Abstract
Simple Summary
The present work primarily focuses on the monitoring of antimicrobial resistance from fecal Escherichia coli from wildlife, an important environmental task due to the spread of resistant bacteria. In total, 128 E. coli isolates recovered from 66 wild animals were analyzed. Their resistance was tested against 17 antibiotics. An approximate percentage of 22.1% of the animals was found to carry multidrug-resistant E. coli, and 0.93% carried strains producing extended-spectrum β-lactamase (ESBL). The highest resistance was observed against ampicillin; all of the isolates were susceptible to amikacin and carbapenems. Therefore, such findings bring concerns about the dissemination of resistant bacteria among wildlife and any further impacts on public health.
Abstract
Antimicrobial resistance (AMR) surveillance in fecal Escherichia coli isolates from wildlife is crucial for monitoring the spread of this microorganism in the environment and for developing effective AMR control strategies. Wildlife can act as carriers of AMR bacteria and spread them to other wildlife, domestic animals, and humans; thus, they have public health implications. A total of 128 Escherichia coli isolates were obtained from 66 of 217 fecal samples obtained from different wild animals using media without antibiotic supplementation. Antibiograms were performed for 17 antibiotics to determine the phenotypic resistance profile in these isolates. Extended-spectrum β-lactamase (ESBL) production was tested using the double-disc synergy test, and 29 E. coli strains were selected for whole genome sequencing. In total, 22.1% of the wild animals tested carried multidrug-resistant E. coli isolates, and 0.93% (2/217) of these wild animals carried E. coli isolates with ESBL-encoding genes (blaCTX-M-65, blaCTX-M-55, and blaEC-1982). The E. coli isolates showed the highest resistance rates to ampicillin and were fully susceptible to amikacin, meropenem, ertapenem, and imipenem. Multiple resistance and virulence genes were detected, as well as different plasmids. The relatively high frequency of multidrug-resistant E. coli isolates in wildlife, with some of them being ESBL producers, raises some concern regarding the potential transmission of antibiotic-resistant bacteria among these animals. Gaining insights into antibiotic resistance patterns in wildlife can be vital in shaping conservation initiatives and developing effective strategies for responsible antibiotic use.
Keywords: wildlife, Escherichia coli, MDR, ESBL, whole genome sequencing
1. Introduction
Antimicrobial resistance (AMR) is a major concern worldwide as it reduces the efficacy of antibiotics and increases the risk of infections without possible treatment. Escherichia coli is a species of bacteria commonly associated with AMR, which is often found colonizing the intestinal tract of wildlife populations. Wildlife can act as a reservoir of resistance genes that can be spread to humans and domestic animals, posing a public health risk [1]. AMR surveillance in Escherichia coli from wildlife has become essential under the ecological concept of One Health [2,3].
E. coli is a relevant opportunistic pathogen often associated with various infections, including urinary tract infections, sepsis, and pneumonia, among others [4]. The widespread use of antibiotics has led to the selection of AMR E. coli isolates, which threaten human and animal health [5].
AMR surveillance in wildlife can also provide valuable information about the presence and spread of AMR bacteria in the environment [6,7]. Wild animals, such as birds and mammals, can acquire AMR bacteria from different contaminated sources and can later contribute to the dissemination of AMR to other animals [8]. The proximity of humans and animals in many rural and urban areas makes monitoring AMR in wildlife populations essential to understand AMR evolution [9].
The presence of extended-spectrum β-lactamases (ESBL) in isolates from wildlife is a matter of significant concern in both the public health and conservation fields. ESBLs are enzymes produced by certain bacteria that confer resistance to a broad range of β-lactam antibiotics, including broad-spectrum cephalosporins [10]. The presence of ESBL-producing isolates in wildlife points to potential environmental contamination with these resistant strains. Fecal shedding from wildlife can introduce ESBLs and other antibiotic-resistant genes into the environment, which may have broader ecological consequences and contribute to the dissemination of resistance genes [10].
Several challenges are associated with AMR surveillance in wildlife, including the need for specialized equipment and expertise and the difficulty of collecting sufficient samples from wildlife populations [11]. In addition, many wildlife populations are difficult to access and sample, making it challenging to obtain representative samples for AMR analysis. Furthermore, the presence of AMR bacteria in wildlife populations may not always indicate the presence of these bacteria in humans or domestic animals, as different populations may have different levels of exposure to antibiotics [12].
Despite these challenges, surveillance in wildlife is an important tool for understanding the spread of AMR bacteria in the environment and developing effective control strategies [13]. In particular, monitoring wildlife populations can provide valuable information about the origin and spread of AMR bacteria and help to identify risk factors [2].
The main objectives of this study were to evaluate the AMR levels of fecal E. coli isolates obtained from different wild animals and to characterize them by genomic analysis. Many previous studies in wildlife have been focused on the detection of specific bacteria/AMR phenotypes (for example, ESBL-E. coli) using specific media for AMR E. coli recovery (antimicrobial-supplemented media). Nevertheless, in this study, we were interested in analyzing the resistance profiles of the E. coli isolates obtained from plates not supplemented with antibiotics to obtain information on the AMR levels and genetic characteristics of non-selected E. coli isolates of wildlife.
2. Materials and Methods
2.1. Study Area and Sampling
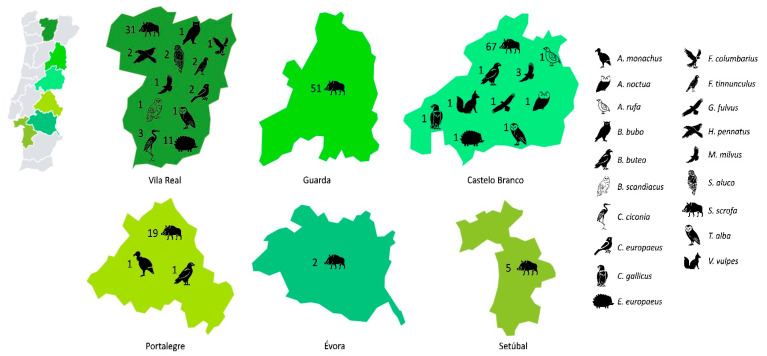
The present study was performed in six different districts of Portugal, namely Vila Real, Guarda, Castelo Branco, Portalegre, Setúbal, and Évora. Fecal samples were collected from 217 different wild animals individually (29 wild birds and 188 mammals) between January 2020 and February 2022 (Figure 1). They were obtained in collaboration with the CRAS (Centro de Recuperação de Animais Selvagens) institution, the CERAS (Centro de Estudos e Recuperação de Animais Selvagens) institution, and during hunts of wild boars organized by different corporations of hunters. Regarding the samples obtained by CRAS and CERAS, the collection of fecal samples was carried out as soon as the animals excreted feces on their own, that is, in the first days of hospitalization, using a spatula and placing the fresh feces directly into a sterilized shipping container. Some of the animals were undergoing antibiotherapy (Supplementary File S1). The wild boar samples were obtained only from those animals which, for the reason of regular hunting, had been legally killed. No animal was killed for the purpose of this study. Fecal samples (~25 g) were collected from the posterior part of the large intestine shortly after death, at evisceration, at the collection point following each driven hunt. They were stored at 4 °C and taken to the laboratory within 12 h.
Figure 1.
A map of Portugal showing the area of study (six districts) and the numbers and different species of animals sampled. The districts are not in scale.
2.2. Bacterial Isolates and Identification
As soon as the samples arrived at the laboratory, 1g of each fecal sample was inoculated in Brain Heart Infusion broth (Liofilchem, Roseto degli Abruzzi, Italy) for enrichment and incubated at 37 °C for 24 h. Then, each sample was streaked on HiCrome Klebsiella Selective Agar Base (HiMedia Laboratories, Einhausen, Germany) and incubated for 24 h at 37 °C. In this medium, both E. coli and Klebsiella spp. grew with the same morphology (purple-magenta color), and no antibiotic was added to specifically select a resistant trait of these species. From each sample, we selected four colonies with this morphology, and we seeded them on Chromogenic Coliform Agar (Oxoid, Cheshire, UK) to differentiate them since E. coli grows with a dark blue color and Klebsiella spp. develops a salmon-red color. The isolates were identified by MALDI-TOF (Bruker, Billerica, MA, USA), and the E. coli isolates were kept for further studies.
2.3. Antimicrobial Susceptibility Testing
The phenotypic resistance characterization of the E. coli isolates was performed using the Kirby–Bauer disk diffusion method by following EUCAST standards (2022) [14], except for ceftazidime, cefotaxime, tetracycline, and nalidixic acid, for which CLSI standards (2021) were followed [15]. Escherichia coli ATCC® 25922 was used as a control strain. The double-disc synergy test was used to evaluate ESBL production. A total of 17 antibiotics were used in the susceptibility testing of E. coli isolates (in μg/disk): amoxicillin–clavulanic acid (20–10), amikacin (30), ampicillin (10), aztreonam (30), cefepime (30), cefotaxime (5), cefoxitin (30), ceftazidime (10), chloramphenicol (30), ciprofloxacin (5), ertapenem (10), gentamicin (10), imipenem (10), meropenem (10), nalidixic acid (30), tetracycline (30), and trimethoprim–sulfamethoxazole (1.25/23.75). Mueller–Hinton Agar (Alliance Bio Expertise, Bruz, France) was the medium used for susceptibility testing, and the zones of inhibition were examined after 18 h at 37 °C. Non-repetitive E. coli isolates were selected for further studies (only one isolate was selected or more than one if they exhibited different antibiotic resistance profiles). Following this criterion, a collection of 128 non-repetitive E. coli isolates were characterized in this study. Isolates that were resistant to three or more antibiotic classes were considered multidrug-resistant (MDR).
