Abstract
The herpes simplex virus type 1 (HSV-1) UL34 protein is likely a type II membrane protein that localizes within the nuclear membrane and is required for efficient envelopment of progeny virions at the nuclear envelope, whereas the UL31 gene product of HSV-1 is a nuclear matrix-associated phosphoprotein previously shown to interact with UL34 protein in HSV-1-infected cell lysates. For these studies, polyclonal antisera directed against purified fusion proteins containing UL31 protein fused to glutathione-S-transferase (UL31-GST) and UL34 protein fused to GST (UL34-GST) were demonstrated to specifically recognize the UL31 and UL34 proteins of approximately 34,000 and 30,000 Da, respectively. The UL31 and UL34 gene products colocalized in a smooth pattern throughout the nuclear rim of infected cells by 10 h postinfection. UL34 protein also accumulated in pleiomorphic cytoplasmic structures at early times and associated with an altered nuclear envelope late in infection. Localization of UL31 protein at the nuclear rim required the presence of UL34 protein, inasmuch as cells infected with a UL34 null mutant virus contained UL31 protein primarily in central intranuclear domains separate from the nuclear rim, and to a lesser extent in the cytoplasm. Conversely, localization of UL34 protein exclusively at the nuclear rim required the presence of the UL31 gene product, inasmuch as UL34 protein was detectable at the nuclear rim, in replication compartments, and in the cytoplasm of cells infected with a UL31 null virus. When transiently expressed in the absence of other viral factors, UL31 protein localized diffusely in the nucleoplasm, whereas UL34 protein localized primarily in the cytoplasm and at the nuclear rim. In contrast, coexpression of the UL31 and UL34 proteins was sufficient to target both proteins exclusively to the nuclear rim. The proteins were also shown to directly interact in vitro in the absence of other viral proteins. In cells infected with a virus lacking the US3-encoded protein kinase, previously shown to phosphorylate the UL34 gene product, UL31 and UL34 proteins colocalized in small punctate areas that accumulated on the nuclear rim. Thus, US3 kinase is required for even distribution of UL31 and UL34 proteins throughout the nuclear rim. Taken together with the similar phenotypes of the UL31 and UL34 deletion mutants, these data strongly suggest that the UL31 and UL34 proteins form a complex that accumulates at the nuclear membrane and plays an important role in nucleocapsid envelopment at the inner nuclear membrane.
Herpes simplex virus type 1 (HSV-1) nucleocapsids, like those of all herpesviruses are assembled in the nucleus and acquire a lipid bilayer envelope by budding through the inner nuclear membrane into the perinuclear space (10). Several viral proteins have been implicated in this initial budding event, including the myristylated UL11 protein, glycoprotein K, which is necessary for envelopment in nondividing cells, and UL34 protein (2, 21, 37). Of these, only UL34 protein has been implicated solely in the initial envelopment step, whereas glycoprotein K and UL11 also play roles in egress through the cytoplasm towards the extracellular space (2, 21, 37).
The UL34 sequence predicts that the protein is oriented as a type II integral membrane protein with an N-terminal cytoplasmic domain of 247 amino acids and a C-terminal transmembrane domain of 22 amino acids (32, 35, 37). The type II membrane topology of HSV-2 UL34 protein has recently been addressed (39). This topology predicts that if the transmembrane domain were anchored in the outer nuclear membrane, the bulk of the protein would lie in the cytoplasm, whereas localization in the inner nuclear membrane would place the bulk of the protein within the nucleoplasm.
The exact role of UL34 protein in the envelopment process remains unclear. One possibility is that UL34 protein interacts directly with capsids and/or tegument components and the nuclear membrane, thereby mediating wrapping of the capsid in the membrane. Alternatively, UL34 protein may be responsible for recruiting other viral or cellular factors to the site of envelopment. Both hypotheses predict that UL34 protein should associate with the nuclear envelope. To date, research on the localization of HSV-1 UL34 protein and its homologues in other herpesviruses has not yielded consistent results. In baculovirus-transduced cells. HSV-1 UL34 protein is found at the nuclear envelope and in the cytoplasm (46), whereas in HSV-1-infected cells, UL34 protein is reportedly detectable at the cell surface (35). HSV-2 UL34 protein has been reported to localize at the endoplasmic reticulum in transfected and infected cells (39). In contrast, the UL34 homologues of both pseudorabies virus (PRV) and Epstein-Barr virus localize primarily to the nuclear envelope of infected cells (15, 23). Whether this variance reflects viral species differences or differences in methods of UL34 expression and detection is unclear.
UL34 protein is also a substrate for the viral protein kinase encoded by the HSV-1 US3 gene (34). The role that UL34 phosphorylation plays in viral infection is unknown (16, 33, 34, 35), but it is not required for the envelopment function of UL34 protein, inasmuch as viruses that fail to express US3 kinase produce infectious progeny on noncomplementing cells (27).
