Abstract
Cell infection by adenovirus serotypes 2 and 5 (Ad2/5) initiates with the attachment of Ad fiber to the coxsackievirus and Ad receptor (CAR) followed by αv integrin-mediated entry. We recently demonstrated that heparan sulfate glycosaminoglycans (HS GAGs) expressed on cell surfaces are involved in the binding and infection of Ad2/5 (M. C. Dechecchi, A. Tamanini, A. Bonizzato, and G. Cabrini, Virology 268:382–390, 2000). The role of HS GAGs was investigated using extracellular soluble domain 1 of CAR (sCAR-D1) and heparin as soluble receptor analogues of CAR and HS GAGs in A549 and recombinant CHO cell lines with differential levels of expression of the two receptors and cultured to various densities. Complete inhibition of binding and infection was obtained by preincubating Ad2/5 with both heparin (10 μg/ml) and sCAR-D1 (200 μg/ml) in A549 cells. Partial inhibition was observed when heparin and sCAR-D1 were preincubated separately with Ad. The level of heparin-sensitive [3H]Ad2/5 binding doubled in sparse A549 cells (50 to 70,000 cells/cm2) with respect to that of cells grown to confluence (200 to 300,000 cells/cm2), in parallel with increased expression of HS GAGs. [3H]Ad2 bound to sparse CAR-negative CHO cells expressing HS GAGs (CHO K1). No [3H]Ad2 binding was observed in CHO K1 cells upon competitive inhibition with heparin and in HS GAG-defective CHO A745, D677, and E606 clones. HS-sensitive Ad2 infection was obtained in CAR-negative sparse CHO K1 cells but not in CHO A745 cells, which were permissive to infection only upon transfection with CAR. These results demonstrate that HS GAGs are sufficient to mediate the initial binding of Ad2/5.
Human adenoviruses (Ads) belonging to subgroup C, namely Ad serotypes 2 and 5 (Ad2/5), have been widely used as gene transfer vectors addressed to the treatment of acquired and genetic diseases, including cystic fibrosis (33). Application of recombinant Ad-derived vectors in gene therapy anticipated the identification of the primary host cell receptor, which is one of the key factors determining the efficiency and targeting of gene transfer. At the present time, new information on the mechanisms of interactions of Ad with a host cell is compelling for successful adaptation of Ad to gene therapy applications and to inspire the design of more efficient vectors (23, 33).
Virus-host cell interactions often require multiple binding events to promote productive cell entry, with coreceptor utilization representing an evolutionary mechanism for extension of the spectra of target cells (12). Infection of all Ad serotypes except those belonging to subgroup B begins with the binding of the fiber to a 42-kDa glycoprotein receptor termed the Coxsackievirus and Ad receptor (CAR) (2, 3, 30, 40). After fiber binding, the Arg-Gly-Asp (RGD) sequence of the penton base interacts with αv integrins (43), which trigger a dynamin-dependent internalization (42) that requires signaling events mediated by phosphoinositide-3-OH-kinase and the Rho family of small GTPases (16, 17). Receptors for Ad besides CAR have been described. Ad2 attaches to hematopoietic cells via αMβ2 and enters through αvβ5 integrins (14). The Ad5 fiber knob appears to interact with the α2 domain of major histocompatibility complex class I (13). Sialic acid mediates the binding of Ad37 (1). Moreover, we recently demonstrated that heparan sulfate glycosaminoglycans (HS GAGs) expressed on cell surfaces are involved in the binding and infection of Ad2/5 (9).
Cell surface HS GAGs are coreceptors for several pathogenic microorganisms (e.g., bacteria, parasites, and viruses) (31), with herpes simplex virus type 1 (HSV-1) being the first extensively investigated (44). The initial interaction of HSV-1 with cells is usually between virion glycoprotein C and cell surface HS GAGs. This facilitates virus entry through membrane fusion mediated by the binding of virion glycoprotein D to any of several cell surface receptors like herpes virus entry mediator, nectin-1α, nectin-1β, and specific HS sites generated by 3-O-sulfotransferases. In the absence of cell surface HS GAGs, HSV-1 can infect cells but entry is very inefficient (36). Similarly to what occurs in HSV-1, HS GAGs serve as primary attachment receptors for adeno-associated parvoviruses type 2 (AAV-2) (38). Fibroblast growth factor receptor and αvβ5 integrins have been implicated as coreceptors (25, 37). Recently, it has been demonstrated that AAV-2 internalization requires αvβ5 integrins together with HS GAGs (34). As for HSV-1, in the absence of HS GAGs, infection efficiency is reduced (26). With respect to Ad2/5, we observed that cleavage or competitive inhibition of HS GAGs reduces binding and infection in CAR-expressing A549 and HeLa cells. Moreover, A549 cells were still permissive to Ad2/5 binding and infection in the presence of a functional blockage of CAR with RmcB monoclonal antibody (9). This finding suggests that Ad2/5 can infect cells upon initial attachment to HS GAGs, also independently of CAR. This hypothesis has been addressed in the present study using soluble receptor analogues of CAR and HS GAGs in A549 and recombinant Chinese hanster ovary (CHO) cell lines with differential levels of expression of the two receptors. Based on our results, we conclude that cell surface HS GAGs are sufficient to mediate Ad2/5 binding and infection in CAR-negative cells.
MATERIALS AND METHODS
Cell lines.