2.4. Whole Genome Sequencing and Analysis
For whole genome analysis, the main selection criterion for the isolates was their capacity to produce ESBLs. There was a specific interest in the genomic background of antimicrobial resistance, particularly within ESBL-producing isolates, and this was an essential factor that influenced the decision. After that, we further evaluated the total AMR profiles. We prioritized isolates with a wider resistance pattern to have a representative sample of the resistance mechanisms in the population. Finally, the animal species from which the isolates were obtained were included. We aimed for our subset to mimic the variety of animal species and sources available in the populations, with as many isolates from different sources and species included as possible. The whole genome analysis from paired raw reads was realized on selected strains by TORMES v1.3. 0, a bioinformatics pipeline that allows for the quality filtering of raw reads (Prinseq), assembly (SPAdes) and subsequent quality filtering (QUAST), taxonomic identification (Kraken2, RDP Classifier, Barrnap), and annotation (Prokka, Prodigal). In addition, it includes specific programs for typing (MLST, fimHTyper, SerotypeFinder) and searching for resistance genes (Blastn, ABRicate and PointFinder in the databases ResFinder, CARD, ARG-ANNOT and PointFinderDB), virulence factors (ABRicate, VFDB), and plasmid detection (Blastn, ABRicate, PlasmidFinder). Finally, R packages (ggplot2 v3.5.1, ggtree v2.1.0, knitr v1.48, plotly v4.10.4, RColorBrewer v1.1-3, reshape2 v1.4.4, and rmarkdown v2.28) were used to generate the report and the plots that appear in it [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Phylotyping was conducted using ClermonTyper, which is available online (http://clermontyping.iame-research.center/) (accessed on 27 July 2024).
3. Results
3.1. E. coli Prevalence in Feces of Wild Animals
A total of 128 non-repetitive E. coli isolates were recovered from 66 of the 217 fecal samples collected (30.7%) using media not supplemented with antibiotics. The 66 animals in which E. coli isolates could be recovered were the following: 32 wild boars (Sus scrofa), 12 hedgehogs (Erinaceus europaeus), 2 common kestrels (Falco tinnunculus), 3 white storks (Ciconia ciconia), 3 red kites (Milvus milvus), 1 eurasian eagle-owl (Bubo bubo), 3 barn owls (Tyto alba), 2 booted eagles (Hieraaetus pennatus), 3 tawny owls (Strix aluco), 1 common buzzard (Buteo buteo), 1 red-legged partridge (Alectoris rufa), 1 eurasian griffon vulture (Gyps fulvus), 1 red fox (Vulpes vulpes), 1 cinereous vulture (Aegypius monachus), 1 snowy owl (Bubo scandiacus), and 1 merlin (Falco columbarius). Of the 66 animals in which E. coli isolates were recovered, 16 were undergoing antibiotherapy. Overall, E. coli isolates were recovered from 45 wild mammals (23.9%) and 21 wild birds (77.8%) (Table 1).
Table 1.
The wild animal species sampled, the number of E. coli isolates obtained, and the number of isolates with an MDR or ESBL phenotype from the respective host species.
| Wild Animals | E. coli Isolates | |||||
|---|---|---|---|---|---|---|
| Classes | Species | No. of Animals Sampled | No. of Animals from Which E. coli Strains Were Obtained | No. | MDR a | ESBL b |
| Birds | Hieraaetus pennatus | 2 | 2 | 6 | 6 | 0 |
| Strix aluco | 3 | 2 | 4 | 4 | 0 | |
| Falco columbarius | 1 | 1 | 2 | 2 | 0 | |
| Milvus milvus | 4 | 3 | 6 | 6 | 0 | |
| Caprimulgus europaeus | 2 | 0 | 0 | 0 | 0 | |
| Falco tinnunculus | 2 | 2 | 6 | 5 | 0 | |
| Tyto alba | 3 | 2 | 5 | 5 | 0 | |
| Bubo bubo | 1 | 1 | 2 | 2 | 0 | |
| Ciconia ciconia | 3 | 3 | 7 | 7 | 0 | |
| Bubo scandiacus | 1 | 1 | 2 | 1 | 0 | |
| Buteo buteo | 2 | 1 | 4 | 3 | 0 | |
| Aegypius monachus | 1 | 1 | 2 | 2 | 0 | |
| Gyps fulvus | 1 | 1 | 2 | 2 | 1 | |
| Athene noctua | 1 | 0 | 0 | 0 | 0 | |
| Alectoris rufa | 1 | 1 | 2 | 2 | 0 | |
| Circaetus gallicus | 1 | 0 | 0 | 0 | 0 | |
| Mammals | Erinaceus europaeus | 12 | 12 | 27 | 22 | 0 |
| Vulpes vulpes | 1 | 1 | 1 | 1 | 1 | |
| Sus scrofa | 175 | 32 | 50 | 22 | 0 | |
| Total | 217 | 66 | 128 | 92 | 2 | |
a Multidrug Resistant; b Extended-Spectrum β-Lactamase.
3.2. Antimicrobial Resistance Profile
Firstly, we verified that 71.9% of the E. coli isolates were MDR and were obtained from 48 of the 217 animals analyzed (22.1%). From the ESBL production test, we detected two E. coli isolates that produced ESBL, obtained from a red fox (Vulpes vulpes) (VV1) and Eurasian griffon vulture (Gyps fulvus) (GF3) (Table 1); these ESBL-E. coli isolates represented 1.6% of the total isolates, 0.9% of animals tested, and 3.0% of animals in which E. coli could be recovered. However, 36 isolates were recovered from animals undergoing antibiotherapy, including the ESBL isolate VV1, which could influence the resistance rates of these E. coli isolates.
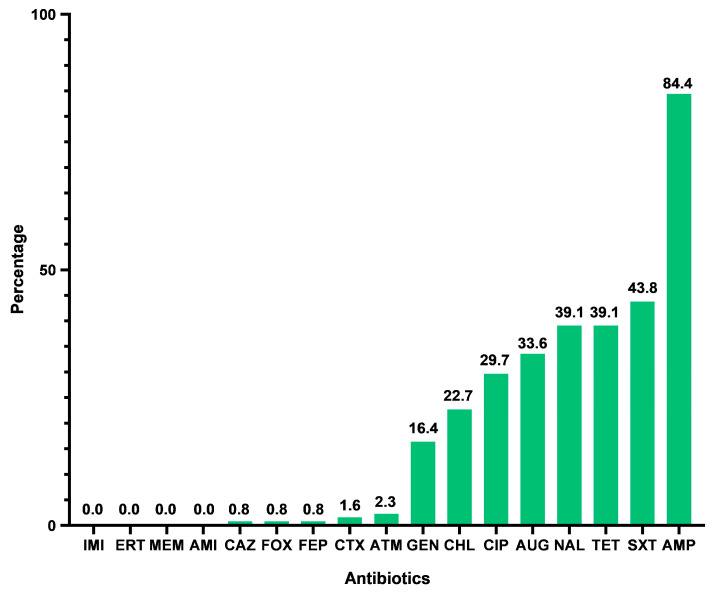
The E. coli isolates, in general, showed high resistance rates to ampicillin (84.4%), trimethoprim–sulfamethoxazole (43.8%), tetracycline (39.1%), nalidixic acid (39.1%), and amoxicillin–clavulanic acid (33.6%); however, they showed low rates of resistance to cefepime (0.8%), cefoxitin (0.8%), and ceftazidime (0.8%) (Figure 2). Among the E. coli isolates recovered from the animals undergoing antibiotherapy, we verified that only seven antibiotics (trimethoprim–sulfamethoxazole, nalidixic acid, amoxicillin–clavulanic acid, ciprofloxacin, gentamicin, cefotaxime, and cefoxitin) had higher resistance rates than the E. coli isolates recovered from the untreated animals. Additionally, the E. coli isolates obtained from treated animals showed the highest resistance rates to ampicillin (91.7%), nalidixic acid (77.8%), amoxicillin–clavulanic acid (63.9%), ciprofloxacin (63.9%), and trimethoprim–sulfamethoxazole (55.6%). The E. coli isolates obtained from untreated animals showed the highest resistance rates to ampicillin (100%), tetracycline (54.8%), nalidixic acid (52.4%), trimethoprim–sulfamethoxazole (47.6%), and amoxicillin–clavulanic acid (45.2%).
Figure 2.
Percentage of antibiotic resistance in collection of 128 E. coli isolates. IMI—imipenem; ERT—ertapenem; MEM—meropenem; AMI—amikacin; CAZ—ceftazidime; FOX—cefoxitin; FEP—cefepime; ATM—aztreonam; CTX—cefotaxime; GEN—gentamicin; CHL—chloramphenicol; CIP—ciprofloxacin; AUG—amoxicillin–clavulanic acid; NAL—nalidixic acid; TET—tetracycline; SXT—trimethoprim–sulfamethoxazole; AMP—ampicillin.
The isolates exhibited resistance to seven different classes of antibiotics (β-lactams, aminoglycosides, tetracyclines, sulfonamides, trimethoprim–sulfamethoxazole, chloramphenicol, and fluoroquinolones). It was also found that the E. coli isolates were resistant to a greater number of antibiotics, such as amoxicillin–clavulanic acid, ampicillin, cefotaxime, chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid, tetracycline, and trimethoprim–sulfamethoxazole. All isolates presented susceptibility to amikacin, meropenem, ertapenem, and imipenem, which are antibiotics that belong to the aminoglycoside and carbapenem classes. More detailed information is given in Supplementary File S1.
3.3. Sequencing, Assembly, and Annotation
To assess the carriage of antibiotic resistance and virulence genes, the genomes of 29 representative isolates from one-third of the animals where E. coli was obtained (20/66) were sequenced and analyzed. These isolates were recovered from the following animals: 8 wild mammals (1 Vulpes vulpes, 1 Sus scrofa, and 6 Erinaceus europaeus) and 12 wild birds (1 Aegypius monachus, 1 Strix aluco, 2 Hieraaetus pennatus, 1 Milvus milvus, 2 Falco tinnunculus, 1 Bubo bubo, 1 Tyto alba, 1 Falco columbarius, 1 Gyps fulvus, and 1 Bubo scandiacus). The average sequencing depth was 588×; the number of assembled contigs ranged between 167 and 7792, and the genome sizes ranged between 4,858,932 and 9,881,410 nucleotides. The average GC content per sample was 50.65%. The draft whole genome sequences of the 29 E. coli isolates were deposited in GenBank under the Bio-Project nº PRJNA1006036, and their accession numbers are SAMN37007325, SAMN37007368, and SAMN42001311.