By using a UL34–glutathione-S-transferase (GST) fusion protein reacted with wild-type-infected cell lysates, the UL34 gene product has been shown to interact with UL31 protein in pull-down assays from infected cell lysates and to stabilize UL31 protein in infected cells (46, 47). The UL31 gene product of HSV-1 has been characterized as a hydrophobic, nucleotidylylated nuclear phosphoprotein with a predicted Mr of 33,951 (7, 8, 31). Like components of the nuclear matrix and karyoskeleton, most HSV-1 UL31 protein remains associated with cells extracted with detergent, DNase, and high concentrations of NaCl (5, 8, 18, 19, 43).
It is likely that UL31 protein plays multiple roles in viral replication. In noncomplementing cells infected with a UL31 deletion virus, the yield of infectious viral progeny was diminished 1,000 to 10,000-fold compared with wild-type virus (9). The UL31 deletion mutant exhibited a striking decrease in production of extranuclear particles, as viewed by electron microscopy, suggesting a marked defect in viral envelopment or a defect in production of budding-competent capsids. In addition, noncomplementing cells infected with the UL31 mutant contained slightly decreased levels of total viral DNA and a three to fivefold reduction in the ratio of monomeric to concatemeric DNA, suggesting minor roles in both DNA replication and processing or packaging of viral DNA.
The goal of these studies was to characterize the intracellular localization of the UL31 and UL34 proteins. We report that both proteins accumulate almost exclusively at the nuclear rim late in infection and that normal nuclear rim targeting of each protein is dependent on expression of the other in infected cells. Moreover, expression of the UL31 and UL34 proteins is necessary and sufficient to target both proteins to the nuclear rim in the absence of other viral proteins. In infected cells, UL31 and UL34 proteins line the nuclear rim smoothly, but this pattern is not reproduced in transfected cells that coexpress these proteins. Normal distribution of UL31 and UL34 proteins in the nuclear membrane depends upon expression of the US3-encoded protein kinase. The interdependence of UL31 and UL34 proteins for nuclear rim targeting and the phenotypes of the respective null mutants are consistent with the hypothesis that the UL31 and UL34 proteins form a complex that accumulates at the nuclear membrane and that this protein complex plays an important role in envelopment of nucleocapsids.
MATERIALS AND METHODS
Cells and viruses.
Cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum or as previously described (44). HEp-2 and Vero cell lines were obtained from the American Type Culture Collection; (certified cell line numbers 23 and 81, respectively). Rabbit skin cells were provided by Bernard Roizman, University of Chicago. The UL34-complementing cell line 143/1099E has been described previously (37).
To construct a cell line capable of rescuing UL31 mutant viruses, approximately 1.4 × 108 subconfluent (approximately 50 to 60% confluent) rabbit skin cells were transfected with pJB233 using 20 μg of plasmid DNA and 200 μl of Superfect transfection reagent (Qiagen, Valencia, Calif.) according to the manufacturer's protocol for production of stably transfected cell lines. Beginning approximately 48 h posttransfection, cells were propagated in medium containing 200 μg of geneticin (Gibco-BRL, Grand Island, N.Y.) per ml. After passage for 2 weeks in selective medium, clonal cell lines were derived by limiting dilution, and individual lines were tested for the ability to propagate infectious R5132 viral progeny. Based on the number and size of UL31 mutant plaques, UL31 clone 7 was determined to rescue replication of the UL31 mutant virus more efficiently than other clones tested and was used to propagate the UL31 mutant virus throughout these studies.
The wild-type viruses HSV-1(F), HSV-2(G), and both US3 mutants R7037 and R7039 (kindly provided by Bernard Roizman) have been described (13, 27, 35). R7037 contains a deletion of portions of the US3 and US4 open reading frames (ORFs), and R7039 contains a deletion of portions of the US2 and US3 ORFs. The construction and growth characteristics of the UL34-null mutant HSV-1(F) vRR1072(tk+) and a virus containing a genetic repair at the UL34 locus in vRR1072(tk+) (VRR1072Rep) have been described previously (37). R5132 (a UL31 mutant virus) and R5132Rep (virus derived from R5132 but containing a restored UL31 gene) have also been described (9). Both R5132 and vRR1072(tk+) required propagation on transformed, complementing cell lines for production of infectious viral progeny. R5132 viral stock was produced on the UL31 clone 7 cell line. vRR1072 viral stock was generated on the previously described 143/1099E cell line (37).
To rule out the presence of revertant viruses, virus stocks were grown for counting on wild-type, noncomplementing Vero cells and, in relevant cases, the appropriate complementary cell line. These studies were performed with virus stocks of the UL31 and UL34 mutant viruses that produced titers at least 10,000-fold higher on complementary cells than on Vero cells.
Plasmids.