Human alveolar type II-derived carcinoma A549 cells, human lymphoblastoid Raji cells, wild-type CHO K1 cells, and mutant derivative CHO A745, CHO D677, and CHO E606 cells were obtained from the American Type Culture Collection. The tissue culture media were Dulbecco's modified Eagle's medium (DMEM) for A549 cells, RPMI 1640 for Raji cells, and Ham's F-12 medium supplemented with 10% fetal bovine serum for CHO cells (BioWhittaker). Stable expression of CAR in the four CHO cell lines was obtained by transfecting cells with a plasmid coding for full-length CAR (pCAR46S) and by using the FuGENE transfection reagent (Roche) according to the manufacturer's instructions in order to obtain CHO K1 CAR, CHO A745 CAR, CHO D677 CAR, and CHO E606 CAR cell lines. The insertless vector pCR3.1-72 was used for transfection of CAR-negative cell lines (CHO K1 il, CHO A745 il, CHO D677 il, and CHO E606 il, where “il” indicates insertless). The construct pCAR46S including the full-length coding region of human CAR cDNA under the control of the cytomegalovirus promoter was prepared as follows. The entire coding region of the human CAR cDNA was amplified by reverse transcription-PCR (RT-PCR) of total RNA from the A549 cell line. Reaction mixtures for first-strand cDNA synthesis were primed with the oligonucleotide CR1173 (5′ GAG ACA TAT GGA GGC TCT 3′), and the region of human CAR cDNA from nucleotides (nt) 53 to 1173 was then amplified by PCR by adding the direct primer CF53 (5′ AGC CAC CAT GGC GCT CCT 3′). Cycling conditions were 30 s at 95°C, 30 s at 52°C, and 1.5 min at 72°C for 33 cycles, followed by a single step at 72°C for 10 min. The 1,121-bp PCR product was gel purified using a GFX PCR DNA kit and a Gel Band purification kit (Amersham Pharmacia Biotech Inc.) and then cloned into the pCR3.1 vector supplied with the bidirectional Eukariotic TA cloning kit (Invitrogen) by following the manufacturer's instructions. The orientation of the insert was checked by PCR, followed by sequencing analysis. A plasmid including the human CAR coding sequence in the direct orientation corresponding to that reported in GenBank file Y07593, except for a silent A→G mutation at nt 1101, was named pCAR46S. Both CHO il and CAR-transfected cells (CHO CAR) were subjected to selection for 2 to 3 weeks with G418 (250 μg/ml), and the single clones were isolated with a cloning cylinder (Sigma). Expression of human CAR mRNA was tested by RT-PCR, and positive clones were checked for CAR protein expression by immunocytochemistry with the anti-CAR monoclonal antibody RmcB (a generous gift of Robert W. Finberg, Dana-Farber Cancer Institute, Boston, Mass.), as described previously (9). See Table 1 for a summary of cell lines.
TABLE 1.
Characteristics of the cell lines used
| Cell type | Presence of:
|
Glycosaminoglycan expression
|
||
|---|---|---|---|---|
| CAR | αv integrins | HS | Chondroitin sulfate | |
| A549 | Yes | Yes | Yes | Yes |
| Raji | Yes | No | No | No |
| CHO K1 il | No | Yes | Yes | Yes |
| CHO A745 il | No | Yes | No | No |
| CHO D677 il | No | Yes | No | Yesa |
| CHO E606 il | No | Yes | Yesb | Yes |
| CHO K1 CAR | Yes | Yes | Yes | Yes |
| CHO A745 CAR | Yes | Yes | No | No |
| CHO D677 CAR | Yes | Yes | No | Yesa |
| CHO E606 CAR | Yes | Yes | Yesb | Yes |
Chondroitin sulfate accumulates to levels 2 to 3 times higher than in K1 cells.
HS is undersulfated by a factor of 2 to 3.
Expression and purification of sCAR-D1.
A cDNA fragment encoding extracellular N-terminal domain 1 (D1) of human CAR (amino acids 22 to 144) was amplified by RT-PCR of total RNA from the A549 cell line. The reaction mixture for first-strand cDNA synthesis was primed with oligo(dT). Primers CAR-c-D2 (CTG AAT TCC ATG GGT ATC ACT ACT CCT GAA GAG A) and CAR-c-R2 (AAC TGC AGT CAG TCG ACC GCA CCT GAA GGC TTA ACA) were designed for cloning D1, which encodes PCR products between the NcoI and SalI sites of the expression vector pTrcHis2b (Invitrogen). The PCR cycling program was 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final single step at 72°C for 10 min. Strain DH5α (Gibco) was transformed with pTrcHis2b constructs, and the sequences were verified to correspond exactly to that reported in GenBank file Y07593, except for the presence of a silent G→A mutation at nt 179, which encodes proline in position 40. In order to obtain the soluble N-terminal D1 of CAR (sCAR-D1) with a removable His tag, primers CAR-c-D3 (ATG CAT ATG GGT ATC ACT ACT CCT GAA) and CAR-c-R3 (CAT GGA TCC TAC GCA CCT GAA GGC TTA ACA A) were designed to adapt the insert, previously cloned in pTrcHis2b, for cloning between the NdeI and BamHI restriction sites of the vector pET15b (Novagen). The PCR cycling program was identical to that used for primers CAR-c-D2 and CAR-c-R2, and the PCR product was cloned in pET15b. The construct was used to transform strain BL21 (DE3) (Novagen) for CAR-D1 expression. To induce protein expression, overnight cultures in Luria-Bertani–ampicillin broth were diluted 50-fold and grown to mid-log phase (optical density [OD] of 0.6 at 600 nm), at which time they were adjusted with 1.3 mM isopropyl β-d-thiogalactopyranoside (IPTG). After shaking for 4 h at 37°C, the bacterial cells were collected by centrifugation. The recombinant protein was recovered from inclusion bodies as previously described by Freimuth et al. (11). Harvested cells were resuspended in STE (100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 1 mM EDTA) containing 100 μg of lysozyme per ml and subjected to three cycles of freezing and thawing. Cell lysate viscosity was reduced by DNase I digestion (in the presence of 2 mM MnCl2), and the cell wall debris was removed by centrifugation at 20,000 × g for 20 min. After centrifugation, the pellet was washed several times in STE containing 0.1% Nonidet P-40. Inclusion bodies were dissolved in a solution containing 8 M urea, 50 mM Tris-HCl (pH 9.2), and 50 mM β-mercaptoethanol (20 ml per liter of initial culture) and then diluted with 15 volumes of 20 mM Tris-HCl (pH 7.4). The slightly hazy solution was cleared by centrifugation at 20,000 × g for 15 min and filtration through a 0.45-μm-pore-size filter. The sCAR-D1 His-tagged protein was purified to be essentially free of contaminating protein with a HisTrap kit according to the instructions of the manufacturer (Amersham Pharmacia). The sCAR-D1 His-tagged protein was subjected to dialysis against phosphate-buffered saline (PBS), and the hexahistidine tag was cleaved from sCAR-D1 by using biotinylated thrombin, which was removed with streptavidin agarose according to the instruction of the kit manufacturer (thrombin cleavage capture kit; Novagen). To ensure that all sCAR-D1 was free from the His tag, solution buffer was exchanged with PD10 columns and sCAR-D1 was subjected to a further passage on His Trap resin, the flowthrough being finally dialyzed against PBS. The native molecular mass of sCAR-D1 was estimated by gel permeation with a Sephacryl S-100 high-resolution column (Amersham Pharmacia). sCAR-D1 (240 mg/0.5 ml of PBS [pH 7.4] containing 0.1 mM EDTA and 1 mM dithiothreitol) was chromatographed with the same running buffer at 0.5 ml/min in parallel with bovine serum albumin (molecular mass, 67 kDa), superoxide dismutase (molecular mass, 30 kDa), and RNase A (molecular mass, 13.7 kDa) as size markers. The mass of sCAR-D1 was estimated to be between 25 and 30 kDa, which is compatible with the dimeric form previously reported by Freimuth et al. (11).