3.4. Whole Genome Typing of E. coli Isolates
Among the 29 strains sequenced, we identified 11 sequence types (ST) by multilocus-sequence typing (MLST) based on the seven-gene Achtman’s scheme and 10 FimH types. MLST assignation was not achieved in two strains since new alleles were found or only a partial match to the known allele was made. Of these, ST162, ST1196, and ST2161 were the most prevalent ST observed with five isolates each. Thirteen different serotypes were also identified, and serotypes O100:H28 and O3:H14 were the most frequently detected among the isolates (Table 2). Regarding phylogroups, 5 strains belonged to phylogroup A (17.2%), 22 to phylogroup B1 (75.9%), 1 to phylogroup F (3.4%), and 1 to phylogroup D (3.4%) (Supplementary File S2).
Table 2.
Comprehensive sequencing data of 29 selected E. coli isolates from wild animals.
| Strain | Animal of Origin | MLST | Phylogroup | Serotype | FimH Type | Resistome | Plasmids | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactams | Other Resistance Genes | Quinolone Resistance Due to Point Mutations | |||||||||
| gyrA | parC | parE | |||||||||
| AC1 | Hieraaetus pennatus (5077/D1290) | ST162 | B1 | O25:H9 | FimH32 | - | aac(3)-IIa; aadA2; aadA5; ant(3″)-Ia; aph(3″)-Ib; aph(3′)-Ia; mdfA; tetB; dfrA17; sul2; catA1; mphB; lnuF; qnrB19; qnrB5 | S83L; D87N | S80I | - | IncFIB; IncFIC(FII); IncI1 |
| AC2 | ST1196 | B1 | O100:H28 | FimH31 | bla TEM-1B | aph(3″)-Ib; aph(6)-Id; mdfA; tetB; mphB | S83L; D87N | S80I | S458A | IncFIB; IncX1 | |
| AC3 | ST10 | A | O101:H10 | FimH30 | bla TEM-1B | aadA2; aadA5; aadA1; mdfA; tetB; dfrA17; sul1; sul3; catA1; cmlA1; mphB; ermC; lnuF; qnrB19; qnrB5; qnrS1 | S83L; D87N | S80I | - | Col(MG828); Col440I (4); IncFIB; IncFIC(FII); IncI1; IncX1; rep10_3_pNE131p1 | |
| AC6 | Hieraaetus pennatus (5121/D1295) | ST162 | B1 | O25:H9 | FimH32 | - | aac(3)-IIa; aadA5; aadA2; ant(3″)-Ia; aph(3″)-Ib; mdfA; tetB; dfrA17; sul2; catA1; mphB; lnuF | S83L; D87N | S80I | - | Col(MG828); Col440I; IncFIB; IncFIC(FII); IncI1 |
| AM3 | Aegypius monachus (4489/21) | - | A | O101:H4 | FimH31 | bla TEM-1B | aadA2; aadA5; aph(3″)-Ib; aph(6)-Id; mdfA; tetB; catA1; mphB; qnrS1 | - | - | - | Col440I; ColRNAI; IncFIB; IncI1; IncR |
| BR1 | Bubo bubo (5127/N905) | ST2161 | B1 | O3:H14 | FimH1257 | bla TEM-1B | aac(3)-Iva; aph(3″)-Ib; aph(4)-Ia; aadA1; mdfA; tetA; dfrA12; sul1; sul3; cmlA1; mphB | S83L; D87N | S80I | - | Col(MG828); Col440I (2); IncFIB; IncFIB(K); IncX1 |
| BR4 | ST1196 | B1 | O100:H28 | FimH31 | bla TEM-1B | aph(3″)-Ib; aph(6)-Id; mdfA; tetB; mphB | S83L; D87N | S80I | S458A | Col440I; IncFIB; IncX1 | |
| CM1 | Strix aluco (5119/N903) | ST1196 | B1 | O100:H28 | FimH31 | bla TEM-1B | aph(3″)-Ib; aph(6)-Id; mdfA; tetB; mphB | S83L; D87N | S80I | S458A | Col440I (2); IncFIB; IncX1 |
| CM3 | ST2161 | B1 | O3:H14 | FimH1257 | bla TEM-1B | aac(3)-Iva; aph(3″)-Ib; aph(4)-Ia; aadA1; mdfA; tetA; dfrA12; sul1; sul3; cmlA1; mphB | S83L; D87N | S80I | - | IncFIB; IncFIB(K); IncX1 | |
| CN3 | Bubo scandiacus (5039/N900) | - | A | O25:H9 | FimH41 | bla TEM-1B | aadA5; aph(3″)-Ib; aph(6)-Id; aadA1; mdfA; tetA; dfrA1; dfrA17; sul2; catA1; lnuF; mphB; qnrS1 | S83L; D87N | S80I; E84G | - | Col440I; IncFIB; IncFIB(pB171); IncI1 |
| E1 | Falco columbarius (5016/D1215) | ST744 | A | O101:H9 | FimH54 | bla TEM-1B | aph(3″)-Ib; aph(3′)-Ia; aph(6)-Id; mdfA; tetB; mphB; qnrB19; qnrB5 | S83L; D87N | A56T; S80I | - | IncFIB |
| E2 | ST155 | B1 | H21 | FimH121 | bla TEM-1A | aac(3)-IIa; aadA1; mdfA; dfrA1; sul1; mefC; mphB; mphG; qnrS1 | - | - | - | IncI1; IncN | |
| GF3 | Gyps fulvus (4499/21) | ST69 | D | O17/O44:H18 | FimH27 | blaCTX-M-55; blaEC-1982 | aph(3″)-Ib; aph(6)-Id; mdfA; tetA; dfrA5; sul2; floR | - | - | - | IncFIB; IncFIC(FII) |
| MM1 | Milvus milvus (4923/D1205) | ST2161 | B1 | O3:H14 | FimH1257 | bla TEM-1B | aac(3)-Iva; aadA2; aph(3″)-Ib; aph(4)-Ia; mdfA; tetA; dfrA12; sul1; sul3; cmlA1; mphB; qnrB19; qnrB5 | S83L; D87N | S80I | - | Col(MG828); ColRNAI; IncFIB; IncFIB(K); IncX1 |
| MM2 | ST2161 | B1 | O3:H14 | FimH1257 | bla TEM-1B | aac(3)-Iva; aadA2; aph(3″)-Ib; aph(4)-Ia; aadA1; mdfA; tetA; dfrA12; sul1; sul3; cmlA1; mphB; qnrB19; qnrB5 | S83L; D87N | S80I | - | Col(MG828); Col440I (3); ColRNAI; IncFIB; IncFIB(K); IncX1 | |
| J5 | Sus scrofa (10P) | ST101 | B1 | O82:H8 | FimH86 | bla TEM-1A | aadA1; sat2; mdfA; tetA; dfrA1; sul3; mphB | - | - | - | IncFIA; IncFIB; IncFII; IncI1 |
| OC6 | Erinaceus europaeus (5049/M521) | ST162 | B1 | O32:H19 | FimH32 | bla TEM-1B | mphB; lnuF; mphB; qnrB19; qnrB5 | S83L; D87N | S80I | - | Col(MG828); Col440I (3); IncFIB; IncFIC(FII) |
| OC10 | Erinaceus europaeus (5045/M525) | ST162 | B1 | O32:H19 | FimH32 | bla TEM-1B | mdfA; tetB; mphB; qnrB19; qnrB5 | S83L; D87N | S80I | - | Col440I (2); ColRNAI; IncFIB; IncFIC(FII) |
| OC14 | Erinaceus europaeus (4927/M511) | ST58 | B1 | O9:H25 | FimH32 | bla TEM-1B | aadA5; aph(3″)-Ib; aph(6)-Id; mdfA; tetB; dfrA17; sul1; sul2; catA1; mphA; mphB | S83L | - | L416F | Col(MG828); Col440I; IncFIB; IncFIC(FII) |
| OC15 | ST58 | B1 | O9:H25 | FimH32 | bla TEM-1B | aadA5; aph(3″)-Ib; aph(6)-Id; mdfA; tetB; dfrA17; sul1; sul2; catA1; mphA; mphB | S83L | - | L416F | Col440I; IncFIB; IncFIC(FII) | |
| OC19 | Erinaceus europaeus (4813/M498) | ST162 | B1 | O11:H16 | FimH32 | bla TEM-1B | ant(3″)-Ia; aadA2; mdfA; tetA; dfrA17; lnuF; mphB; qnrB19; qnrB5 | S83L; D87N | S80I | - | Col(MG828); Col440I; IncFIB; IncFIC(FII); IncFII; IncX2 |
| OC22 | Erinaceus europaeus (4906/M508) | ST58 | B1 | O9:H25 | FimH32 | bla TEM-1B | aadA5; aph(3″)-Ib; aph(6)-Id; mdfA; tetB; dfrA17; sul1; sul2; catA1; ermC; mphA; mphB | S83L | - | L416F | Col(MG828); Col440I (2); IncFIB; IncFIC(FII); IncI1; rep10_3_pNE131p1 |
| OC23 | ST10 | A | O101:H10 | FimH30 | bla TEM-1B | aadA2; aadA5; aadA1; mdfA; tetB; dfrA17; sul1; sul3; catA1; cmlA1; lnuF; mphB; qnrS1 | S83L; D87N | S80I | - | Col440I (2); IncFIB; IncFIC(FII); IncI1; IncX1 | |
| OC26 | Erinaceus europaeus (5072/M353) | ST1196 | B1 | O100:H28 | FimH31 | bla TEM-1B | aph(3″)-Ib; aph(6)-Id; mdfA; tetB; mphB | S83L; D87N | S80I | S458A | Col440I (2); IncFIB; IncX1 |
| P3 | Falco tinnunculus (4873/D1201) | ST58 | B1 | O9:H25 | FimH32 | bla TEM-1B | aadA5; aph(3″)-Ib; aph(6)-Id; mdfA; tetB; dfrA17; sul1; sul2; catA1; mphA; mphB; qnrB19; qnrB5 | S83L | - | L416F | Col440I; IncFIB; IncFIC(FII) |
| P5 | Falco tinnunculus (4874/D1202) | ST2161 | B1 | O3:H14 | FimH1257 | bla TEM-1B | aac(3)-Iva; aph(3″)-Ib; aph(4)-Ia; aadA1; mdfA; tetA; dfrA12; sul1; sul3; cmlA1; mphB; qnrB19 | S83L; D87N | S80I | - | Col(MG828); Col440I (2); IncFIB; IncFIB(K); IncX1 |
| P6 | ST457 | F | O11:H25 | FimH145 | bla TEM-1B | aac(3)-IId; ant(3″)-Ia; aadA17; mdfA; sul3; lnuF; mefC; mphG; mphB; qnrB19; qnrB5 | S83L; D87Y | S80I | S458A | Col(MG828); Col440I (2); IncFIA; IncFIB; IncI1; IncI2; IncX1 | |
| TA1 | Tyto alba (4965/N893) | ST1196 | B1 | O100:H28 | FimH31 | bla TEM-1B | aph(3″)-Ib; aph(6)-Id; mdfA; tetB; mphB | S83L; D87N | S80I | S458A | Col(MG828); Col440I; IncFIB; IncX1 |
