Abstract
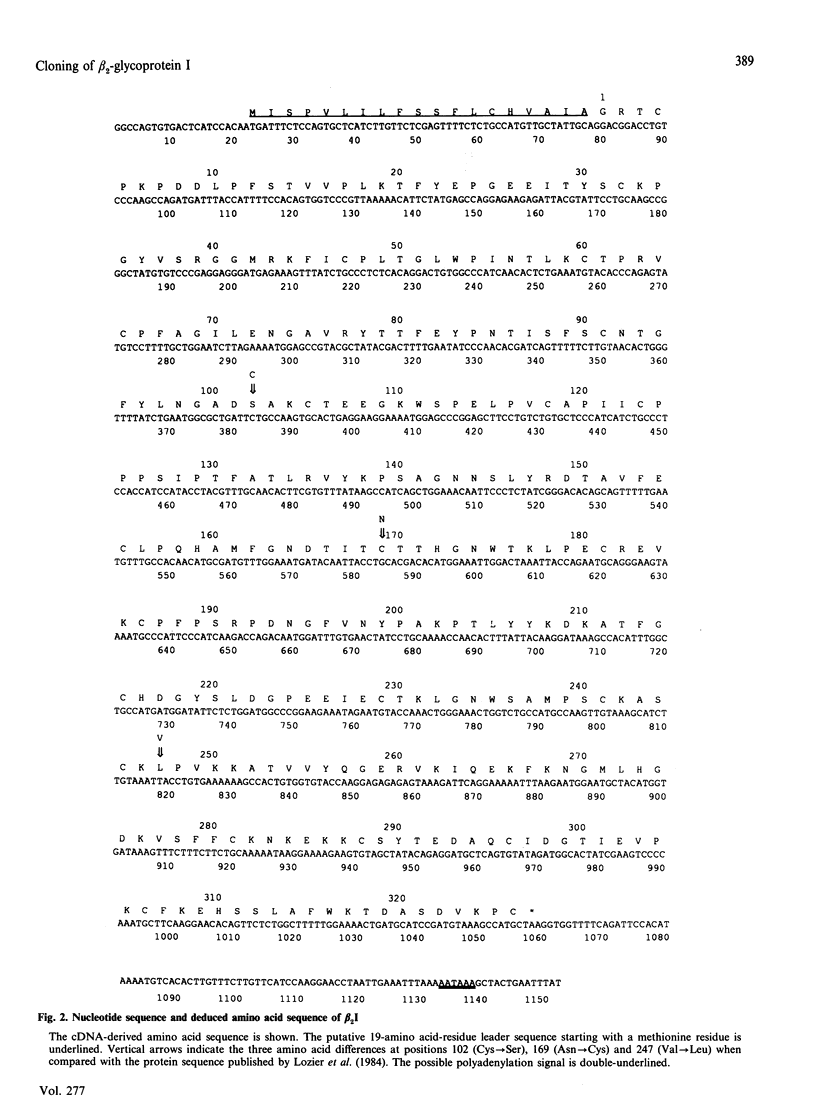
The nucleotide and complete amino acid sequence for the human beta 2-glycoprotein I (beta 2I) was derived by sequencing the cDNA clone pB2I-1. In addition to the 326 amino acid residues of the mature protein this clone codes for a putative leader peptide and contains sequence representing 5' and 3' untranslated regions. When this amino acid sequence was compared with the previously published primary sequence, three major amino acid substitutions were found, two involving cysteine residues. These substitutions lead to a new alignment of the complement control protein (CCP) repeats present in beta 2I and a prediction of the complete disulphide bond organization. Northern-blot analysis indicates that hepatocytes are a major site of biosynthesis for this protein. A transcription signal of about 1.5 kb was detected by using RNA from HepG2 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Chan Y. L., Wool I. G. The primary structure of rat beta 2-glycoprotein I. Nucleic Acids Res. 1989 Aug 11;17(15):6401–6401. doi: 10.1093/nar/17.15.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. N., Baron M., Norman D. G., Day A. J., Willis A. C., Sim R. B., Campbell I. D. Secondary structure of a complement control protein module by two-dimensional 1H NMR. Biochemistry. 1991 Jan 29;30(4):997–1004. doi: 10.1021/bi00218a016. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. A strategy for the rapid multiple alignment of protein sequences. Confidence levels from tertiary structure comparisons. J Mol Biol. 1987 Nov 20;198(2):327–337. doi: 10.1016/0022-2836(87)90316-0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Estaller C., Schwaeble W., Dierich M., Weiss E. H. Human complement factor H: two factor H proteins are derived from alternatively spliced transcripts. Eur J Immunol. 1991 Mar;21(3):799–802. doi: 10.1002/eji.1830210337. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Galli M., Comfurius P., Maassen C., Hemker H. C., de Baets M. H., van Breda-Vriesman P. J., Barbui T., Zwaal R. F., Bevers E. M. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990 Jun 30;335(8705):1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- Janatova J., Reid K. B., Willis A. C. Disulfide bonds are localized within the short consensus repeat units of complement regulatory proteins: C4b-binding protein. Biochemistry. 1989 May 30;28(11):4754–4761. doi: 10.1021/bi00437a036. [DOI] [PubMed] [Google Scholar]