Viruses.
Ad2/5 were obtained as stocks from the American Type Culture Collection and passaged on A549 cells. The viruses were purified from infected cells by four freeze-thaw cycles followed by two successive bandings on CsCl gradients according to the method of Precious and Russell (24). Purified viruses were dialyzed against 10 mM Tris-HCl (pH 7.4)–1 mM MgCl2. The dialysate was aliquoted with the addition of 10% glycerol and stored at −80°C until use. The concentration of purified Ad was determined by absorbance measured at 260 nm according to the method of Mittereder et al. (21), who assumed the conversion factor of 1 OD unit of Ad5 corresponding to 1.1 × 1012 virions.
Cell infection assay.
Ad infection was tested by fluorescent focus assay and quantitated as the number of fluorescent foci per well (in fluorescent focal units [FFU]) as described previously (9) according to the method of Wickham et al. (43). Cells grown in 1-cm2 chamber slide wells were infected with 200 μl of ice-cold serum-free DMEM containing Ad5 or Ad2 at the appropriate infection doses reported in the figure legends. After incubation for 1 h at 4°C, the unbound virus was removed and the cells were left at 37°C for 24 h (A549 cells) or 72 h (CHO cells) before being fixed with acetone. Anti-Ad primary antibody directed against hexon protein (Chemicon) was used at a 1:100 dilution. The secondary antibody was fluorescein isothiocyanate-conjugated rabbit anti-mouse antibody (Sigma) at a 1:100 dilution. In competition experiments, Ad was preincubated with heparin or sCAR-D1 at the concentrations indicated in the figure legends for 1 h at 37°C in 20 μl of serum-free DMEM containing 0.1% bovine serum albumin (wt/vol). When both heparin and sCAR-D1 were used, Ad was preincubated for 1 h with heparin before sCAR-D1 was added for a further 30 min. The Ad suspension was then diluted 1:10 with ice-cold serum-free DMEM and added to the cells as described above. GAGs utilized in inhibition experiments were heparin, low-molecular-weight heparin, de-N-sulfated heparin from porcine intestinal mucosa, chondroitin sulfate A from bovine trachea, and keratan sulfate from bovine cornea (Sigma).
[methyl-3H]thymidine Ad labeling and binding assay.
[methyl-3H]thymidine Ad2/5 labeling was carried out as described previously (9) according to the method of Roelvink et al. (28). Specific activity ranged between 4 × 10−5 and 9 × 10−4 cpm/virion. The binding experiments performed with cells grown to confluence, as described in the previous report (9), were also carried out with sparse cells. Briefly, A549 or CHO cells seeded at densities ranging from 20 to 30,000 cells/cm2 were detached after 2 days (sparse cells) or after 5 to 8 days to obtain confluent cells. Cell densities are reported in the figure legends. In all cases, A549 or CHO cells were detached with 5 mM EGTA in PBS and suspended at the concentration of 4 × 106/ml in PBS++ (PBS, 3 mM MgCl2, 1 mM CaCl2). Raji cells were suspended under the same conditions. Radioactive Ads (15,000 to 20,000 cpm) were incubated for 1 h at 4°C with 106 cells in Eppendorf tubes precoated with 5% bovine serum albumin in PBS++. Cells were washed twice with PBS++, and the pellet was suspended in 100 μl of PBS++ and placed in a liquid scintillation counter. Specific binding was calculated by subtracting the signal obtained in the presence of a 50-fold excess of nonlabeled Ad. The average amount of nonspecific binding was 20% of the total amount of Ad bound. Competition experiments were performed as described for the infection assay.
Proliferation assay.
A cell proliferation assay kit (Chemicon International) was used for quantification of cell proliferation and viability, according to the manufacturer's instructions. The assay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases, which is a function of the expansion of the number of viable cells. The production of formazan was measured by absorbance at 440 nm. Cells cultured in 96-well microtiter plates were incubated with Ad in the presence of sCAR-D1 and heparin, as described above for the cell infection assay but without washing to prolong the exposure time. Therefore, after incubation for 1 h at 4°C, cells were brought to 37°C for 24 h with Ad, sCAR-D1, or heparin, as specified in Fig. 3C. After this period, the tetrazolium salt was added (10 μl to each well) and absorbance was measured at the times indicated in Fig. 3C.
