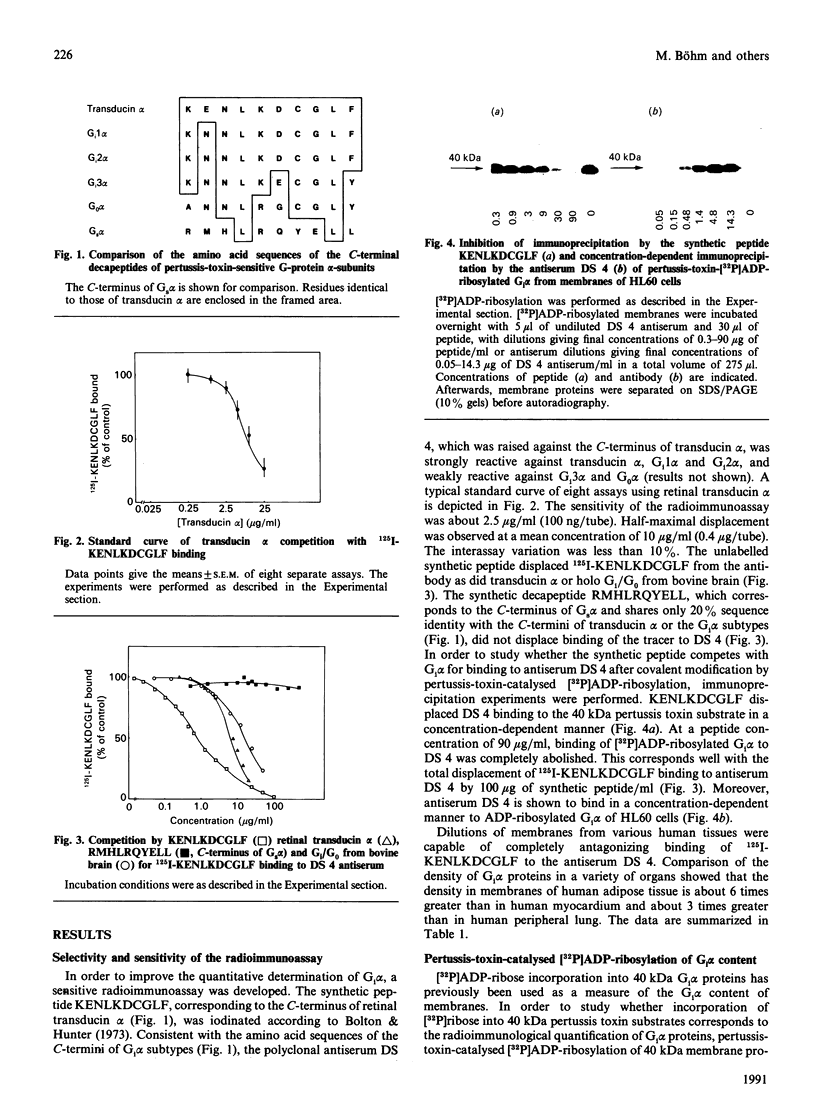
Abstract
The quantitative determination of pertussis-toxin-sensitive guanine-nucleotide-binding proteins (G-proteins) in cell membranes is still a problem. Pertussis-toxin-catalysed [32P]ADP-ribosylation strongly relies on the substrate quality of the alpha-subunits and is influenced by the concentration of nucleotides, beta gamma-subunits, the physicochemical properties of the membranes influencing the availability of Gi alpha for pertussis toxin, and covalent modification of Gi alpha. Quantification of immunoreactive material on Western blots can be only imprecisely performed by two-dimensional densitometry. In order to generate a method for quantification of pertussis-toxin-sensitive G-proteins in membranes we have developed a fast and sensitive radioimmunoassay. The C-terminal decapeptide of retinal transducin alpha (KENLKDCGLF) was 125I-labelled and used as tracer. Polyclonal antiserum (DS 4) was raised against this peptide. Gi alpha proteins were determined by competition of solubilized membranes for 125I-KENLKDCGLF binding to DS 4 using dilutions of retinal transducin alpha as standard. The interassay variation was less than 10%, with a sensitivity of 2.5 micrograms/ml. The density of Gi alpha was highest in human adipose tissue, followed by HL60 cells, lung, mononuclear leucocytes, thrombocytes and left ventricular myocardium. A striking difference was observed between the density of Gi alpha and the amount of incorporation of [32P]ADP-ribose into the 40 kDa membrane proteins by pertussis toxin in the same samples. This is also demonstrated by comparison of the weak [32P]ATP-ribosylation of pertussis toxin substrates with the density of immunoreactive Gi alpha on Western blots in tissues such as lung. This study shows that the Gi alpha content can be exactly determined by a sensitive and fast radioimmunoassay using iodinated synthetic peptide homologues of Gi alpha proteins. Radioimmunological quantification of Gi alpha might be able to detect the 'true' Gi alpha content of membranes without being hampered by influences on the [32P]ADP-ribosylation reaction. It is concluded that this newly developed method may become an important tool for studying expression of Gi alpha proteins in a variety of tissues or cell types, and for precisely quantifying the changes caused by pathological conditions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Properties of a novel nitric oxide-stimulated ADP-ribosyltransferase. Arch Biochem Biophys. 1990 Jun;279(2):286–290. doi: 10.1016/0003-9861(90)90493-i. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Manclark C. R. Adenine nucleotides promote dissociation of pertussis toxin subunits. J Biol Chem. 1986 Mar 25;261(9):4324–4327. [PubMed] [Google Scholar]