| VV1 | Vulpes vulpes (4492/21) | ST2179 | B1 | O9a:H9 | FimH32 | bla TEM-1B ; bla CTX-M-65 | aac(3)-Iid; mdfA; mphB; qnrS2 | S83L | S80I | - | IncFIB; IncFII(29); IncFII(pCoo) |
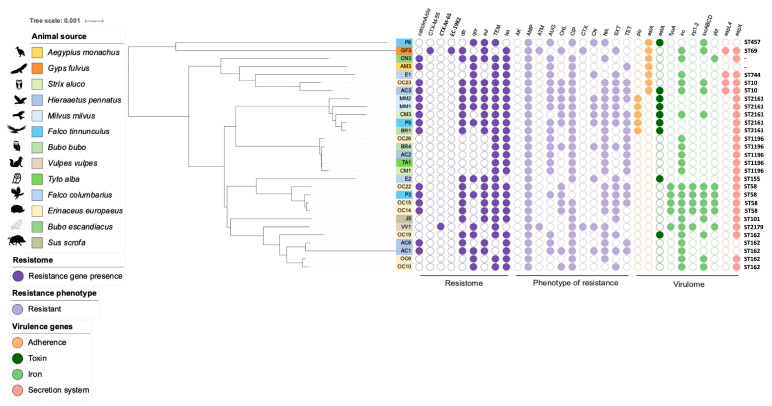
A pangenome analysis of the 29 E. coli genomes generated 2143 core genes, 2810 shell genes, and 13,081 cloud genes. In the core genome cladogram (Figure 3) we can observe the genetic distance between the different isolates. For instance, three E. coli isolates (AC1, AC2, and AC3) were obtained from the same wild animal fecal sample [bird Hieraaetus pennatus (5077/D1290)]; however, the difference in branch lengths between these isolates suggests significant genetic divergence. Furthermore, AC1 is more genetically related to AC6, an isolate recovered on other animal fecal samples of the same species, than to AC2 and AC3, since they are next to each other on the tree. The same happens between isolates OC22 and OC23; both were isolated from the same fecal sample [mammal Erinaceus europaeus (4906/M508)] but are genetically distant. In fact, they are more related to the isolates detected in wild birds (E2, P3, and AC3) than in the other mammals.
Figure 3.
A pangenome tree based on the core genes’ alignment representation of the genomes of 29 selected E. coli isolates. The presence of resistance genes, resistance phenotypes, and virulence genes is represented by colored circles, and white circles represent their absence. The final column represents the MLST of each isolate. More information on virulence genes is presented in Supplementary File S2.
3.5. Antibiotic Resistance Genes
In agreement with their β-lactam resistance phenotypes, 27 of the 29 selected isolates that were sequenced harbored at least one acquired β-lactamase gene: blaTEM-1B (n = 24), blaTEM-1A (n = 2), blaCTX-M-65 (n = 1), blaEC-1982 (n = 1), and blaCTX-M-55 (n = 1). The genes encoding CTX-M-65 and CTX-M-55 were detected in two ESBL-positive isolates of the wild animals, namely Vulpes vulpes and Gyps fulvus, respectively (Table 2).
Several other antibiotic resistance genes (ARGs) related to phenicols (floR, catA1, and cmlA1), aminoglycosides (aadA1, aadA2, aadA5, aadA17, aph(3′)-Ia, aph(3″)-Ib, aph(4)-Ia, aph(6)-Id, aac(3)-Iia, aac(3)-Iid, aac(3)-Iva, aac(3)-IIa, ant(3″)-Ia, and sat2), tetracyclines (tetA and tetB), trimethoprim (dfrA1, dfrA5, dfrA12, and dfrA17), sulfonamides (sul1, sul2, and sul3), macrolide–lincosamide–streptogramin B (mphA, mphB, mphG, mefC, lnuF, and ermC), quinolones (qnrS1, qnrS2, qnrB5, and qnrB19), and the gene responsible for encoding a multidrug transporter (mdfA) were found in several isolates. Also, regarding quinolones resistance, different amino acid changes in the GyrA (S83L, D87N, and D87Y), ParC (S80I, A56T, and E84G), and ParE proteins (S458A and L416F) were also found (Table 2).
3.6. Plasmid Replicons
At least one plasmid replicon was identified in all isolates, and they corresponded to five incompatibility group plasmids (IncF, IncI, IncR, IncX, and IncN). Col plasmids were identified in 72.4% of the isolates (21/29). The Col440I plasmid was the most frequently detected, and some of the isolates had multiple copies of it. The Col(MG828) (12/29) and ColRNAI (4/29) plasmids were also found. Plasmid IncFIB was frequently identified among the studied isolates (28/29). Most of the isolates (all but one) carried multiple plasmids (Table 2).
3.7. Virulence Factors
The 29 sequenced genomes were screened for genes related to virulence factors in the following categories: adherence, iron utilization, secretion systems, and toxins. A total of 22 adherence genes were detected (Supplementary File S2). All strains were positive for the ompA gene, a major protein in the E. coli outer membrane. Regarding the type 1 fimbriae, the fimA/B/C/D/E/F/G/H/I cluster was detected in all but one isolate (E1) where the fimA gene was missing from the cluster genes. We also detected kpsD in the P6 isolate, which is associated with the K1 capsule. All strains harbored the yagW/ecpD, yagX/ecpC, yagY/ecpB, yagZ/ecpA, and ykgK/ecpR genes, which are related to the E. coli common pilus. Additional information is available in Supplementary File S2.
A total of 41 iron utilization genes were detected (Supplementary File S2). The fes gene that catalyzes the hydrolysis of ferric enterobactin was also identified in all isolates. The entA/B/C/D/E/F/S cluster genes were present in 27 strains, and the remaining isolate (P6) lacked the entD gene from this operon. The fepA/B/C/D/G cluster of genes was present in all isolates. The complete iroB/C/D/E/N cluster was detected in 19 strains, but in the AM3 isolate, the iroD gene was detected alone, as in the P6 isolate, where the iroE gene was also verified independently. One of the twenty-eight studied isolates (P6) harbored the iron uptake cluster genes chuS/T/U/V/W/X/Y (missing chuA) and also carried the shuA gene, an outer membrane receptor that binds to hemoglobin. Additional information is available in Supplementary File S2. Heat-stable enterotoxin 1 (astA) was the only toxin gene present among nine isolates.
A total of 18 secretion system genes were detected. The genes found were involved either in the type II secretion system (T2SS) or the type III secretion system (T3SS). The T2SS widely conserved operon, gspC/D/E/F/G/H/I/J/K/L/M, was present in 26 strains. Among the genes related to T3SS, we detected the espL1/4, espX1/4/5, espR1/4, and espY1/2/3/4 genes (Supplementary File S2).
4. Discussion
The main goal of this study was to evaluate the antimicrobial resistance in E. coli from wildlife using a cultivation strategy without antibiotic selection to dimension the actual AMR prevalence among the non-selected E. coli isolates from the intestinal tracts of the animals. This information is essential for devising effective strategies to control AMR and for monitoring its progress over time. Given the proximity of humans and animals in various rural and urban settings, monitoring AMR in wildlife populations is crucial to prevent the transmission of MDR bacteria in both directions.
E. coli was detected in approximately one-third of the fecal samples analyzed; this was expected as E. coli is commonly detected in animals’ intestinal tracts [45,46], and it is more frequently detected in wild bird samples than in mammals. Different rates of E. coli recovery were reported in other studies, with higher recovery rates among birds than among mammals in general [8,47].
The relatively frequent detection of MDR or virulent E. coli isolates in wild animals is a cause for apprehension since they are able to transmit zoonotic diseases to humans and different animals. Some strains of E. coli, such as those with relevant mechanisms of antibiotic resistance (such as ESBL or carbapenemases) or those of virulent serotypes (as O157:H7), can cause zoonotic infections, resulting in various health risks for humans and animals [48].