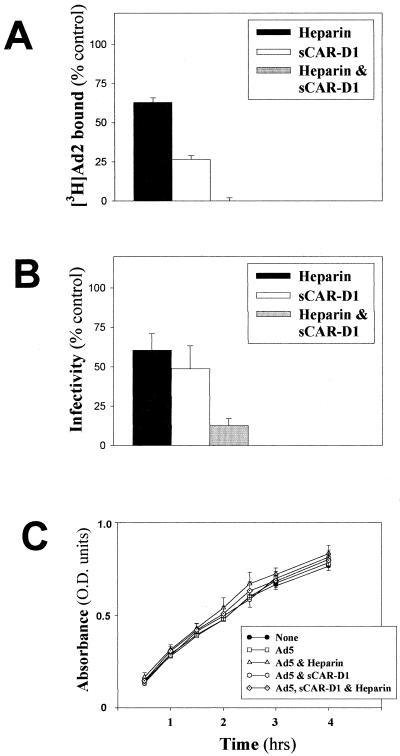
FIG. 3.
Effect of sCAR-D1 and heparin on binding and infection of A549 cells. (A) Binding assay. [3H]Ad2 (1,200 × 106 virions) was preincubated with sCAR-D1 (200 μg/ml) and/or heparin (10 μg/ml) for 1 h at 37°C before being subjected to a binding assay, as described in Materials and Methods. The amount of Ad bound in the absence of soluble receptor analogues [(counts per minute of virus bound/counts per minute of total virus added) × 100] was 5.6 ± 0.4 (mean ± SD, n = 6). Results shown are representative of three independent experiments. (B) Infection assay. Ad2 (90 × 106 virions) was preincubated with sCAR-D1 and/or heparin for 1 h at 37°C before being added to confluent A549 cell monolayers as described in Materials and Methods. The number of foci counted in the absence of sCAR-D1 and heparin (100% infectivity) was 4,816 ± 384 per well (mean ± SD, n = 3). Results shown are representative of three independent experiments. (C) Proliferation assay. The absorbance of formazan was measured in A549 cells preincubated for 24 h with medium (filled circles), Ad5 (40 × 106 virions) (open circles), Ad5 and heparin (10 μg/ml) (open triangles), Ad5 and sCAR-D1 (200 μg/ml), or Ad5 with both heparin (10 μg/ml) and sCAR-D1 (200 μg/ml) (dotted diamonds) as described in Materials and Methods. Data are means ± standard errors of the means (n = 3), and the time course is representative of two independent experiments. O.D., optical density.
Flow cytometry analysis.
Cells (2 × 107/ml) in 0.5% casein–PBS were incubated with 20 μg of F58-10E4 immunoglobulin M monoclonal antibody (Seikagaku America, Falmouth, Mass.) per ml directed against HS for 2 h at 4°C and then with 50 μg of fluorescein-conjugated goat anti-mouse immunoglobulin M antibody (Sigma) per ml for 30 min at 4°C in PBS. After being washed, cells were fixed with 4% formaldehyde in PBS and analyzed using a FACScan flow cytometer (Becton Dickinson).
RESULTS
Competitive inhibition of Ad binding and infection.
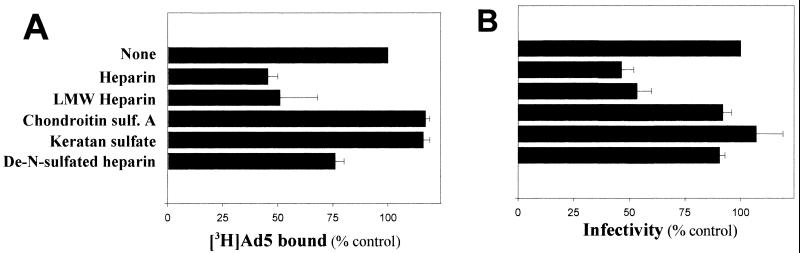
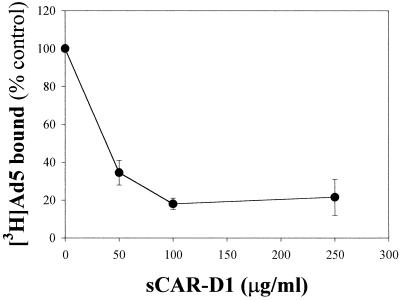
We previously demonstrated that HS GAGs expressed on cell surfaces are involved in the binding and infection of Ad2/5 by different experimental approaches, including the competitive inhibition of Ad interactions with heparin (9). To demonstrate that interactions of Ad2/5 with cell receptors are related to HS GAGs and are not simply due to charge interactions, other soluble GAGs were tested in comparison with heparin in both binding and infection experiments. Figure 1 indicates that only the HS GAG analogue heparin is relevant to inhibition of Ad binding and infection. The distal N-terminal extracellular CAR-D1 has been identified and further characterized as the domain involved in binding to Ad2 (5, 11, 29). The ability of sCAR-D1 to inhibit the binding of Ad2/5 to the native cell receptor was tested in cells expressing CAR but lacking cell surface HS GAGs such as Raji cells (10, 22). Increasing concentrations of sCAR-D1 were preincubated with [3H]Ad5 before attachment to cells. The dose-response experiment whose results are shown in Fig. 2 indicates that sCAR-D1 inhibits almost completely Ad5 binding at concentrations starting from 100 μg/ml. These results validate the idea that sCAR-D1 is an appropriate tool for abolishing CAR-specific Ad binding. To assess the relative contribution of each receptor to Ad-host cell interaction, competitive inhibition was performed with sCAR-D1 and/or heparin as the soluble receptor analogue in A549 cells. No binding was observed upon preincubation of [3H]Ad2 with both heparin (10 μg/ml) and sCAR-D1 (200 μg/ml), as shown in Fig. 3A. Competition with heparin or sCAR-D1 produced partial inhibition. Similar results were obtained with [3H]Ad5. The infection experiments presented in Fig. 3B confirm the additive effect observed in binding experiments when virus was preincubated with both sCAR-D1 and heparin. The presence of a residual infectivity could be explained by entry of the virus through receptor-independent fluid-phase pinocytosis, as demonstrated with fluorescent Ads in A549 cells (15). As soluble factors such as heparin or sCAR-D1 may potentially affect Ad infection by interfering with cell growth, cells were incubated up to 28 h with heparin and sCAR-D1 and proliferation was tested. As shown in Fig. 3C, neither heparin nor sCAR-D1 modified cell proliferation. This finding is also consistent with previously reported data showing that heparin does not reduce infection efficiency of Ad3, which does not utilize HS GAGs as receptors (9). These results strengthen the finding that HS GAGs are indeed involved in Ad2/5 binding and infection, opening the possibility of HS GAGs being receptors independent of CAR.