All cloning was performed by standard methods (38). A 924-bp PCR amplicon containing the HSV-1(F) UL31 ORF was generated from HSV-1(F) DNA using primers 5′-ACCTATGTATGACACCGACCCCCAT (bases 67359 to 67383 of the HSV-1 genome) and 3′-CTACGGCGGAGGAAACTCGTCGAA (bases 66459 to 66484 of the HSV-1 genome) (17). The amplicon was cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.) so that HSV-1 sequences were flanked by EcoRI restriction sites. The EcoRI fragment containing UL31 was subcloned into the EcoRI site of the vector pcDNA3 (Invitrogen) so that transcription of UL31 was driven by the human cytomegalovirus (HCMV) immediate-early gene promoter-enhancer; the resultant plasmid was designated pJB233. Full-length UL31 was also subcloned into the EcoRI site of the pGEX4T-1 vector (Amersham Pharmacia Biotech, Piscataway, N.J.) in frame with the gene encoding GST. Sequencing of the construct confirmed that the UL31 and GST ORFs were maintained. This construct was designated pJB187.
Two methods were used to generate UL34 expression constructs. The primers used to amplify a 965-bp fragment containing the UL34 gene of HSV-1(F) were 5′-CGTGCTGTTGGGCGGGACGGTTCGCGAACC (bases 69558 to 69587 of the HSV-1 genome) and 3′-AGGGCTGTGTGGGGCGAAGGCGTCCGGAAC (bases 70494 to 70523 of the HSV-1 genome) (17). The 965-bp amplicon was cloned into pCR2.1, where it was flanked by HindIII and XbaI restriction sites and was subsequently subcloned into the HindIII and XbaI sites of pcDNA3. The resulting UL34-pcDNA3 construct was designated pJB234.
Alternatively, plasmid pRR1034, which encodes a GAL4-UL34 fusion protein, was constructed by ligating the BspEI-NcoI HSV-1(F) genomic fragment containing the UL34 ORF into the NcoI and SmaI sites of pAS2-I (Clontech, Palo Alto, Calif.). Plasmid pRR1100, which encodes a UL34-GST fusion protein, was constructed by ligating the NcoI-SalI fragment of pRR1034 into the NcoI and XhoI sites of a modified pGEX vector. Plasmid pRR1062, which contains the UL34 ORF under the transcriptional control of the HCMV major immediate-early promoter, was constructed by first digesting pRR1034 with NcoI and treating with Klenow enzyme and deoxynucleoside triphosphates (dNTPs) to produce a blunt end, digesting again with SalI, and ligating the UL34-containing fragment into the EcoRV and XhoI sites of pcDNA3 (Invitrogen). Plasmid pRR1066 was constructed by digesting the HindIII N fragment of the HSV-1(F) genome with ApoI and treating with Klenow enzyme and dNTPs to produce a blunt end, digesting with XbaI, and ligating the fragment into RSV.5(hyg) (26). Plasmid pRR1098, which contains the US3 ORF under the transcriptional control of the HCMV major immediate-early promoter, was constructed by first digesting pRR1066 with BpmI and treating with Klenow enzyme and dNTPs to produce a blunt end, digesting again with ApoI, and ligating the US3-containing fragment into the EcoRI and EcoRV sites of pcDNA3 (Invitrogen).
The lamin B receptor-green fluorescent protein (LBR-GFP) fusion protein construct was provided by Jan Ellenberg and has been described (14).
Production of antisera.
The anti-UL34 antibody (Aves Labs Inc., Tigard, Oreg.) was obtained by inoculating a chicken with bacterially expressed (from plasmid pRR1100), column-purified UL34-GST fusion protein. Antibodies were purified from eggs by Aves Labs Inc.
To produce an antiserum against UL31 protein, the BL21 strain of Escherichia coli was transformed with pJB187. Expression of a soluble UL31-GST fusion protein of approximately Mr 52,000 was induced at 30°C with 0.3 mM isopropyl-β-d-thiogalactoside for 2 h (data not shown). The UL31-GST fusion protein was purified by affinity chromatography on glutathione-conjugated Sepharose 4B beads (Amersham Pharmacia Biotech). New Zealand White rabbits were immunized with the purified protein as described (1).
SDS-PAGE and immunoblotting.
For confirmation of specificity of the UL34 antibody, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrically transferred to nitrocellulose by standard methods (38). Blots were probed using a 1:1,000 dilution of chicken anti-UL34 antibody and a 1:500 dilution of alkaline phosphatase-conjugated goat anti-chicken immunoglobulin antibody (Aves Labs Inc.) as described (37).