Only 2 of the 215 tested animals [Vulpes vulpes (4492/21) and Gyps fulvus (4499/21)] carried E. coli-producing ESBL (0.93%). It should be highlighted that we recovered these isolates in a culture medium not supplemented with antibiotics, and consequently, the rate of ESBL carriage in the animals could be underestimated. The low number of wild animals carrying ESBL-positive E. coli isolates (n = 2) suggests that the wildlife population may have had limited exposure to these resistant bacteria or their genetic elements carrying ESBL genes, which could indicate that ESBLs are not yet widely spread in the environment where the wildlife was sampled. This result is potentially positive in terms of public health and environmental implications, although surveillance should be continued in the future.
Among the seventeen antibiotics used to test the antibiotic susceptibility of E. coli in general, ampicillin had the highest percentage of resistance (84.4%). High resistance rates to ampicillin are frequently detected among E. coli strains isolated from wild animals [45,49]. The use of antibiotics in some of the wild animals could have an influence on the percentage of antibiotic resistance since treatment with antibiotics exerts a selective pressure favorable to the survival and proliferation of bacteria possessing mechanisms of resistance to these drugs [50]. The only difference in the highest resistance rates between the treated and untreated animals is that the treated animals were more resistant to ciprofloxacin, and the untreated animals were more resistant to tetracycline. The fact that the treated animals were more resistant to ciprofloxacin is justifiable since one of the antibiotics used to treat these animals is enrofloxacin, which belongs to the same class as ciprofloxacin. Fortunately, most of the research on wild animals has reported, like us, low or no levels of resistance to carbapenem antibiotics [6,45,51,52]. Carbapenems are potent antibiotics often considered a last resort for treating severe bacterial infections. The absence of carbapenem resistance observed in this study in wild animals suggests that this ecosystem has not been contaminated with carbapenem-resistant bacteria, which could be detrimental to wildlife populations.
The genetic relatedness of the E. coli isolates in our study was determined by MLST, serotyping, Fim-H typing, and phylotyping of the whole genome sequences of the selected isolates. Our findings show the genetic diversity of the strains as we observed 11 different STs, which is consistent with the results of other studies. However, the most common STs were ST162, ST1196, and ST2161, with each being found in 5 of the 29 selected isolates. ST162 is commonly found among wildlife isolates [53,54], but ST1196 and ST2161 are primarily observed in humans or animals, mainly from poultry samples [55,56,57,58,59]. Still, a survey conducted in Andean condors detected E. coli strains belonging to ST1196 [60]. The most frequent serotypes detected were O100:H28 and O3:H14. Due to their presence in multiple reservoirs and their potential to cause infections, serotypes O100 and O3 are frequently found in E. coli isolates from animals and humans. The presence of both serotypes in isolates from wild animals highlights the importance of surveillance and control strategies across veterinary and human health sectors. Its ability to spread between animals and humans and the potential for antimicrobial resistance highlights the need for continued monitoring, effective public health interventions, and good education on appropriate food handling practices. Understanding the epidemiological significance of these serotypes is crucial to preventing outbreaks and ensuring public health safety.
The identification of multiple phylogroups within the E. coli isolates indicates genetic diversity. The presence of strains belonging to phylogroups A, B1, D, and F aligns with the common phylogroups found in E. coli. Commensal strains usually belong to phylogroup A, phylogroup B1 is often found in environmental isolates, and phylogroups D and F may include both commensal and pathogenic strains depending on the specific strain’s virulence factors [61]. The isolate belonging to the phylogroup D was isolated from the species Gyps fulvus, which is a species that is exposed to diverse environments potentially harboring and spreading various bacterial strains. This animal was captured by the recovery center of CERAS but was not undergoing antibiotic treatment when the fecal sample was collected, which means it was part of the animal microbiota since no selective pressure was being induced. The circulation of pathogenic E. coli strains in the environment poses a zoonotic risk since such strains may infect livestock and humans or be transmitted from wildlife to livestock or human populations, particularly where vultures or other wildlife interact with human populations, farms, or urban environments. Furthermore, the phylogenetic tree showed a great genetic diversity between the 29 E. coli isolates, where some isolates detected on mammals were more genetically related to wild bird isolates. This suggests an interesting ecological context since the bacterial strains may be adapted to a specific ecological niche that is conducive to both wild mammals and wild birds. Also, the genetic relatedness may reflect a shared evolutionary history, indicating that these strains have a common ancestry that predates the divergence between these isolates.
The sequence analysis allowed for the detection of a large number of ARGs in the 29 selected E. coli isolates. Genes responsible for the production of β-lactamase enzymes were detected, such as blaTEM-1B, blaTEM-1A, blaEC-1982, blaCTX-M-65, and blaCTX-M-55. The blaTEM-1B and blaTEM-1A genes have already been frequently reported in wildlife [62,63,64,65,66,67]. blaEC genes encode class C β-lactamase enzymes, which provide resistance to cephalosporins. Many variants of this gene have been identified, but only a few are expressed at levels sufficient to confer antibiotic resistance. [68]. The presence of these variants has been detected in isolates from humans [69] and cattle [70]. The presence of blaCTX-M-55 and blaCTX-M-65 in bacteria isolated from wild animals has been documented in several studies [66,71,72,73], which indicates that wild animals may be considered reservoirs for antibiotic-resistant bacteria. Aminoglycoside resistance was confirmed by the presence of several genes that have already been reported among wild animals [74,75,76,77]. However, we detected the sat2 gene in one isolate, though it is not usually found in wild animals besides being part of a class 2 integron that carries the classic dfrA1, sat2, and aadA1 gene cassettes. Nonetheless, some studies in wild animals have already reported this gene cassette array [78]. Reports have been frequently made among domestic animals and food products [79,80]. All E. coli isolates carried the mdfA resistance gene, which confers resistance to a diverse group of cationic compounds and multiple antimicrobial classes, including chloramphenicol, macrolide, lincosamide, and streptogramin, certain aminoglycosides, and fluoroquinolones [81]. The detection of the mdfA gene in this study is consistent with other genomic studies characterizing E. coli isolates from wildlife [76]. The tetA and tetB genes, the most widespread and dominant resistant genes detected in enterobacteria across the One Health interface [38,39], were responsible for tetracycline resistance. Other resistance genes that confer resistance to sulfonamides, phenicols, trimethoprim, macrolides, lincosamides, and streptogramin B were also observed. Moreover, we detected chromosomal target mutations in 34 E. coli isolates, specifically in the gyrA, parC, and parE genes, which confer resistance to critically important fluoroquinolones [76,82,83]. Besides the mutations responsible for fluoroquinolone resistance, we also detected plasmid-mediated quinolone resistance (PMQR) genes, such as the qnrB5, qnrB19, qnrS1, and qnrS2 genes. While qnrS1 and qnrB19 are relatively more common in wild animals [84], qnrS2 [85] and qnrB5 [86] are also present but less frequently reported.
The detection of plasmids in all isolates aligns with the well-established understanding that plasmids are common in E. coli and other bacteria. Plasmids are extrachromosomal genetic elements that can carry a variety of genes, including those related to antibiotic resistance, virulence, and metabolic functions. The identification of different incompatibility groups (IncF, IncI, IncR, IncX, and IncN) in the isolates is consistent with the diversity of plasmids found in E. coli populations [76,87,88], including those of wildlife [3]. Different incompatibility groups represent distinct plasmids, each with its own replication and maintenance mechanisms. Col plasmids can carry genes for the production of colicins, which are bacteriocins that can inhibit the growth of closely related bacteria [89,90]. The detection of Col plasmids in a significant proportion (75.9%) of the isolates is in line with the known presence of colicinogenic plasmids in E. coli [76,91]. The identification of the IncFIB plasmid in most isolates is also consistent with the prevalence of IncF-type plasmids in E. coli populations [92]. IncF plasmids are known for carrying various genes, including virulence factors and antibiotic resistance genes [93]. In summary, these results regarding the presence of plasmids and their diversity in E. coli isolated from wildlife align with the general knowledge in the literature. The variety of incompatibility groups, the presence of common plasmid types, and the occurrence of multiple plasmids in most strains are consistent with the versatility of plasmids in E. coli populations.
Regarding the virulence factors, the detection of adherence genes such as ompA and fimA/B/C/D/E/F/G/H/I align with these genes’ known role in bacterial adherence to host cells and tissues. These genes are associated with forming fimbriae and outer membrane proteins that facilitate adhesion [94,95,96]. The presence of genes related to iron utilization, such as fes, entA/B/C/D/E/F/S, and fepA/B/C/D/G, is consistent with the need for bacteria to acquire iron from the host environment. These genes are involved in iron acquisition, often through siderophores or heme utilization systems [97]. The detection of the astA gene, which encodes heat-stable enterotoxin 1, in nine isolates is consistent with the presence of this toxin in some strains of E. coli. This toxin is associated with gastrointestinal disease [58,98]. The identification of genes related to the type II secretion system (T2SS) and type III secretion system (T3SS) is consistent with the presence of these secretion systems in E. coli [99,100]. T2SS is involved in the secretion of various proteins, including toxins, and T3SS is known for delivering effectors into host cells. Overall, these results align with the understanding that different strains of E. coli can possess a range of virulence factors related to adherence, iron utilization, toxin production, and secretion systems.
5. Conclusions
This study found a relatively high prevalence of MDR E. coli isolates among the tested animals (22.3%), corresponding to 71.9% of the total isolates obtained. However, ESBL-positive E. coli was only detected in two wild animals. The E. coli strains exhibited high rates of resistance to certain antibiotics, specifically ampicillin, trimethoprim–sulfamethoxazole, tetracycline, nalidixic acid, and amoxicillin–clavulanic acid. On the contrary, the resistance rates to cefepime, cefoxitin, and ceftazidime were relatively low. Positively, all E. coli strains presented susceptibility to carbapenem antibiotics and also amikacin.
Overall, the results indicate a concerning level of antibiotic resistance in the E. coli strains isolated from the wildlife. The detection of MDR and ESBL-producing strains opens the possibility of AMR bacteria transmission among wildlife, other animals, or humans. The finding of full susceptibility to carbapenems is optimistic. Still, continued surveillance and responsible use of antibiotics are essential to prevent the further development and spread of antibiotic resistance in both wildlife and human populations. Additionally, understanding antibiotic resistance patterns in wildlife can help inform conservation efforts and guide strategies for managing antibiotic use in veterinary medicine and agriculture.