FIG. 1.
Heparin inhibits Ad binding and infection of A549 cells. (A) Binding assay. [3H]Ad5 (1,000 × 106 virions) was preincubated for 1 h at 37°C with heparin, low-molecular-weight (LMW) heparin, chondroitin sulfate (sulf.) A, keratan sulfate, or de-N-sulfated heparin (10 μg/ml) before being subjected to a binding assay as described in Materials and Methods. The amount of Ad bound in the absence of GAGs [(counts per minute of virus bound/counts per minute of total virus added) × 100] was 4.6 ± 0.5 (mean ± standard deviation [SD], n = 4). Results shown are representative of two independent experiments. (B) Infection assay. Ad5 (120 × 106 virions) was preincubated with GAGs for 1 h at 37°C before being added to confluent A549 cell monolayers, as described in Materials and Methods. The number of foci counted in the absence of GAGs (100% infectivity) was 4,700 ± 627 per well (mean ± SD, n = 4). Results shown are representative of two independent experiments.
FIG. 2.
sCAR-D1 inhibits Ad binding to Raji cells. [3H]Ad5 (500 × 106 virions) was preincubated with increasing concentrations of sCAR-D1 for 1 h at 37°C and then cooled to 4°C and incubated with 106 cells for 1 h. Ad bound in the absence of sCAR-D1 [(counts per minute of virus bound/counts per minute of total virus added) × 100] was 1.5 ± 0.2 (mean ± SD, n = 6). Results shown are representative of three independent experiments.
Ad binding to HS GAGs as a function of cell density.
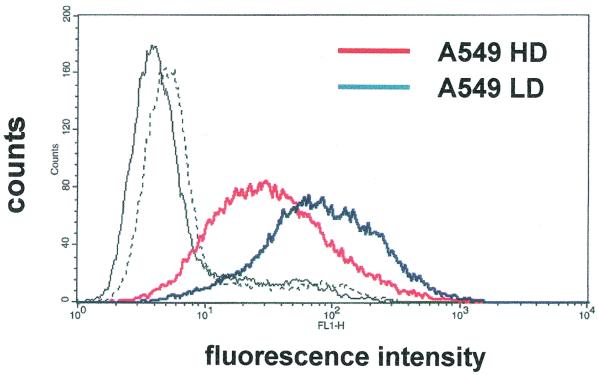
To help in understanding the relevance of HS GAGs as Ad receptors, we devised a strategy to modulate the expression of HS GAGs. Cells modify the level of expression and the degree and pattern of sulfation of HS GAGs in response to proliferation, differentiation, and transformation (18). Changes in the expression of HS GAGs have been obtained by growing corneal fibroblast cells at different densities, where ligand binding to HS GAGs was higher in sparse cells than in confluent cells (27). Therefore, A549 cells were grown under different confluence conditions, both at high and low densities (200 to 300,000 and 50 to 70,000 cells/cm2, respectively), and HS GAGs expression was measured by flow cytometer analysis with the F58-10E4 antibody, which is directed against the HS moiety of the GAGs (8). As shown in Fig. 4, confluence leads to a decline in HS GAG levels in A549 cells. Therefore, we tested the effect of heparin on Ad binding both in A549 cells grown to confluence and in sparse cells. The dose-response experiment reported in Fig. 5 indicates that Ad2 binding to HS GAGs is doubled in sparse cells with respect to that in confluent cells. The effect of heparin in confluent cells reproduces that observed in the experiments described in Fig. 3A and previously reported (9), performed under the same density conditions. The results shown in Fig. 5 demonstrate that Ad binding to HS GAGs can be up-modulated by growing A549 cells at low density.
FIG. 4.
Expression of HS GAGs in A549 cells. Fluorescence intensity is plotted against the number of events (counts). A549 HD indicates A549 cells grown at high density (from 200,000 to 300,000 cells/cm2) in the absence (solid black line) and in the presence (solid red line) of the primary antibody F58-10E4 directed against the HS GAGs. A549 LD indicates A549 cells grown at low density (from 50,000 to 70,000 cells/cm2) in the absence (dashed black line) and in the presence (solid blue line) of the primary antibody.
FIG. 5.
Inhibition of Ad binding by heparin as a function of density in A549 cells. [3H]Ad2 (1,200 × 106 virions) was preincubated with heparin for 1 h at 37°C before being subjected to a binding assay, as described in Materials and Methods. Ad bound in the absence of heparin [(counts per minute of virus bound/counts per minute of total virus added) × 100] was 5.3 ± 0.3 in confluent cells (from 200,000 to 300,000 cells/cm2) and 7.1 ± 0.4 in sparse cells (from 50,000 to 70,000 cells/cm2). Data are means ± SD. Results shown are representative of three independent experiments.
Ad binding to CAR-negative cell lines.