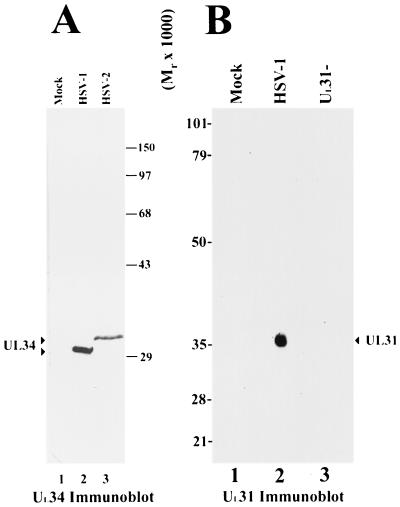
For confirmation of the specificity of UL31 rabbit polyclonal antiserum, HEp-2 cells were mock infected or infected with 5.0 PFU/cell of HSV-1(F) or the UL31 null mutant virus R5132. At 16 h postinfection (h.p.i.), cells were lysed in cold PBSA (phosphate-buffered saline [PBS, pH 7.4] supplemented with 1.0% Triton X 100, 1.0% sodium deoxycholate, 10 μM TLCK [α-tosyl-l-lysine chloromethyl ketone], and 10 μM TPCK [tolylsulfonyl phenyalanyl chloromethyl ketone]). Proteins from approximately 105 HEp-2 cells were electrophoretically separated on a 10% denaturing SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked overnight with a mixture of PBS containing 5% nonfat dry milk, 1% bovine serum albumin (BSA), and 1% Tween 20. The membrane was reacted overnight with UL31 antiserum diluted 1:500 in cold PBS containing 1.0% Tween 20 and 1.0% BSA that was preadsorbed against SDS-denatured protein from approximately 1.4 × 108 R5132-infected HEp-2 cells. Rabbit immunoglobulin bound to the nitrocellulose sheet was visualized by reaction with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) followed by fixation of chromogenic substrate (Bio-Rad, Hercules, Calif.). To confirm that cells were infected, the membrane was digitally scanned (as shown in Fig. 1B) and then reprobed with UL21 protein-specific antiserum prepared and diluted 1:1,000 as described (2).
FIG. 1.
Digitized scanned image of immunoblots probed with antisera directed against UL34 and UL31 proteins. (A) Vero cells were either mock infected (lane 1) or infected for 18 h with HSV-1(F) (lane 2) or HSV-2(G) (lane 3). Cellular lysates were prepared, separated on an SDS-polyacrylamide gel, blotted to nitrocellulose, and probed with anti-UL34 antibody. (B) HEp-2 cells were mock infected (lane 1), infected with wild-type HSV-1 (F) virus (lane 2), or infected with UL31 null mutant virus (lane 3). Lysates were prepared at 16 h.p.i., subjected to electrophoresis on an SDS–10% polyacrylamide gel, blotted to nitrocellulose, and probed with antiserum raised against a purified UL31-GST fusion protein. Sizes of protein standards are indicated between panels A and B.
Transient transfections.
HEp-2 cells were seeded onto glass coverslips at 50 to 70% confluence. Cells were transfected by two different transfection methods that yielded equivalent results. The first method, with Superfect reagent, has been described previously and was used to generate the data presented in Fig. 5 (36). The second method, utilizing calcium phosphate, was used to generate the data presented in Fig. 8. A total of 30 μg of plasmid DNA (pRR1062 and/or pRR1098) was diluted in 270 μl of distilled water. Diluted DNAs were adjusted to 125 mM CaCl2 and 1 X HEPES-buffered saline (HBS; 140 mM NaCL2 50 mM HEPES, 860 mM Na2HPO4 in distilled water [pH 7.05]) by sequential addition of 30 μl of filter-sterilized CaCl2 and 2 X HBS. The mixture was incubated for 20 min at room temperature, and 100 μl per well was added directly to the growth medium. Cells were incubated at 37°C for various time periods and subsequently fixed with 2% formaldehyde in PBS. After permeabilization with detergent, the cells were assayed for expression of UL34 protein (Fig. 2 and Fig. 8), the US3-encoded kinase (Fig. 7 and Fig. 8), or GFP expression by confocal microscopy as described below.
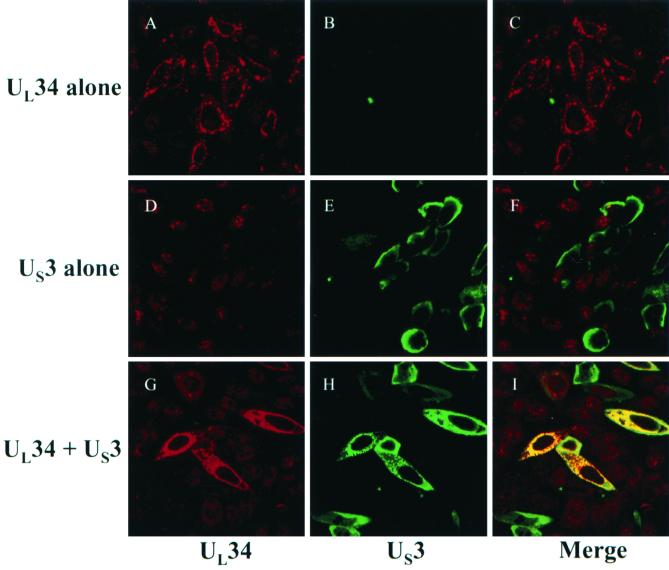
FIG. 5.
Digital confocal images of transfected HEp-2 cells fixed and permeabilized with ice-cold methanol at 18 h posttransfection. (A) Cells were transfected with UL31/pcDNA3 and probed with anti-UL31-GST antibody. (B) Nomarski DIC image of same microscopic field as in panel A. (C) Merge of images in A and B. (D) HEp-2 cells were transfected with UL34/pcDNA3 and stained with antiserum directed against UL34 protein. (E) Nomarski DIC image of same field as in panel D. (F) Merge of images in D and E. (G) Cells were cotransfected with UL31 and UL34 constructs and costained with UL31-specific and UL34-specific antisera. UL31-specific immunofluorescence is shown in panel G. (H) UL34-specific immunofluorescence in same cotransfected cells as in panel H. (I) Merge of UL31- and UL34-specific immunofluorescence, showing colocalization of transiently expressed UL31 and UL34 proteins as visualized by a yellow color upon merging of the separate images. (J) Nomarski DIC image of cotransfected cell shown in panels G to K. (K) Nomarski DIC image merged with the UL31- and UL34-specific immunofluorescence. Original magnification, X600.