Further investigations are necessary to gain a deeper understanding of the antibiotic resistance patterns in wildlife and its potential implications. On the one hand, there is a need to identify the specific mechanisms responsible for antibiotic resistance in the E. coli strains isolated from wildlife by understanding the genetic basis of resistance, which can provide insights into how these resistance genes are acquired and spread among bacterial populations. On the other hand, studying the transmission dynamics of antibiotic-resistant E. coli between wildlife, humans, and domestic animals is also essential to assess the potential risks of zoonotic transmission. This includes identifying possible reservoirs and vectors of resistant bacteria and understanding the transmission routes. Also, adopting a One Health approach that considers the interconnections between human health, animal health, and the environment is essential to identify the potential sources of antibiotic resistance in wildlife. Investigating the impact of antibiotic resistance on the health and survival of wildlife species is vital for understanding the ecological consequences of resistance development. This can also provide insights into potential risks to biodiversity and ecosystem dynamics. Thus, implementing robust surveillance and monitoring programs is critical to track changes in antibiotic resistance over time in wildlife populations since regularly monitoring resistance patterns can help detect emerging threats and guide effective intervention strategies.
Acknowledgments
We would like to thank the CERAS interns and volunteers, especially Luís de Matos, who collaborated in collecting and sending the samples as well as organizing the data. National funds were provided to Carolina Sabença through the FCT under the Ph.D. grant 2020.06967.BD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vetsci11100469/s1, Supplementary File S1: Phenotypic resistance profiles of E. coli isolates obtained from wild animals. Supplementary File S2: Virulence genes data of 29 selected E. coli isolates from wild animals.
Author Contributions
Conceptualization, C.S.; methodology, C.S., M.R.-R. and R.B.-H.; validation, R.S., L.S., F.S., F.L., A.C.A. and M.V.-P.; investigation, C.S., M.R.-R. and R.B.-H.; resources, R.S., L.S., F.S., F.L., A.C.A. and M.V.-P.; data curation, C.S.; writing—original draft preparation, C.S.; writing—review and editing, C.S., M.R.-R., R.S., L.S., F.S., F.L., A.C.A., M.V.-P., R.B.-H., C.T., G.I., R.d.C. and P.P.; supervision, G.I., C.T. and P.P.; funding acquisition, R.d.C. and P.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed in the current study are available in the National Center for Biotechnology Information repository, https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1006036; https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/bioproject/?term=PRJNA1006036 (accessed on 3 September 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the projects UIDB/00772/2020 (Doi:10.54499/UIDB/00772/2020) and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT), by CIBERINFEC (CB21/13/00084), Instituto de Salud Carlos III, Madrid, Spain, and by FCT/MCTES (LA/P/0008/2020 DOI 10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI 10.54499/UIDP/50006/2020 and UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020), through national funds.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aslam B., Khurshid M., Arshad M.I., Muzammil S., Rasool M., Yasmeen N., Shah T., Chaudhry T.H., Rasool M.H., Shahid A., et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021;11:771510. doi: 10.3389/fcimb.2021.771510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velazquez-Meza M.E., Galarde-López M., Carrillo-Quiróz B., Alpuche-Aranda C.M. Antimicrobial resistance: One Health approach. Vet. World. 2022;15:743–749. doi: 10.14202/vetworld.2022.743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso C.A., de Toro M., de la Cruz F., Torres C. Genomic insights into drug resistance and virulence platforms, CRISPR-Cas systems and phylogeny of commensal E. coli from wildlife. Microorganisms. 2021;9:999. doi: 10.3390/microorganisms9050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villafuerte D., Aliberti S., Soni N.J., Faverio P., Marcos P.J., Wunderink R.G., Rodriguez A., Sibila O., Sanz F., Martin-Loeches I., et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology. 2020;25:543–551. doi: 10.1111/resp.13663. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso G., Midiri A., Gerace E., Biondo C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens. 2021;10:1310. doi: 10.3390/pathogens10101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plaza-Rodríguez C., Alt K., Grobbel M., Hammerl J.A., Irrgang A., Szabo I., Stingl K., Schuh E., Wiehle L., Pfefferkorn B., et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2021;7:627821. doi: 10.3389/fvets.2020.627821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtsson-Palme J., Abramova A., Berendonk T.U., Pedro Coelho L., Forslund S.K., Gschwind R., Heikinheimo A., Hugo Jarquín-Díaz V., Ali Khan A., Klümper U., et al. Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environ. Int. 2023;178:108089. doi: 10.1016/j.envint.2023.108089. [DOI] [PubMed] [Google Scholar]
- 8.Smith S., Wang J., Fanning S., McMahon B.J. Antimicrobial resistant bacteria in wild mammals and birds: A coincidence or cause for concern? Ir. Vet. J. 2014;67:8. doi: 10.1186/2046-0481-67-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smoglica C., Vergara A., Angelucci S., Festino A.R., Antonucci A., Marsilio F., Di Francesco C.E. Antibiotic-Resistant Bacteria Dissemination in the Wildlife, Livestock, and Water of Maiella National Park, Italy. Animals. 2023;13:432. doi: 10.3390/ani13030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng C.-H., Liu C.-W., Liu P.-Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics. 2023;12:661. doi: 10.3390/antibiotics12040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argudín M.A., Deplano A., Meghraoui A., Dodémont M., Heinrichs A., Denis O., Nonhoff C., Roisin S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics. 2017;6:12. doi: 10.3390/antibiotics6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold K.E., Williams N.J., Bennett M. “Disperse abroad in the land”: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016;12:20160137. doi: 10.1098/rsbl.2016.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. [(accessed on 20 May 2022)]. Available online: http://www.eucast.org.
- 15.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. [Google Scholar]
- 16.Quijada N.M., Rodríguez-Lázaro D., Eiros J.M., Hernández M. TORMES: An automated pipeline for whole bacterial genome analysis. Bioinformatics. 2019;35:4207–4212. doi: 10.1093/bioinformatics/btz220. [DOI] [PubMed] [Google Scholar]
- 17.Tange O. GNU Parallel 2018. 2018 [Cited 2023 Nov 28] [(accessed on 17 July 2023)]. Available online: https://zenodo.org/records/1146014.
- 18.Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurk S., Bankevich A., Antipov D., Gurevich A.A., Korobeynikov A., Lapidus A., Prjibelski A.D., Pyshkin A., Sirotkin A., Sirotkin Y., et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013;20:714–737. doi: 10.1089/cmb.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seemann T. Barrnap: BAsic Rapid Ribosomal RNA Predictor. 2018. [(accessed on 17 July 2023)]. Available online: https://github.com/tseemann/barrnap.
- 22.Wood D.E., Lu J., Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seemann T. mlst: Scan Contig Files Against Traditional PubMLST Typing schemes. 2022. [(accessed on 17 July 2023)]. Available online: https://github.com/tseemann/mlst.
- 25.Seemann T. ABRicate: Mass Screening of Contigs for Antimicrobial Resistance or Virulence Genes. 2020. [(accessed on 17 July 2023)]. Available online: https://github.com/tseemann/abricate.
- 26.Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., Aarestrup F.M., Larsen M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McArthur A.G., Waglechner N., Nizam F., Yan A., Azad M.A., Baylay A.J., Bhullar K., Canova M.J., De Pascale G., Ejim L., et al. The Comprehensive Antibiotic Resistance Database. Antimicrob. Agents Chemother. 2013;57:3348. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S.K., Padmanabhan B.R., Diene S.M., Lopez-Rojas R., Kempf M., Landraud L., Rolain J.M. ARG-annot, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014;58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Yang J., Yu J., Yao Z., Sun L., Shen Y., Jin Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyatt D., Chen G.L., LoCascio P.F., Land M.L., Larimer F.W., Hauser L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 32.Joensen K.G., Tetzschner A.M.M., Iguchi A., Aarestrup F.M., Scheutz F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015;53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roer L., Tchesnokova V., Allesoe R., Muradova M., Chattopadhyay S., Ahrenfeldt J., Thomsen M.C.F., Lund O., Hansen F., Hammerum A.M., et al. Development of a web tool for Escherichia coli subtyping based on fimh alleles. J. Clin. Microbiol. 2017;55:2538–2543. doi: 10.1128/JCM.00737-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattoli A., Zankari E., García-Fernández A., Voldby Larsen M., Lund O., Villa L., Møller Aarestrup F., Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zankari E., Allesøe R., Joensen K.G., Cavaco L.M., Lund O., Aarestrup F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017;72:2764–2768. doi: 10.1093/jac/dkx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [(accessed on 17 July 2023)]. Available online: https://www.r-project.org/ [Google Scholar]
- 37.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York, NY, USA: 2016. [(accessed on 17 July 2023)]. Available online: https://ggplot2.tidyverse.org. [Google Scholar]
- 38.Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.Y. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 39.Xie Y. Dynamic Documents with R and Knitr. 2nd ed. Chapman and Hall/CRC; Boca Raton, FL, USA: 2015. [Google Scholar]
- 40.Sievert C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny. Chapman and Hall/CRC; Boca Raton, FL, USA: 2020. [Google Scholar]
- 41.Neuwirth E. RColorBrewer: ColorBrewer Palettes. 2022. [(accessed on 17 July 2023)]. Available online: https://cran.r-project.org/web/packages/RColorBrewer/index.html.
- 42.Wickham H. Reshaping data with the reshape package. J. Stat. Softw. 2007;21:1–20. doi: 10.18637/jss.v021.i12. [DOI] [Google Scholar]
- 43.Allaire J., Xie Y., Dervieux C., McPherson J., Luraschi J., Ushey K., Atkins A., Wickham H., Cheng J., Chang W., et al. rmarkdown: Dynamic Documents for R. 2023. [(accessed on 17 July 2023)]. Available online: https://github.com/rstudio/rmarkdown.
