Abstract
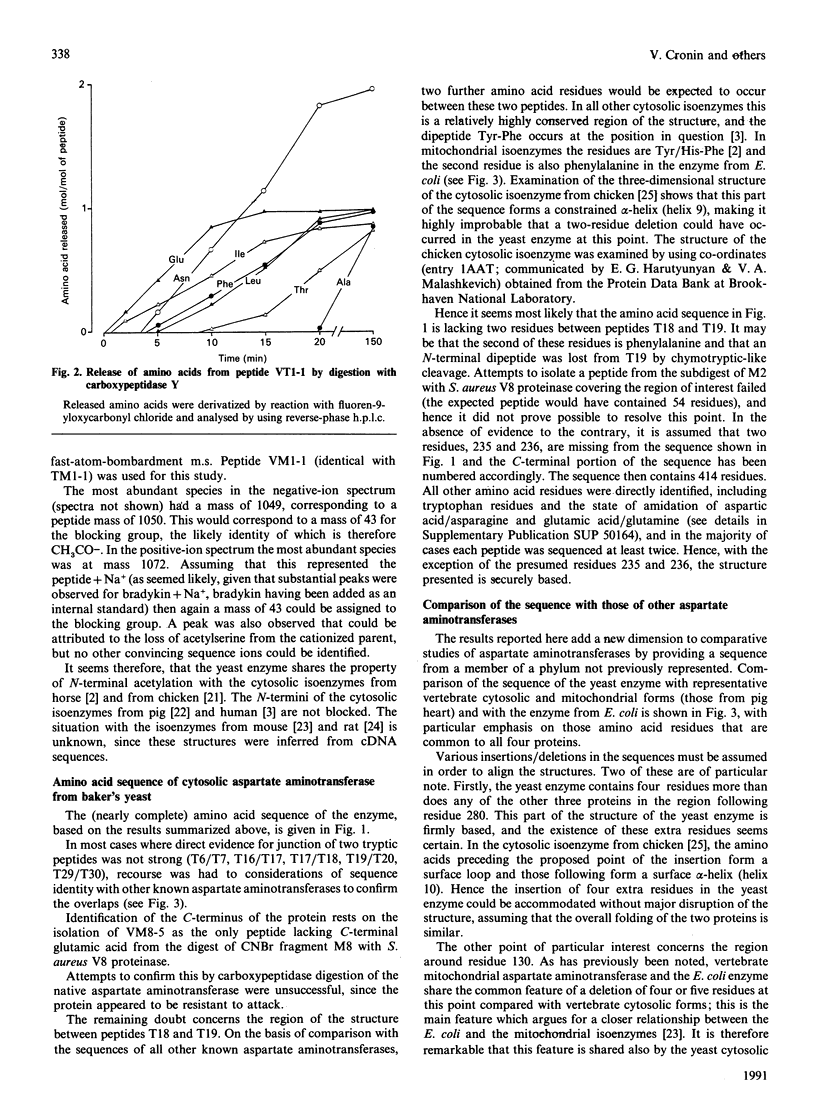
1. The single (cytosolic) aspartate aminotransferase was purified in high yield from baker's yeast (Saccharomyces cerevisiae). 2. Amino-acid-sequence analysis was carried out by digestion of the protein with trypsin and with CNBr; some of the peptides produced were further subdigested with Staphylococcus aureus V8 proteinase or with pepsin. Peptides were sequenced by the dansyl-Edman method and/or by automated gas-phase methods. The amino acid sequence obtained was complete except for a probable gap of two residues as indicated by comparison with the structures of counterpart proteins in other species. 3. The N-terminus of the enzyme is blocked. Fast-atom-bombardment m.s. was used to identify the blocking group as an acetyl one. 4. Alignment of the sequence of the enzyme with those of vertebrate cytosolic and mitochondrial aspartate aminotransferases and with the enzyme from Escherichia coli showed that about 25% of residues are conserved between these distantly related forms. 5. Experimental details and confirmatory data for the results presented here are given in a Supplementary Publication (SUP 50164, 25 pages) that has been deposited at the British Library Document Supply Centre, Boston Spa. Wetherby, West Yorkshire LS23 7 BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1991) 273, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra D., Bossa F., Doonan S., Fahmy H. M., Hughes G. J., Martini F., Petruzzelli R., Wittmann-Liebold B. The cytosolic and mitochondrial aspartate aminotransferases from pig heart. A comparison of their primary structures, predicted secondary structures and some physical properties. Eur J Biochem. 1980 Jul;108(2):405–414. doi: 10.1111/j.1432-1033.1980.tb04736.x. [DOI] [PubMed] [Google Scholar]
- CAMMARATA P. S., COHEN P. P. Fractionation and properties of glutamic oxalacetic transaminase. J Biol Chem. 1951 Nov;193(1):53–62. [PubMed] [Google Scholar]
- Cozzani I., Pellegrini M., Barsacchi R., Dibenedetto G., Sgarrella F. Purification and preliminary characterization of aspartate aminotransferase from yeast. Ital J Biochem. 1974 Nov-Dec;23(6):380–392. [PubMed] [Google Scholar]
- Cubellis M. V., Rozzo C., Nitti G., Arnone M. I., Marino G., Sannia G. Cloning and sequencing of the gene coding for aspartate aminotransferase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. Eur J Biochem. 1989 Dec 8;186(1-2):375–381. doi: 10.1111/j.1432-1033.1989.tb15219.x. [DOI] [PubMed] [Google Scholar]
- De la Morena E., Santos I., Grisolia S. Homogenous crystalline phosphoglycerate phosphomutase of high activity. A simple method for lysis of yeast. Biochim Biophys Acta. 1968 Feb 5;151(2):526–528. doi: 10.1016/0005-2744(68)90121-6. [DOI] [PubMed] [Google Scholar]