FIG. 8.
Digital confocal images showing transiently tranfected HEp-2 cells. Cells were transfected with a UL34 expression construct (A to C) or a US3 expression construct (D to F) or cotransfected with UL34 and US3 expression constructs (G to I). At 24 h posttransfection cells were fixed with formaldehyde and immunostained with chicken anti-UL34 antibody detected with donkey anti-chicken Ig-Texas Red conjugate and rabbit anti-US3 detected with goat anti-rabbit Ig-FITC conjugate. Shown are confocal Z-sections. The high level of background UL34 staining seen in cells transfected with US3 alone (panels D to F) was not a regular feature of these experiments. Original magnification, X630.
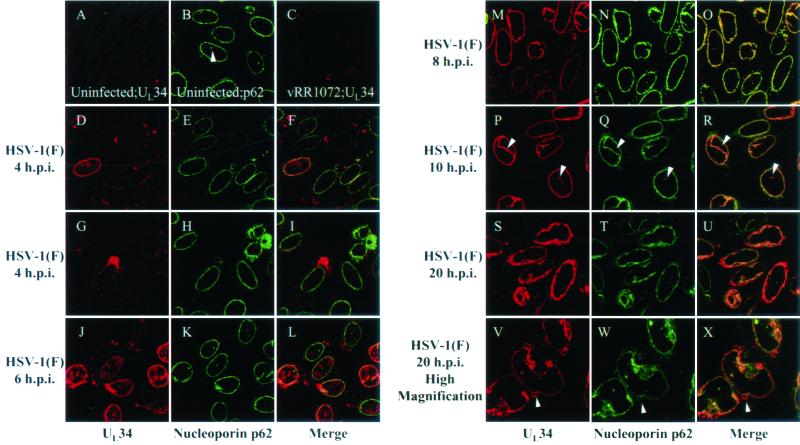
FIG. 2.
Digitized confocal optical sections of a time course of UL34 protein localization. HEp-2 cells were mock infected (A and B), infected at an MOI of 10 with UL34 null virus (vRR1072) for 10 h (C), or infected at an MOI of 10 with HSV-1(F) for 4 h (D to I), 6 h (J to L), 8 h (M to O), 10 h (P to R), or 20 h (S to X). All cells were fixed with formaldehyde, permeablized, and immunostained with chicken anti-UL34 antibody, detected with donkey anti-chicken Ig-Texas Red conjugate either alone (C) or with mouse anti-nucleoporin p62 detected with goat anti-mouse Ig-FITC conjugate (A, B, and D to X). Arrows indicate positions of invaginations and projections of the nuclear envelope (B and P to R) or membranous nuclear peripheral structures (V to X). Original magnifications: X630 (A to U); X1000 (V to X).
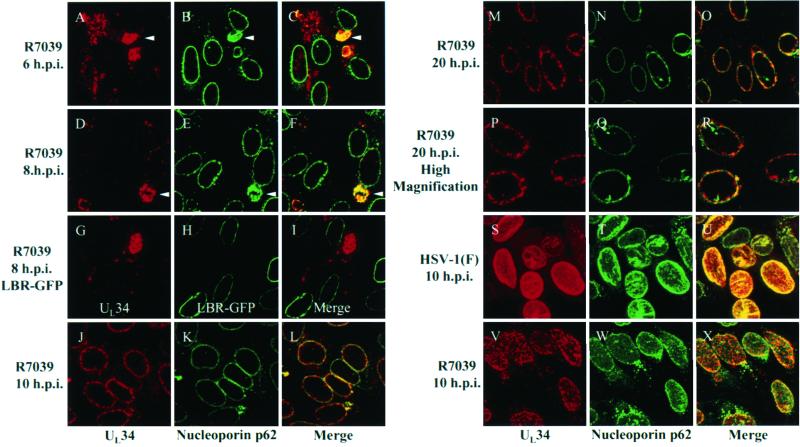
FIG. 7.