- 44.Wang L.G., Lam T.T.Y., Xu S., Dai Z., Zhou L., Feng T., Guo P., Dunn C.W., Jones B.R., Bradley T., et al. Treeio: An R Package for Phylogenetic Tree Input and Output with Richly Annotated and Associated Data. Mol. Biol. Evol. 2020;37:599–603. doi: 10.1093/molbev/msz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foti M., Siclari A., Mascetti A., Fisichella V. Study of the spread of antimicrobial-resistant Enterobacteriaceae from wild mammals in the National Park of Aspromonte (Calabria, Italy) Environ. Toxicol. Pharmacol. 2018;63:69–73. doi: 10.1016/j.etap.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Prandi I., Bellato A., Nebbia P., Stella M.C., Ala U., von Degerfeld M.M., Quaranta G., Robino P. Antibiotic resistant Escherichia coli in wild birds hospitalised in a wildlife rescue centre. Comp. Immunol. Microbiol. Infect. Dis. 2023;93:101945. doi: 10.1016/j.cimid.2023.101945. [DOI] [PubMed] [Google Scholar]
- 47.Carroll D., Wang J., Fanning S., Mcmahon B.J. Antimicrobial Resistance in Wildlife: Implications for Public Health. Zoonoses Public Health. 2015;62:534–542. doi: 10.1111/zph.12182. [DOI] [PubMed] [Google Scholar]
- 48.Rahman M.T., Sobur M.A., Islam M.S., Ievy S., Hossain M.J., Zowalaty M.E.E., Rahman A.M.M.T., Ashour H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms. 2020;8:1405. doi: 10.3390/microorganisms8091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bamunusinghage N.P.D., Neelawala R.G., Magedara H.P., Ekanayaka N.W., Kalupahana R.S., Silva-Fletcher A., Kottawatta S.A. Antimicrobial Resistance Patterns of Fecal Escherichia coli in Wildlife, Urban Wildlife, and Livestock in the Eastern Region of Sri Lanka, and Differences Between Carnivores, Omnivores, and Herbivores. J. Wildl. Dis. 2022;58:380–383. doi: 10.7589/JWD-D-21-00048. [DOI] [PubMed] [Google Scholar]
- 50.Larsson D.G.J., Flach C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022;20:257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asai T., Usui M., Sugiyama M., Izumi K., Ikeda T., Andoh M. Antimicrobial susceptibility of Escherichia coli isolates obtained from wild mammals between 2013 and 2017 in Japan. J. Vet. Med. Sci. 2020;82:345–349. doi: 10.1292/jvms.19-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guyomard-Rabenirina S., Reynaud Y., Pot M., Albina E., Couvin D., Ducat C., Gruel G., Ferdinand S., Legreneur P., Le Hello S., et al. Antimicrobial Resistance in Wildlife in Guadeloupe (French West Indies): Distribution of a Single blaCTX–M–1/IncI1/ST3 Plasmid Among Humans and Wild Animals. Front. Microbiol. 2020;11:1524. doi: 10.3389/fmicb.2020.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagy B.J., Balázs B., Benmazouz I., Gyüre P., Kövér L., Kaszab E., Bali K., Lovas-Kiss Á., Damjanova I., Majoros L., et al. Comparison of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolates From Rooks (Corvus frugilegus) and Contemporary Human-Derived Strains: A One Health Perspective. Front. Microbiol. 2022;12:785411. doi: 10.3389/fmicb.2021.785411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sellera F.P., Cardoso B., Fuentes-Castillo D., Esposito F., Sano E., Fontana H., Fuga B., Goldberg D.W., Seabra L.A.V., Antonelli M., et al. Genomic Analysis of a Highly Virulent NDM-1-Producing Escherichia coli ST162 Infecting a Pygmy Sperm Whale (Kogia breviceps) in South America. Front. Microbiol. 2022;13:915375. doi: 10.3389/fmicb.2022.915375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aworh M.K., Kwaga J., Okolocha E., Harden L., Hull D., Hendriksen R.S., Thakur S. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook. 2020;2:1–11. doi: 10.1186/s42522-020-00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majewski P., Gutowska A., Smith D.G.E., Hauschild T., Majewska P., Hryszko T., Gizycka D., Kedra B., Kochanowicz J., Glowiński J., et al. Plasmid Mediated mcr-1.1 Colistin-Resistance in Clinical Extraintestinal Escherichia coli Strains Isolated in Poland. Front. Microbiol. 2021;12:547020. doi: 10.3389/fmicb.2021.547020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aworh M.K., Kwaga J.K.P., Hendriksen R.S., Okolocha E.C., Harrell E., Thakur S. Quinolone-resistant Escherichia coli at the interface between humans, poultry and their shared environment- a potential public health risk. One Health Outlook. 2023;5:2. doi: 10.1186/s42522-023-00079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou W., Lin R., Zhou Z., Ma J., Lin H., Zheng X., Wang J., Wu J., Dong Y., Jiang H., et al. Antimicrobial resistance and genomic characterization of Escherichia coli from pigs and chickens in Zhejiang, China. Front. Microbiol. 2022;13:1018682. doi: 10.3389/fmicb.2022.1018682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grilo T., Freire S., Miguel B., Martins L.N., Menezes M.F., Nordmann P., Poirel L., Sousa M.J.R., Aires-de-Sousa M. Occurrence of plasmid-mediated fosfomycin resistance (fos genes) among Escherichia coli isolates, Portugal. J. Glob. Antimicrob. Resist. 2023;35:342–346. doi: 10.1016/j.jgar.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Fuentes-Castillo D., Esposito F., Cardoso B., Dalazen G., Moura Q., Fuga B., Fontana H., Cerdeira L., Dropa M., Rottmann J., et al. Genomic data reveal international lineages of critical priority Escherichia coli harbouring wide resistome in Andean condors (Vultur gryphus Linnaeus, 1758) Mol. Ecol. 2020;29:1919–1935. doi: 10.1111/mec.15455. [DOI] [PubMed] [Google Scholar]
- 61.Bhowmik A., Shah S.T., Goswami S., Sirajee A.S., Ahsan S. Predominance of Multidrug Resistant Escherichia coli of Environmental Phylotype in Different Environments of Dhaka, Bangladesh. Trop. Med. Infect. Dis. 2023;8:226. doi: 10.3390/tropicalmed8040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furlan J.P.R., Lopes R., Gonzalez I.H.L., Ramos P.L., Stehling E.G. Comparative analysis of multidrug resistance plasmids and genetic background of CTX-M-producing Escherichia coli recovered from captive wild animals. Appl. Microbiol. Biotechnol. 2020;104:6707–6717. doi: 10.1007/s00253-020-10670-4. [DOI] [PubMed] [Google Scholar]
- 63.Martínez-Álvarez S., Châtre P., Cardona-Cabrera T., François P., Sánchez-Cano A., Höfle U., Zarazaga M., Madec J.Y., Haenni M., Torres C. Detection and genetic characterization of blaESBL-carrying plasmids of cloacal Escherichia coli isolates from white stork nestlings (Ciconia ciconia) in Spain. J. Glob. Antimicrob. Resist. 2023;34:186–194. doi: 10.1016/j.jgar.2023.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Sabença C., Igrejas G., Poeta P., Robin F., Bonnet R., Beyrouthy R. Multidrug Resistance Dissemination in Escherichia coli Isolated from Wild Animals: Bacterial Clones and Plasmid Complicity. Microbiol. Res. 2021;12:123–137. doi: 10.3390/microbiolres12010009. [DOI] [Google Scholar]
- 65.Benavides J.A., Godreuil S., Opazo-Capurro A., Mahamat O.O., Falcon N., Oravcova K., Streicker D.G., Shiva C. Long-term maintenance of multidrug-resistant Escherichia coli carried by vampire bats and shared with livestock in Peru. Sci. Total Environ. 2022;810:152045. doi: 10.1016/j.scitotenv.2021.152045. [DOI] [PubMed] [Google Scholar]
- 66.Dalazen G., Fuentes-Castillo D., Pedroso L.G., Fontana H., Sano E., Cardoso B., Esposito F., Moura Q., Matinata B.S., Silveira L.F., et al. CTX-M-producing Escherichia coli ST602 carrying a wide resistome in South American wild birds: Another pandemic clone of One Health concern. One Health. 2023;17:100586. doi: 10.1016/j.onehlt.2023.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeballos-Gross D., Rojas-Sereno Z., Salgado-Caxito M., Poeta P., Torres C., Benavides J.A. The Role of Gulls as Reservoirs of Antibiotic Resistance in Aquatic Environments: A Scoping Review. Front. Microbiol. 2021;12:703886. doi: 10.3389/fmicb.2021.703886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt J., Zdarska V., Kolar M., Mlynarcik P. Analysis of BlaEC family class C beta-lactamase. FEMS Microbiol. Lett. 2023;370:fnad097. doi: 10.1093/femsle/fnad097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tegha G., Ciccone E.J., Krysiak R., Kaphatika J., Chikaonda T., Ndhlovu I., van Duin D., Hoffman I., Juliano J.J., Wang J. Genomic epidemiology of Escherichia coli isolates from a tertiary referral center in lilongwe, Malawi. Microb. Genom. 2021;7:000490. doi: 10.1099/mgen.0.000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adator E.H., Walker M., Narvaez-Bravo C., Zaheer R., Goji N., Cook S.R., Tymensen L., Hannon S.J., Church D., Booker C.W., et al. Whole genome sequencing differentiates presumptive extended spectrum beta-lactamase producing Escherichia coli along segments of the one health continuum. Microorganisms. 2020;8:448. doi: 10.3390/microorganisms8030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zurfluh K., Albini S., Mattmann P., Kindle P., Nüesch-Inderbinen M., Stephan R., Vogler B.R. Antimicrobial resistant and extended-spectrum β-lactamase producing Escherichia coli in common wild bird species in Switzerland. Microbiologyopen. 2019;8:e845. doi: 10.1002/mbo3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carvalho I., Tejedor-Junco M.T., González-Martín M., Corbera J.A., Suárez-Pérez A., Silva V., Igrejas G., Torres C., Poeta P. Molecular diversity of Extended-spectrum β-lactamase-producing Escherichia coli from vultures in Canary Islands. Environ. Microbiol. Rep. 2020;12:540–547. doi: 10.1111/1758-2229.12873. [DOI] [PubMed] [Google Scholar]