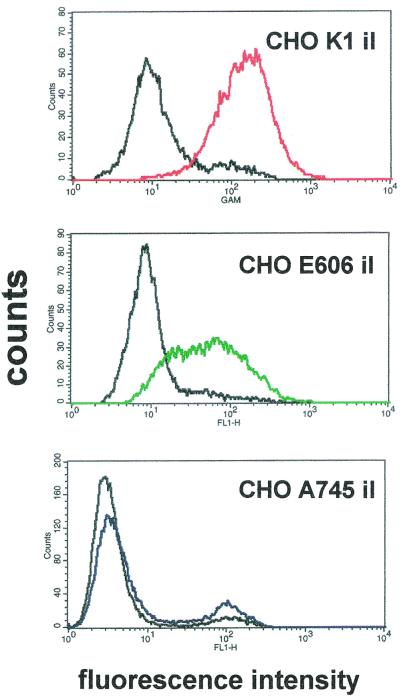
The role of HS GAGs as independent receptors for Ad2/5 can be more precisely defined in a cell line lacking the constitutive expression of CAR. CHO cell lines have been widely used to understand the role of GAGs as cell receptors, as a large collection of mutants of CHO cells defective in glycosylation or proteoglycan biosynthesis has been characterized (31). As summarized in Table 1, we used cells expressing different GAG moieties, like CHO cells expressing HS and chondroitin sulfate (CHO K1), the mutant clone lacking both HS and chondroitin sulfate (CHO A745), that defective in HS synthesis but expressing higher levels of chondroitin sulfate (CHO D677), and that expressing HS undersulfated by a factor of 2 to 3 (CHO E606). All these clones express constitutively αv integrins but lack CAR. Therefore, the four clones have been rendered cells that stably express CAR (CHO CAR clones). As CAR-negative cells, clones which have been transfected with an insertless plasmid (CHO il) were used. HS GAG expression has been checked in these clones by flow cytometry analysis with the antibody F58-10E4. As shown in Fig. 6, the transfection procedure did not change the expression of HS GAGs reported previously for the original cell lines, as K1 and E606 cells are positive while A745 cells are negative for HS GAGs (for references see reference 31). No signal was obtained in D677 il cells (not shown). Interestingly, the median fluorescence of the E606 il cells expressing underdesulfated HS GAGs is lower than that of K1 il cells. Ad binding to recombinant CHO clones grown to confluence is reported in Table 2. Expression of CAR increases Ad binding by 8- to 10-fold in CHO cells, demonstrating that our recombinant CHO CAR clones express a functional receptor. Under these experimental conditions no significant differences were observed between CHO K1 CAR cells and the other mutants lacking HS GAGs, confirming that CAR expression is sufficient for binding, as reported also for Raji cells (Fig. 1). As HS GAGs with structural diversities can be expressed and regulated within the same cell type (18) and recalling that Ad binding to HS GAGs can be up-modulated in sparse A549 cells, as reported in Fig. 5, we tested Ad binding to CAR-negative CHO clones grown at low density. Ad2 binding to the CAR-negative CHO K1 il cells increased by an average of fourfold in cells grown at low density, as shown in Fig. 7. Ad2 binding to sparse CHO K1 il cells was completely inhibited by heparin, suggesting that the increased Ad binding observed in sparse cells was due to the interaction with HS GAGs. Moreover, Ad binding to HS GAGs defective CHO clones did not change as a function of cell density, indicating that the GAG involved in Ad binding to CHO K1 il cells is HS. Therefore, the experiments in CAR-defective CHO K1 il cells grown at low density demonstrate that HS GAGs are regulated receptors sufficient to mediate Ad2/5 binding in cells lacking CAR expression.
FIG. 6.
Expression of HS GAGs in CHO il cells. Fluorescence intensity is plotted against the number of events (counts). The fluorescence measured in the absence of the primary antibody F58-10E4 is shown by solid black lines.
TABLE 2.
[3H]Ad5 binding to recombinant CHO cells grown to confluencea
| CHO cells | Mean of Ad bound (% of virus added) ± SD
|
|
|---|---|---|
| Transfected with insertless vector | Transfected with CAR | |
| K1 | 1.0 ± 0.4 (12) | 11.7 ± 1.0 (4) |
| A745 | 0.3 ± 0.1 (8) | 10.1 ± 1.9 (4) |
| D677 | 0.7 ± 0.2 (4) | 11.9 ± 1.2 (4) |
| E606 | 0.6 ± 0.2 (4) | 9.7 ± 1.5 (4) |
Binding experiments were performed as described in Materials and Methods. Cell density ranged from 80,000 to 120,000 cells/cm2. Values in parentheses are numbers of experiments performed.
FIG. 7.
Ad binding to CAR-defective recombinant CHO cells as a function of cell density. [3H]Ad2 binding to CHO il cells was performed as described in Materials and Methods. Binding to confluent cells (from 80,000 to 120,000 cells/cm2) and to sparse cells (from 20,000 to 30,000 cells/cm2) was performed as described in Materials and Methods. Data are means ± SD of results of 12 independent experiments paired for high and low densities with K1 cells, of 8 experiments with A745 cells, and of 4 experiments with D677 and E606 cells.
Ad infection in CAR-negative cell lines.
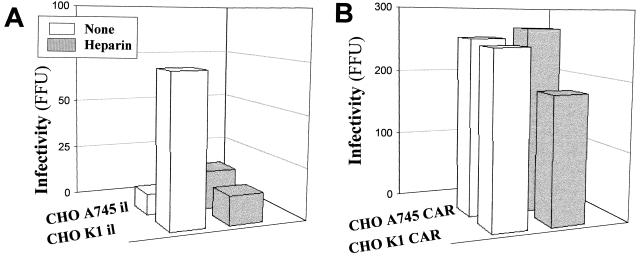
That Ad2/5 use HS GAGs as autonomous receptors for attachment to host cells raises the question of whether HS GAGs are sufficient to initiate the multistep process leading to infection and viral replication. To address this issue we performed infection experiments in CHO cells grown at low density. Ad2 is able to infect CAR-defective CHO K1 il cells in a time-dependent fashion, as shown in Fig. 8. CHO K1 CAR cells were also infected, while only a few scattered fluorescent foci were observed in CHO A745 il cells lacking HS GAGs and CAR, even 96 h postinfection (data not shown). Competitive-inhibition experiments with heparin were performed to confirm that infection in CHO K1 il cells was mediated by initial attachment to HS GAGs. Preincubation of Ad2 with heparin (10 μg/ml) inhibited Ad2 infection in CHO K1 il cells, as shown in Fig. 9A, supporting the role of HS GAGs in infection. According to the results obtained in cells expressing both CAR and HS GAGs like A549 cells (Fig. 3B), heparin partially reduced infection in CHO K1 CAR cells, as shown in Fig. 9B. CHO A745 cells are indeed permissive to infection upon transfection with CAR, as shown in Fig. 9B. As expected, heparin did not ihibit infection in this cell line. Taken together, the results presented here demonstrate that HS GAGs are receptors able to mediate Ad2/5 binding and infection in the absence of CAR.