Digital confocal images showing localization of UL34 protein in cells infected with a US3 null virus. (A to F and J to R) HEp-2 cells were infected at an MOI of 10 with US3 null HSV-1(F) (R7039) for 6 h (A to C), 8 h (D to F), 10 h (J to L), and 20 h (M to R). Infected cells were fixed with formaldehyde and immunostained with chicken anti-UL34 antibody detected with donkey anti-chicken Ig-Texas Red conjugate and with mouse anti-nucleoporin p62 detected with goat anti-mouse Ig-FITC conjugate. (G to I) HEp-2 cells were transfected with an LBR-GFP expression construct and allowed to express for 24 h. Transfected cells were subsequently infected for 8 h with US3 null virus R7039, fixed with formaldehyde, and immunostained with chicken anti-UL34 detected with donkey anti-chicken Ig-Texas Red conjugate. (S to X) HEp-2 cells were infected with HSV-1(F) (S to U) or R7039 (V to X) at an MOI of 10 for 10 h fixed, permeabilized, and immunostained with anti-UL34 and anti-nucleoporin p62. Confocal Z-sections are shown in panels A to R, and confocal Z-stacks are shown in panels S to X. Arrows in panels A through F indicate positions of perinuclear UL34-nucleoporin p62 structures. Original magnifications: A to O and S to X, X630; P to R, X1,000.
In experiments using LBR-GFP as a nuclear envelope marker, HEp-2 cells were seeded on coverslips and transfected with 30 μg of LBR-GFP expression plasmid as above. Cells were allowed to express transgenes for 24 h and were subsequently infected for 8 h at a multiplicity of infection (MOI) of 10 with R7037 or R7039, fixed with 2% formaldehyde for 15 min, and assayed for UL34 and GFP expression by confocal microscopy.
IFA.
For the indirect immunofluorescence assay (IFA), to remove antibodies from the rabbit polyclonal antiserum that recognized epitopes other than those within UL31 protein, 2 ml of the UL31 rabbit antiserum diluted 1:10 in cold PBS supplemented with 1.0% BSA was adsorbed against HEp-2 cells infected with the UL31 deletion virus. To preadsorb the antisera, approximately 3.75 × 108 HEp-2 cells were infected with 5.0 PFU of R5132 virus per cell and harvested at 16 h.p.i. Protein from the infected cells was precipitated in 10 volumes of ice-cold acetone and air dried. The diluted UL31 antiserum was added to the dried protein, and the mixture was incubated overnight at 4°C with continuous agitation. Cold 1.0% BSA–PBS was added to generate a final dilution of 1:50 for use in IFAs.
For optimal sensitivity and specificity when performing indirect immunofluorescence assays with UL31 antisera, cells were fixed and permeabilized in ice-cold methanol. The anti-UL34-GST antiserum was used to stain cells fixed in either methanol or formaldehyde, as indicated in the text. For methanol fixation, medium was removed, and the cells were washed extensively with PBS and then fixed and permeabilized in ice-cold methanol at −20°C for 20 min. After 20 min, the methanol was removed, and the cells were again washed extensively with PBS prior to indirect immunofluorescence staining. For formaldehyde fixation, cells were fixed with 2% formaldehyde in PBS for 15 min, followed by three postfixation washes in PBS. Fixed cells were permeablized for 15 min in immunofluorescence (IF) buffer (0.5% Triton X-100 [Fisher Scientific, Pittsburgh, Pa.], 0.5% deoxycholate [Fisher Scientific], 1% BSA, 0.05% sodium azide [Sigma Chemical, St. Louis, Mo.] in PBS) and washed three times in PBS. Infected cells were blocked for 1 h in BlockHenII (Aves Labs Inc.) diluted 1:10 in PBS, followed by three washes with PBS.
Indirect immunofluorescence was performed as previously described (36). Cells were incubated for approximately 1 h with primary antibodies diluted in 1.0% BSA-supplemented cold PBS or IF buffer as indicated in the text. Antibodies used in the IFA studies included (i) UL31 protein-GST-preadsorbed rabbit antiserum diluted 1:50 in 1.0% BSA–PBS; (ii) UL34 protein-specific chicken antiserum diluted 1:4,000 either in 1.0% BSA in cold PBS or in IF buffer; (iii) mouse monoclonal antibody directed against ICP4 diluted 1:1,000 (Goodwin Institute for Cancer Research antibody no. 1114; Plantation, Fla.) in 1% BSA–PBS; (iv) anti-US3 rabbit polyclonal antibody (provided by Bernard Roziman) diluted 1:500 in IF buffer; and (v) anti-nucleoporin p62 mouse monoclonal antibody (Transduction Laboratories, Lexington, Ky.) diluted 1:1,000 in IF buffer. Secondary antibodies were diluted in either cold PBS or IF buffer supplemented with 1% BSA and allowed to bind for approximately 1 h followed by extensive washing with PBS. In Fig. 2, donkey anti-chicken IgY-Texas Red (Jackson ImmunoResearch). goat anti-mouse IgG-fluoroisothiocyanate (FITC) conjugate (Gibco-BRL), and goat anti-rabbit IgG-FITC conjugate (Gibco-BRL) were each diluted 1:200 in IF buffer. The bound primary antibodies in Fig. 3 were visualized with Texas Red-conjugated donkey anti-rabbit Ig antibody and FITC-conjugated donkey anti-mouse Ig antibody (Jackson ImmunoResearch) diluted 1:200. The bound primary antibodies in Fig. 4 were visualized with FITC-conjugated donkey anti-rabbit Ig antibody and Texas Red-conjugated donkey anti-chicken Ig antibodies (Jackson ImmunoResearch) diluted 1:200 or indodicarbocyanine (Cy5)-conjugated goat anti-mouse Ig antibodies (Amersham Pharmacia Biotech) diluted 1:1,000 in cold 1% BSA–PBS. In all experiments, the coverslips were washed extensively with PBS and rinsed in distilled water. Excess liquid was removed by blotting on bibulous paper. The moist coverslips were placed on a drop of mounting reagent (Vectashield [Vector Laboratories, Burlingame, Calif.] or SlowFade II [Molecular Probes, Eugene, Oreg.]) on glass microscope slides and, when necessary, sealed with clear nail polish for storage at 4°C in the dark.