- 73.Zeng Z., Yang J., Gu J., Liu Z., Hu J., Li X., Chen X., Sun Z., Li J. Prevalence and antimicrobial susceptibility of CTX-M-type-producing Escherichia coli from a wildlife zoo in China. Vet. Med. Sci. 2022;8:1294–1299. doi: 10.1002/vms3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohsin M., Raza S., Schaufler K., Roschanski N., Sarwar F., Semmler T., Schierack P., Guenther S. High prevalence of CTX-M-15-Type ESBL-Producing E. coli from migratory avian species in Pakistan. Front. Microbiol. 2017;8:2476. doi: 10.3389/fmicb.2017.02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X., Zou W., Zeng J., Xie S., An T., Luo X., Chen D., Feng L., Cheng G., Cai R., et al. Prevalence of antimicrobial resistance and integron gene cassettes in Escherichia coli isolated from yaks (Poephagus grunniens) in Aba Tibetan Autonomous Prefecture, China. Microb. Pathog. 2017;111:274–279. doi: 10.1016/j.micpath.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Awosile B., Fritzler J., Levent G., Rahman M.K., Ajulo S., Daniel I., Tasnim Y., Sarkar S. Genomic Characterization of Fecal Escherichia coli Isolates with Reduced Susceptibility to Beta-Lactam Antimicrobials from Wild Hogs and Coyotes. Pathogens. 2023;12:929. doi: 10.3390/pathogens12070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devnath P., Karah N., Graham J.P., Rose E.S., Asaduzzaman M. Evidence of Antimicrobial Resistance in Bats and Its Planetary Health Impact for Surveillance of Zoonotic Spillover Events: A Scoping Review. Int. J. Environ. Res. Public Health. 2023;20:243. doi: 10.3390/ijerph20010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonso C.A., Alcalá L., Simón C., Torres C. Novel sequence types of extended-spectrum and acquired AmpC beta-lactamase producing Escherichia coli and Escherichia clade V isolated from wild mammals. FEMS Microbiol. Ecol. 2017;93:fix097. doi: 10.1093/femsec/fix097. [DOI] [PubMed] [Google Scholar]
- 79.Alonso C.A., Michael G.B., Li J., Somalo S., Simón C., Wang Y., Kaspar H., Kadlec K., Torres C., Schwarz S. Analysis of blaSHV-12-carrying Escherichia coli clones and plasmids from human, animal and food sources. J. Antimicrob. Chemother. 2017;72:1589–1596. doi: 10.1093/jac/dkx024. [DOI] [PubMed] [Google Scholar]
- 80.Hammad A.M., Ishida Y., Shimamoto T. Prevalence and molecular characterization of ampicillin-resistant Enterobacteriaceae isolated from traditional egyptian domiati cheese. J. Food Prot. 2009;72:624–630. doi: 10.4315/0362-028X-72.3.624. [DOI] [PubMed] [Google Scholar]
- 81.Edgar R., Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J. Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massella E., Reid C.J., Cummins M.L., Anantanawat K., Zingali T., Serraino A., Piva S., Giacometti F., Djordjevic S.P. Snapshot study of whole genome sequences of Escherichia coli from healthy companion animals, livestock, wildlife, humans and food in italy. Antibiotics. 2020;9:782. doi: 10.3390/antibiotics9110782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ong K.H., Khor W.C., Quek J.Y., Low Z.X., Arivalan S., Humaidi M., Chua C., Seow K.L.G., Guo S., Tay M.Y.F., et al. Occurrence and Antimicrobial Resistance Traits of Escherichia coli from Wild Birds and Rodents in Singapore. Int. J. Environ. Res. Public Health. 2020;17:5606. doi: 10.3390/ijerph17155606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skarżyńska M., Zaja̧c M., Bomba A., Bocian Ł., Kozdruń W., Polak M., Wia̧cek J., Wasyl D. Antimicrobial Resistance Glides in the Sky—Free-Living Birds as a Reservoir of Resistant Escherichia coli With Zoonotic Potential. Front. Microbiol. 2021;12:656223. doi: 10.3389/fmicb.2021.656223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Literak I., Micudova M., Tausova D., Cizek A., Dolejska M., Papousek I., Prochazka J., Vojtech J., Borleis F., Guardone L., et al. Plasmid-mediated quinolone resistance genes in fecal bacteria from rooks commonly wintering throughout Europe. Microb. Drug Resist. 2012;18:567–573. doi: 10.1089/mdr.2012.0075. [DOI] [PubMed] [Google Scholar]
- 86.Halová D., Papoušek I., Jamborova I., Masarikova M., Cizek A., Janecko N., Oravcova V., Zurek L., Clark A.B., Townsend A., et al. Plasmid-mediated quinolone resistance genes in enterobacteriaceae from american crows: High prevalence of bacteria with variable qnrB genes. Antimicrob. Agents Chemother. 2014;58:1257–1258. doi: 10.1128/AAC.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarabai H., Krejci S., Karyakin I., Bitar I., Literak I., Dolejska M. Clinically relevant antibiotic resistance in Escherichia coli from black kites in southwestern Siberia: A genetic and phenotypic investigation. mSphere. 2023;8:e0009923. doi: 10.1128/msphere.00099-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ortega-Balleza J.L., Guerrero A., Castro-Escarpulli G., Martínez-Vázquez A.V., Cruz-Hernández M.A., Luna-Santillana E.d.J.d., Acosta-Cruz E., Rodríguez-Sánchez I.P., Rivera G., Bocanegra-García V. Genomic Analysis of Multidrug-Resistant Escherichia coli Strains Isolated in Tamaulipas, Mexico. Trop. Med. Infect. Dis. 2023;8:458. doi: 10.3390/tropicalmed8100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Majeed H., Gillor O., Kerr B., Riley M.A. Competitive interactions in Escherichia coli populations: The role of bacteriocins. ISME J. 2011;5:71–81. doi: 10.1038/ismej.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parker J.K., Gu R., Estrera G.A., Kirkpatrick B., Rose D.T., Ai Mavridou D., Mondy K.E., Davies B.W. Carbapenem-Resistant and ESBL-Producing Enterobacterales Emerging in Central Texas. Infect. Drug Resist. 2023;16:1249–1261. doi: 10.2147/IDR.S403448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moussa J., Nassour E., Tahan E., El Chaar M., Jisr T., Tokajian S. Carbapenem Resistance Determinants and Their Transmissibility among Clinically Isolated Enterobacterales in Lebanon. J. Infect. Public Health. 2023;16:1947–1953. doi: 10.1016/j.jiph.2023.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Montealegre M.C., Talavera Rodríguez A., Roy S., Hossain M.I., Islam M.A., Lanza V.F., Julian T.R. High Genomic Diversity and Heterogenous Origins of Pathogenic and Antibiotic-Resistant Escherichia coli in Household Settings Represent a Challenge to Reducing Transmission in Low-Income Settings. mSphere. 2020;5:e00704-19. doi: 10.1128/mSphere.00704-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stephens C., Arismendi T., Wright M., Hartman A., Gonzalez A., Gill M., Pandori M., Hess D. F Plasmids Are the Major Carriers of Antibiotic Resistance Genes in Human-Associated Commensal Escherichia coli. mSphere. 2020;5:e00709-20. doi: 10.1128/msphere.00709-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Connell H., Agace W., Klemm P., Schembri M., Mårild S., Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krishnan S., Prasadarao N.V. Outer membrane protein A and OprF: Versatile roles in Gram-negative bacterial infections. FEBS J. 2012;279:919–931. doi: 10.1111/j.1742-4658.2012.08482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Day C.J., Lo A.W., Hartley-Tassell L.E., Pilar Argente M., Poole J., King N.P., Tiralongo J., Jennings M.P., Schembri M.A. Discovery of bacterial fimbria–glycan interactions using whole-cell recombinant Escherichia coli expression. MBio. 2021;12:e03664-20. doi: 10.1128/mBio.03664-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mey A.R., Gómez-Garzón C., Payne S.M. Iron Transport and Metabolism in Escherichia, Shigella, and Salmonella. EcoSal Plus. 2021;9:eESP00342020. doi: 10.1128/ecosalplus.ESP-0034-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alonso C.A., Mora A., Díaz D., Blanco M., González-Barrio D., Ruiz-Fons F., Simón C., Blanco J., Torres C. Occurrence and characterization of stx and/or eae-positive Escherichia coli isolated from wildlife, including a typical EPEC strain from a wild boar. Vet. Microbiol. 2017;207:69–73. doi: 10.1016/j.vetmic.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 99.Yang J., Baldi D.L., Tauschek M., Strugnell R.A., Robins-Browne R.M. Transcriptional regulation of the yghJ-pppA-yghG-gspCDEFGHIJKLM cluster, encoding the type II secretion pathway in enterotoxigenic Escherichia coli. J. Bacteriol. 2007;189:142–150. doi: 10.1128/JB.01115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carter M.Q., Quiñones B., Laniohan N., Carychao D., Pham A., He X., Cooley M. Pathogenicity assessment of Shiga toxin-producing Escherichia coli strains isolated from wild birds in a major agricultural region in California. Front. Microbiol. 2023;14:1214081. doi: 10.3389/fmicb.2023.1214081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed in the current study are available in the National Center for Biotechnology Information repository, https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1006036; https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/bioproject/?term=PRJNA1006036 (accessed on 3 September 2024).