- Kroll J., Larsen J. K., Loft H., Ezban M., Wallevik K., Faber M. DNA-binding proteins in Yoshida ascites tumor fluid. Biochim Biophys Acta. 1976 Jun 15;434(2):490–501. doi: 10.1016/0005-2795(76)90239-7. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lozier J., Takahashi N., Putnam F. W. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil H. P., Simpson R. J., Chesterman C. N., Krilis S. A. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990 Jun;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimpf J., Bevers E. M., Bomans P. H., Till U., Wurm H., Kostner G. M., Zwaal R. F. Prothrombinase activity of human platelets is inhibited by beta 2-glycoprotein-I. Biochim Biophys Acta. 1986 Oct 29;884(1):142–149. doi: 10.1016/0304-4165(86)90237-0. [DOI] [PubMed] [Google Scholar]
- Nimpf J., Wurm H., Kostner G. M. Beta 2-glycoprotein-I (apo-H) inhibits the release reaction of human platelets during ADP-induced aggregation. Atherosclerosis. 1987 Feb;63(2-3):109–114. doi: 10.1016/0021-9150(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Patthy L. Detecting homology of distantly related proteins with consensus sequences. J Mol Biol. 1987 Dec 20;198(4):567–577. doi: 10.1016/0022-2836(87)90200-2. [DOI] [PubMed] [Google Scholar]
- Polz E., Kostner G. M. The binding of beta 2-glycoprotein-I to human serum lipoproteins: distribution among density fractions. FEBS Lett. 1979 Jun 1;102(1):183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- Polz E., Wurm H., Kostner G. M. Investigations on beta 2-glycoprotein-I in the rat: isolation from serum and demonstration in lipoprotein density fractions. Int J Biochem. 1980;11(3-4):265–270. doi: 10.1016/0020-711x(80)90229-3. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Schousboe I. Binding of beta 2-glycoprotein I to platelets: effect of adenylate cyclase activity. Thromb Res. 1980 Jul 1;19(1-2):225–237. doi: 10.1016/0049-3848(80)90421-1. [DOI] [PubMed] [Google Scholar]
- Schousboe I. Purification, characterization and identification of an agglutinin in human serum. Biochim Biophys Acta. 1979 Aug 28;579(2):396–408. doi: 10.1016/0005-2795(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Schousboe I. beta 2-Glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood. 1985 Nov;66(5):1086–1091. [PubMed] [Google Scholar]
- Walsh M. T., Watzlawick H., Putnam F. W., Schmid K., Brossmer R. Effect of the carbohydrate moiety on the secondary structure of beta 2-glycoprotein. I. Implications for the biosynthesis and folding of glycoproteins. Biochemistry. 1990 Jul 3;29(26):6250–6257. doi: 10.1021/bi00478a020. [DOI] [PubMed] [Google Scholar]
- Wurm H. beta 2-Glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int J Biochem. 1984;16(5):511–515. doi: 10.1016/0020-711x(84)90168-x. [DOI] [PubMed] [Google Scholar]