FIG. 3.
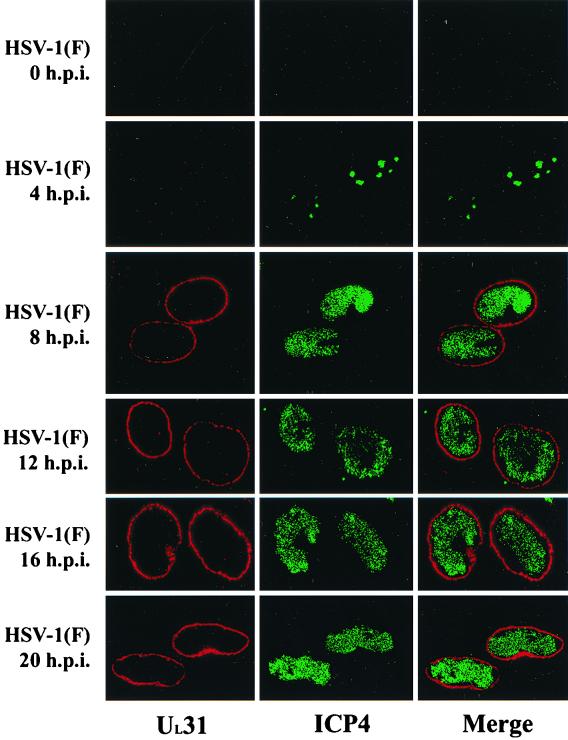
Digital confocal images of HEp-2 cells infected with wild-type HSV-1(F) at an MOI of 5. Cells were fixed and permeablized in ice-cold methanol at 0, 4, 8, 12, 16, and 20 h.p.i. Fixed cells were immunostained with UL31 rabbit antiserum and ICP4 monoclonal antibody. UL31-specific immunofluorescence was detected with a Texas Red-conjugated donkey anti-rabbit Ig antibody, and ICP4-specific immunofluorescence was detected with FITC-conjugated donkey anti-mouse Ig antibody. Original magnification, X600.
FIG. 4.
Digital confocal image of HEp-2 cells infected with various viruses and fixed in methanol at 16 h.p.i. Using an MOI of 5, HEp-2 cells were infected with wild-type HSV-1 (WT), UL34 null mutant virus vRR1072(tk+) (34−), repaired UL34 mutant virus vRR1072Rep (34R), UL31 null mutant virus R5132 (31−), or a repaired UL31 mutant virus (R5132Rep) (UL31R). Cells were stained with UL31 protein-specific antiserum (green), UL34 protein-specific antiserum (red), and ICP4-specific ascites fluid (blue). Bound immunoglobulins specific for UL31 protein, UL34, and ICP4 by reaction with FITC-conjugated donkey anti-rabbit Ig, Texas Red-conjugated donkey anti-chicken Ig, and Cy5-conjugated goat anti-mouse Ig antibody, respectively. Cells were analyzed by confocal laser scanning microscopy by acquiring images through the middle of the cell. For illustrative purposes, Cy5 staining was pseudocolored blue in the figure. Areas of UL31 and UL34 staining colocalization are indicated by a yellow color upon merging of the separate images (Merge). Original magnification, X600.
Confocal fluorescence microscopy.
Slides were analyzed with a confocal laser scanning microscope equipped with krypton and argon lasers under an oil immersion (60X, 63X, or 100X) objective and a 10X ocular objective in light filtered to wavelengths appropriate for excitation of Texas Red, FITC, and/or Cy5 fluorochromes, or transmitted light images were analyzed by Nomarski differential interference contrast imaging. There was minimal emission of more than one fluorochrome at a given excitation wavelength. Digital images were acquired with Fluoview version 2.1.39 software and printed with a Codonics NP 1600 dye sublimation printer. For illustrative purposes, a blue color was digitally applied to the Cy5-specific staining in the digital images made with Fluoview software.
GST pull-down reactions.
Constructs containing full-length UL31 (pJB233) and UL34 genes (pJB234) were transcribed and translated in vitro utilizing a transcription-translation-coupled reticulocyte lysate system (TNT System; Promega, Madison. Wis.). The expressed proteins were reacted with 30 to 40 μg of purified GST. UL31-GST, or UL34-GST fusion protein bound to glutathione-Sepharose beads. Proteins were continuously agitated with the beads at 4°C for 10 to 16 h in cold PBS containing 0.25% Triton X-100 and washed extensively with cold PBS supplemented with 0.25% Triton X-100. Proteins remaining bound to the glutathione-Sepharose-conjugated GST fusion proteins were electrophoretically separated on a denaturing SDS–12% polyacrylamide gel. The polyacrylamide gel was immersed for 30 min in a 20% solution of sodium salicylate dissolved in distilled water, dried, and exposed to film overnight at −80°C. Radioactivity within the dried gel was measured in a Molecular Dynamics Storm 860 PhosphorImager, and counts were analyzed with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
Identification of HSV UL34 and UL31 proteins in infected cell lysates.
