptional factors predicted is publicly available from online databases (JASPAR, GTRD, ChIPBase V2.0 and AnimalTFDB 3.0). The other data are provided within the article and supplementary information, otherwise available from the corresponding author upon reasonable request. Source data are provided with the paper.