- Doonan S. Aspartate aminotransferases and malate dehydrogenases: patterns of evolution. Biochem Soc Trans. 1990 Apr;18(2):167–169. doi: 10.1042/bst0180167. [DOI] [PubMed] [Google Scholar]
- Doonan S., Marra E., Passarella S., Saccone C., Quagliariello E. Transport of proteins into mitochondria. Int Rev Cytol. 1984;91:141–186. doi: 10.1016/s0074-7696(08)61316-9. [DOI] [PubMed] [Google Scholar]
- Doonan S., Martini F., Angelaccio S., Pascarella S., Barra D., Bossa F. The complete amino acid sequences of cytosolic and mitochondrial aspartate aminotransferases from horse heart, and inferences on evolution of the isoenzymes. J Mol Evol. 1986;23(4):328–335. doi: 10.1007/BF02100642. [DOI] [PubMed] [Google Scholar]
- Doyle J. M., Schininà M. E., Bossa F., Doonan S. The amino acid sequence of cytosolic aspartate aminotransferase from human liver. Biochem J. 1990 Sep 15;270(3):651–657. doi: 10.1042/bj2700651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford G. C., Eichele G., Jansonius J. N. Three-dimensional structure of a pyridoxal-phosphate-dependent enzyme, mitochondrial aspartate aminotransferase. Proc Natl Acad Sci U S A. 1980 May;77(5):2559–2563. doi: 10.1073/pnas.77.5.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham I. G., Dacey S. A., Taylor P. P., Smith T. J., Hunter M. G., Finlay M. E., Primrose S. B., Parker D. M., Edwards R. M. The cloning and sequence analysis of the aspC and tyrB genes from Escherichia coli K12. Comparison of the primary structures of the aspartate aminotransferase and aromatic aminotransferase of E. coli with those of the pig aspartate aminotransferase isoenzymes. Biochem J. 1986 Mar 15;234(3):593–604. doi: 10.1042/bj2340593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf-Hausner U., Wilson K. J., Christen P. The covalent structure of mitochondrial aspartate aminotransferase from chicken. Identification of segments of the polypeptide chain invariant specifically in the mitochondrial isoenzyme. J Biol Chem. 1983 Jul 25;258(14):8813–8826. [PubMed] [Google Scholar]
- Hollenberg C. P., Riks W. F., Borst P. The glutamate dehydrogenases of yeast: extra-mitochondrial enzymes. Biochim Biophys Acta. 1970 Jan 27;201(1):13–19. doi: 10.1016/0304-4165(70)90004-8. [DOI] [PubMed] [Google Scholar]
- Horio Y., Tanaka T., Taketoshi M., Nagashima F., Tanase S., Morino Y., Wada H. Rat cytosolic aspartate aminotransferase: molecular cloning of cDNA and expression in Escherichia coli. J Biochem. 1988 May;103(5):797–804. doi: 10.1093/oxfordjournals.jbchem.a122349. [DOI] [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Kondo K., Wakabayashi S., Yagi T., Kagamiyama H. The complete amino acid sequence of aspartate aminotransferase from Escherichia coli: sequence comparison with pig isoenzymes. Biochem Biophys Res Commun. 1984 Jul 18;122(1):62–67. doi: 10.1016/0006-291x(84)90439-x. [DOI] [PubMed] [Google Scholar]