FIG. 8.
Time course of Ad infection in recombinant CHO cells. Ad2 (90 × 109 virions) was added to sparse CHO cells for 1 h at 4°C, and the cells were washed and incubated at 37°C for the times specified in the graph, as described in Materials and Methods. Data are means ± SD (n = 3) of the number of fluorescent foci per well (FFU).
FIG. 9.
Effect of heparin on Ad infection of recombinant CHO cells. CHO K1 or A745 cells were transfected with an insertless vector (A) or with a vector encoding CAR (B). Ad2 (90 × 109 virions) was preincubated with heparin (10 μg/ml) for 1 h at 37°C before being added to sparse CHO cells for 1 h at 4°C, and the cells were washed and incubated at 37°C for 72 h, as specified under Materials and Methods. Histograms show means of FFU from two separate experiments performed in triplicate.
DISCUSSION
We previously proposed the involvement of HS GAGs as receptors for Ad2/5 binding and infection (9), but their role with respect to CAR was not defined. The results presented here demonstrate that HS GAGs are receptors sufficient for Ad2/5 binding, which leads to infection. This is based on the following evidence: (i) Ad2 binds and infects CAR-negative CHO K1 cells, (ii) binding and infection of CAR-negative CHO K1 cells are inhibited by heparin; and (iii) CAR-negative CHO clones defective in HS GAGs are resistent to binding and infection. That HS GAGs are sufficient to mediate the initial attachment leading to infection and replication has already been described for other viruses, like HSV-1 and AAV-2 (44, 38). Moreover, the susceptibility of HS GAG-defective CHO cells to Ad2/5 upon transfection with CAR indicates that HS GAGs are not absolutely required for Ad2/5-CAR interactions. However, the present data do not exclude the possibility that the interaction of Ad2/5 with cell surface HS GAGs can also facilitate Ad-CAR binding, i.e., by bringing the virus closer to membrane domains containing CAR or by increasing the stability and avidity of the Ad-CAR complex. It should be recalled that HS GAGs expressed on the surfaces of adherent cells modulate ligand-receptor encounters, immobilizing the ligand, increasing its local concentration, changing its conformation, or presenting it to a signaling receptor (4).
We report here that Ad2/5 binding to HS GAGs is regulated by cell density. CHO cells grown at low density are rendered permissive to Ad2 infection through binding to HS GAGs. In addition, HS GAG-mediated Ad2/5 binding is up-modulated in sparse A549 cells, in parallel with increased HS GAG expression. Different levels of cell-cell contacts have been shown to modulate HS GAG expression. In particular, low-density culture conditions increases syndecan levels in both vascular smooth muscle cells and corneal stromal fibroblasts (7, 27). In addition, the role of cell surface HS GAGs varies depending on the relative abundance of the polysaccharide chains, their size, and their nature, as enormous structural heterogeneity can be generated through specific HS chain modifications during their biosynthesis (4). HS structure variations have been schematically described as modifications in the length, the degree of sulfation, and the positions of the sulfate groups in the disaccharide repeats, resulting in carbohydrate chains with different levels of flexibility and conformations, with domains at high and low levels of sulfation (18). It should be noted that the F58-10E4 antibody recognizing the N-sulfated residues of HS GAGs revealed a lower signal in cells expressing undersulfated HS GAGs (CHO E606) than that in CHO K1 cells, the explanation being that the signal can depend on both the number of repeated HS disaccharides and their degree of sulfation (8). Therefore, the increased signal observed in A549 cells grown at low density can be due both to the increased amount of HS disaccharide repeats and to the sulfation of the residues. Moreover, HS fine-structure variations are relevant to the recognition of different ligand proteins. For instance, human immunodeficiency virus type 1 Tat protein binds to HS chains only above a minimal critical size, the binding affinity being also modulated by length variations (32). Dengue and respiratory syncytial viruses bind to HS as a function of their degree of sulfation (6, 20). Basic fibroblast growth factor binds to 6-O-sulfated but not to 2-O-sulfated HS disaccharide chains (19). Very interestingly, interaction of HS GAGs with HSV-1 glycoprotein C mediates binding insufficient for infection, while the activation of the 3-O-sulfotransferase results in the synthesis of 3-O-sulfated HS GAGs, allowing HSV-1 entry through interaction with viral glycoprotein D, indicating that subtle modifications of HS GAGs result in interaction with specific proteins, which are functional in different cell processes (35). Therefore, we can consider the possibility that both CHO and A549 cells grown at low density express structurally different types of HS chains, relevant to Ad2/5 recognition.
The C-terminal knob of Ad5 fiber is known to be the structure mediating the attachment of the virus to the N-terminal CAR-D1 (5, 29). In particular, 6 amino acid residues located on the side of the trimeric knob are recognized to be critical for binding, allowing the potential interaction of three CAR molecules for each trimeric knob (29). We cannot say which viral structure interacts with cell HS GAGs. For instance, it seems unlikely that the sulfated groups of HS GAGs interact with the fairly negative charges of the hexon protein. Moreover, the charge interactions of HS GAGs with proteins seem restricted to specific recognition structures. HS GAGs are known to bind preferentially to the consensus sequences BBXB and BBBXXB, where B is a basic amino acid like Lys, Arg, or His (39). On the other hand, structural changes among GAGs can be critical, as demonstrated also in the present report by the lack of effect of chondroitin sulfate and keratan sulfate on heparin in Ad binding and infection. The results presented here show that preincubation of Ad2/5 with sCAR-D1 does not inhibit virus binding to HS GAGs in A549 cells. Therefore, occupation of the CAR binding site with the sCAR-D1 does not interfere with the interactions between Ad2/5 and HS GAGs, suggesting that the HS GAG binding site is not close to that mediating the attachment to CAR.