- Bégin-Heick N. Quantification of the alpha and beta subunits of the transducing elements (Gs and Gi) of adenylate cyclase in adipocyte membranes from lean and obese (ob/ob) mice. Biochem J. 1990 May 15;268(1):83–89. doi: 10.1042/bj2680083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Gierschik P., Jakobs K. H., Schnabel P., Kemkes B., Erdmann E. Localization of a "postreceptor" defect in human dilated cardiomyopathy. Am J Cardiol. 1989 Oct 1;64(12):812–814. doi: 10.1016/0002-9149(89)90773-x. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Feldman A. M., Cates A. E., Veazey W. B., Hershberger R. E., Bristow M. R., Baughman K. L., Baumgartner W. A., Van Dop C. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988 Jul;82(1):189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Codina J., Simons C., Birnbaumer L., Spiegel A. Antisera against a guanine nucleotide binding protein from retina cross-react with the beta subunit of the adenylyl cyclase-associated guanine nucleotide binding proteins, Ns and Ni. Proc Natl Acad Sci U S A. 1985 Feb;82(3):727–731. doi: 10.1073/pnas.82.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Spiegel A., Jakobs K. H. Purification and immunochemical characterization of the major pertussis-toxin-sensitive guanine-nucleotide-binding protein of bovine-neutrophil membranes. Eur J Biochem. 1987 May 15;165(1):185–194. doi: 10.1111/j.1432-1033.1987.tb11210.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Green A., Johnson J. L., Milligan G. Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1990 Mar 25;265(9):5206–5210. [PubMed] [Google Scholar]
- Hara-Yokoyama M., Furuyama S. Endogenous inhibitor of the ADP-ribosylation of (a) G-protein(s) as catalyzed by pertussis toxin is present in rat liver. FEBS Lett. 1988 Jul 4;234(1):27–30. doi: 10.1016/0014-5793(88)81295-x. [DOI] [PubMed] [Google Scholar]
- Hodges T. D., Bailey J. C., Fleming J. W., Kovacs R. J. Selective parasympathectomy increases the quantity of inhibitory guanine nucleotide-binding proteins in canine cardiac ventricle. Mol Pharmacol. 1989 Jul;36(1):72–77. [PubMed] [Google Scholar]
- Jacobson M. K., Loflin P. T., Aboul-Ela N., Mingmuang M., Moss J., Jobson E. L. Modification of plasma membrane protein cysteine residues by ADP-ribose in vivo. J Biol Chem. 1990 Jul 5;265(19):10825–10828. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim L. K., Sekura R. D., Kaslow H. R. Adenine nucleotides directly stimulate pertussis toxin. J Biol Chem. 1985 Mar 10;260(5):2585–2588. [PubMed] [Google Scholar]
- Longabaugh J. P., Vatner D. E., Graham R. M., Homcy C. J. NADP improves the efficiency of cholera toxin catalyzed ADP-ribosylation in liver and heart membranes. Biochem Biophys Res Commun. 1986 May 29;137(1):328–333. doi: 10.1016/0006-291x(86)91214-3. [DOI] [PubMed] [Google Scholar]
- Mattera R., Codina J., Sekura R. D., Birnbaumer L. The interaction of nucleotides with pertussis toxin. Direct evidence for a nucleotide binding site on the toxin regulating the rate of ADP-ribosylation of Ni, the inhibitory regulatory component of adenylyl cyclase. J Biol Chem. 1986 Aug 25;261(24):11173–11179. [PubMed] [Google Scholar]
- Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988 Oct 1;255(1):1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina y Vedia L., Nolan R. D., Lapetina E. G. Endogenous ADP-ribosylation in human platelets. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1323–1328. doi: 10.1016/s0006-291x(88)81019-2. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Neumann J., Schmitz W., Scholz H., von Meyerinck L., Döring V., Kalmar P. Increase in myocardial Gi-proteins in heart failure. Lancet. 1988 Oct 22;2(8617):936–937. doi: 10.1016/s0140-6736(88)92601-3. [DOI] [PubMed] [Google Scholar]
- Ohisalo J. J., Milligan G. Guanine-nucleotide-binding proteins Gi and Gs in fat-cells from normal, hypothyroid and obese human subjects. Biochem J. 1989 Jun 15;260(3):843–847. doi: 10.1042/bj2600843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M., Gierschik P., Milligan G., Klee W., Spiegel A. Antibodies against the carboxyl-terminal 5-kDa peptide of the alpha subunit of transducin crossreact with the 40-kDa but not the 39-kDa guanine nucleotide binding protein from brain. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4095–4099. doi: 10.1073/pnas.82.12.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T. Pertussis toxin in the analysis of receptor mechanisms. Biochem Pharmacol. 1990 May 15;39(10):1499–1504. doi: 10.1016/0006-2952(90)90513-k. [DOI] [PubMed] [Google Scholar]
- Reithmann C., Gierschik P., Müller U., Werdan K., Jakobs K. H. Pseudomonas exotoxin A prevents beta-adrenoceptor-induced upregulation of Gi protein alpha-subunits and adenylyl cyclase desensitization in rat heart muscle cells. Mol Pharmacol. 1990 May;37(5):631–638. [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F., Birnbaumer L., Field J. B. Incubation of bovine thyroid slices with thyrotropin is associated with a decrease in the ability of pertussis toxin to adenosine diphosphate-ribosylate guanine nucleotide regulatory component(s). Mol Endocrinol. 1987 Jul;1(7):482–490. doi: 10.1210/mend-1-7-482. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F., Mattera R., Grenet D., Sekura R. D., Birnbaumer L., Field J. B. Adenosine diphosphate ribosylation of G proteins by pertussis and cholera toxin in isolated membranes. Different requirements for and effects of guanine nucleotides and Mg2+. Mol Endocrinol. 1987 Jul;1(7):472–481. doi: 10.1210/mend-1-7-472. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Endo H. Identification in human erythrocytes of mono(ADP-ribosyl) protein hydrolase that cleaves a mono(ADP-ribosyl) Gi linkage. FEBS Lett. 1990 Feb 26;261(2):381–384. doi: 10.1016/0014-5793(90)80597-c. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Eukaryotic mono(ADP-ribosyl)transferase that ADP-ribosylates GTP-binding regulatory Gi protein. J Biol Chem. 1988 Apr 15;263(11):5485–5489. [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Kanaho Y., Hewlett E. L., Moss J. Effects of guanyl nucleotides and rhodopsin on ADP-ribosylation of the inhibitory GTP-binding component of adenylate cyclase by pertussis toxin. J Biol Chem. 1984 Dec 25;259(24):15320–15323. [PubMed] [Google Scholar]
- Van Meurs K. P., Angus C. W., Lavu S., Kung H. F., Czarnecki S. K., Moss J., Vaughan M. Deduced amino acid sequence of bovine retinal Go alpha: similarities to other guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]