- Mehta P. K., Hale T. I., Christen P. Evolutionary relationships among aminotransferases. Tyrosine aminotransferase, histidinol-phosphate aminotransferase, and aspartate aminotransferase are homologous proteins. Eur J Biochem. 1989 Dec 8;186(1-2):249–253. doi: 10.1111/j.1432-1033.1989.tb15202.x. [DOI] [PubMed] [Google Scholar]
- Obaru K., Nomiyama H., Shimada K., Nagashima F., Morino Y. Cloning and sequence analysis of mRNA for mouse aspartate aminotransferase isoenzymes. J Biol Chem. 1986 Dec 25;261(36):16976–16983. [PubMed] [Google Scholar]
- Poulos C. X., Wilkinson D. A., Cappell H. Homeostatic regulation and Pavlovian conditioning in tolerance to amphetamine-induced anorexia. J Comp Physiol Psychol. 1981 Oct;95(5):735–746. doi: 10.1037/h0077838. [DOI] [PubMed] [Google Scholar]
- SCHREIBER G., ECKSTEIN M., MAASS G., HOLZER H. DIE PHYSIKALISCH-CHEMISCHEN EIGENSCHAFTEN VON APOASPARTATAMINOTRANSFERASE AUS BIERHEFE. Biochem Z. 1964 Jul 8;340:21–34. [PubMed] [Google Scholar]
- Seville M., Vincent M. G., Hahn K. Modeling the three-dimensional structures of bacterial aminotransferases. Biochemistry. 1988 Nov 1;27(22):8344–8349. doi: 10.1021/bi00422a009. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov S. V., Myasnikov A. N., Severin E. S., Myagkova M. A., Torchinsky Y. M., Braunstein A. E. Primary structure of cytoplasmic aspartate aminotransferase from chicken heart and its homology with pig heart isoenzymes. FEBS Lett. 1979 Oct 15;106(2):385–388. doi: 10.1016/0014-5793(79)80537-2. [DOI] [PubMed] [Google Scholar]
- Williams D. H., Bradley C. V., Santikarn S., Bojesen G. Fast-atom-bombardment mass spectrometry. A new technique for the determination of molecular weights and amino acid sequences of peptides. Biochem J. 1982 Jan 1;201(1):105–117. doi: 10.1042/bj2010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Kagamiyama H., Nozaki M. Aspartate: 2-oxoglutarate aminotransferase from bakers' yeast: crystallization and characterization. J Biochem. 1982 Jul;92(1):35–43. doi: 10.1093/oxfordjournals.jbchem.a133929. [DOI] [PubMed] [Google Scholar]
- Yagi T., Shounaka M., Yamamoto S. Distribution of aspartate aminotransferase activity in yeasts, and purification and characterization of mitochondrial and cytosolic isoenzymes from Rhodotorula minuta [corrected]. J Biochem. 1990 Jan;107(1):151–159. doi: 10.1093/oxfordjournals.jbchem.a123000. [DOI] [PubMed] [Google Scholar]