The ability of Ad2/5 to use several receptors, like CAR, αv and αMβ2 integrins, class I major histocompatibility complex, and HS GAGs, either independently of or in cooperation with each other to infect a host cell, is not unusual with respect to that of other viruses, like HSV-1, AAV-2, or human immunodeficiency virus type 1. Despite ongoing progress in elucidating virus-cell interactions, there are still major deficits in our knowledge of Ad tropism, i.e., why Ad2/5 cause mild upper respiratory tract infections, as the apical surface of respiratory epithelia do not appear to have CAR and αv integrins available (41, 45). Therefore, factors other than CAR must also operate in determining host tropism, and further information on the structural features of Ad receptors interactions will increase our understanding of its mechanisms. Moreover, in consideration of the wide range of tissues that can be infected by Ads and their high efficiency in the nuclear delivery of foreign genes, further studies will provide the rational bases to design efficient Ad-derived vectors targeted to specific cell types, with minimal deleterious host reactions.
ACKNOWLEDGMENTS
We are indebted to Robert W. Finberg (Dana-Farber Cancer Institute) for donation of the RmcB antibody. We are grateful to E. Nicolis, R. Rolfini, and A. Tamanini for helpful discussions and to Federica Quiri and Angela Bozzoli for excellent technical assistance.
The financial support of Telethon—Italy (grant A.153) and the Fondo Riservato Centro Fibrosi Cistica from the Azienda Ospedaliera di Verona are gratefully acknowledged. P. Melotti is supported by Telethon (grant A.153 to G.C.). Support was in part received from AIRC—Milan (grant to M.C.).
REFERENCES
- 1.Arnberg N, Edlund K, Kidd A H, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernfield M, Gotte M, Park P W, Reizes O, Fitzgerald M L, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 5.Bewley M C, Springer K, Zhang Y-B, Freimuth P, Flanagan J M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 7.Cizmeci-Smith G, Stahl R C, Showalter L J, Carey D J. Differential expression of transmembrane proteoglycans in vascular smooth muscle cells. J Biol Chem. 1993;268:18740–18747. [PubMed] [Google Scholar]
- 8.David G, Bai X M, Van der Schueren B, Cassiman J-J, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dechecchi M C, Tamanini A, Bonizzato A, Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- 10.Ebbinghaus C, Al-Jaibajil A, Operschall E, Schoffel A, Petr I, Greber U F, Hemmi S. Functional and selective targeting of adenovirus to high-affinity FCγ receptor 1-positive cells by using a bispecific hybrid adapter. J Virol. 2001;75:480–489. doi: 10.1128/JVI.75.1.480-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class 1 alpha-2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 16.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li E, Stupack D G, Brown S L, Klemke R, Schaepfer D D, Nemerow G R. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000;275:14729–14735. doi: 10.1074/jbc.275.19.14729. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl U, Kusche-Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 19.Maccarana M, Casu B, Lindhal U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993;268:23898–23905. [PubMed] [Google Scholar]
- 20.Martinez I, Melero J A. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J Gen Virol. 2000;81:2715–2722. doi: 10.1099/0022-1317-81-11-2715. [DOI] [PubMed] [Google Scholar]
- 21.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mounkes L C, Zhong W, Cipres-Palacin G, Heath T D, Debs R J. Proteoglycans mediate cationic liposome-DNA complex based gene delivery in vitro and in vivo. J Biol Chem. 1998;273:26164–26170. doi: 10.1074/jbc.273.40.26164. [DOI] [PubMed] [Google Scholar]
- 23.Parks R J. Improvements in adenoviral vector technology: overcoming barriers for gene therapy. Clin Genet. 2000;58:1–11. doi: 10.1034/j.1399-0004.2000.580101.x. [DOI] [PubMed] [Google Scholar]
- 24.Precious B, Russell W C. Growth, purification and titration of adenovirus. In: Mahy B W J, editor. Virology: a practical approach. Oxford, United Kingdom: IRL Press; 1995. pp. 193–205. [Google Scholar]
- 25.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Handa A, Kirby M, Brown K E. The interaction of heparan sulfate and adeno-associated virus 2. Virology. 2000;269:137–147. doi: 10.1006/viro.2000.0205. [DOI] [PubMed] [Google Scholar]
- 27.Richardson T P, Trinkaus-Randall V, Nugen M A. Regulation of basic fibroblast growth factor binding and activity by cell density and heparan sulfate. J Biol Chem. 1999;274:13534–13540. doi: 10.1074/jbc.274.19.13534. [DOI] [PubMed] [Google Scholar]
- 28.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roelvink P W, Lee G M, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 30.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rusnati M, Tulipano G, Spillman D, Tanghetti E, Oreste P, Zoppetti G, Giacca M, Presta M. Multiple interactions of HIV-1 Tat protein with size-defined heparin oligosaccharides. J Biol Chem. 1999;274:28198–28205. doi: 10.1074/jbc.274.40.28198. [DOI] [PubMed] [Google Scholar]
- 33.Russell W C. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 34.Sanlioglu S, Benson P K, Yang J, Atkinson E M, Reynolds T, Engelhardt J F. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by Rac1 and phosphatidylinositol-3-kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla D, Liu J, Blaiklock P, Shworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 36.Spear P G, Eisenberg R J, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 37.Summerford C, Bartlett J S, Samulski R J. αvβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 38.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Templeton D M. Proteoglycans in cell regulation. Crit Rev Clin Lab Sci. 1992;29:141–184. doi: 10.3109/10408369209114599. [DOI] [PubMed] [Google Scholar]
- 40.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Haung S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickham T J, Mathias P, Cheresh D A, Nemerow G. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 44.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistence of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]