To determine the specificity of the antiserum directed against the protein containing UL34 protein fused to GST (UL34-GST), Vero cells were either mock infected or infected for 18 h with wild-type viruses [HSV-1(F) strain or HSV-2(G) strain], and cellular lysates were prepared, separated on an SDS-polyacrylamide gel, transferred to nitrocellulose, and probed with the anti-UL34-GST antibody (Fig. 1A). Strong signals were observed in lysates from cells infected with both HSV-1 and HSV-2 viruses (lanes 2 and 3, respectively), but not from mock-infected cells (lane 1). The reactive protein in HSV-1(F)-infected cells migrated at an Mr of ∼30,000, as expected for UL34 protein. The reactive species from HSV-2(G)-infected cells migrated substantially more slowly at an Mr of ∼32,000. Inasmuch as the sequences of HSV-1 and HSV-2 UL34 protein are predicted to differ in length by a single amino acid, the observed difference in migration suggests a corresponding difference in posttranslational processing of the two proteins.
To determine the specificity of the rabbit antiserum directed against UL31-GST fusion protein, HEp-2 cells were mock infected or infected with wild-type HSV-1(F) or an HSV-1 UL31 null mutant virus (9). Cellular lysates were harvested at 16 h.p.i., and proteins were resolved on a denaturing SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was subsequently probed with the novel anti-UL31-GST rabbit antiserum.
A broad band containing a protein with an apparent Mr of 35,000 to 37,000 was detected in the lane containing the HSV-1(F)-infected cell lysate but absent from lysates of cells that were mock infected or infected with the UL31 null mutant (Fig. 1B). Given the observation that UL21 protein was readily detectable in lanes 2 and 3 by subsequent immunoblot analysis (2) (not shown), the absence of UL31 protein immunoreactivity in cells infected with the UL31 null virus cannot be attributed to either poor infection with the UL31 mutant virus or inability of this virus to express viral gene products. We therefore conclude that the 35,000- to 37,000-Mr protein is the product of the UL31 gene. The observation that the UL31 gene product migrates as a broad band is consistent with previous results indicating that the protein migrates with an apparent Mr of 34,000 and is extensively modified (7, 8, 9).
Localization of the HSV-1(F) UL34 gene product changes during the course of infection.
Given the divergent conclusions concerning the localization of various UL34 protein homologues (see the introduction) and the possibility that UL34 protein localization might be expected to evolve upon expression of viral or cellular interacting partners, a time course of UL34 protein localization was performed. Parallel cultures of HEp-2 cells were mock infected or infected for 10 h with a UL34 null virus [vRR1072(tk+)] or for 4, 6, 8, 10, or 20 h with HSV-1(F). At each time point, cells were fixed and processed for indirect immunofluorescence staining using the chicken anti-UL34-GST antibody to detect UL34 and a mouse antinucleoporin p62 monoclonal antibody as a nuclear envelope marker (4). Bound Ig was detected with a Texas Red-conjugated anti-chicken Ig secondary antibody and a FITC-conjugated anti-mouse Ig secondary antibody. Immunostained cells were then analyzed by confocal microscopy (Fig. 2).
No UL34 protein-specific staining was evident in mock-infected cells (Fig. 2A) or in cells infected with the UL34 null mutant vRR1072 (Fig. 2C). At 4 h.p.i., UL34 protein could be detected in only a fraction of HSV-1-infected cells (Fig. 2D to 2I). In cells expressing detectable levels, UL34 protein localized to large, pleiomorphic structures near the nucleus and smaller structures throughout the cytoplasm. In a smaller fraction of cells, UL34 protein colocalized with nucleoporin p62, indicating an association with the nuclear envelope (Fig. 2D to 2F). However, in the few cells where UL34 protein associated with the nuclear envelope, there was also intranuclear UL34 protein staining which did not colocalize with nucleoporin p62 and therefore did not appear to be associated with the nuclear envelope (Fig. 2F).
By 6 h.p.i., UL34 protein-specific staining at the nuclear envelope was more prominent. However, UL34 protein remained detectable in intranuclear, perinuclear, and cytoplasmic structures (Fig. 2J to 2L). At 8 and 10 h.p.i., UL34 protein-specific staining localized exclusively and uniformly in a rim-like pattern at the nuclear envelope coincident with nucleoporin p62 staining (Fig. 2M to 2R). In contrast to earlier time points, virtually all infected cells showed comparable levels of UL34 protein expression. In addition to staining at the nuclear rim, both UL34 protein and nucleoporin p62 were detected in structures extending into the interior of the nucleus. Depending on their orientation to the confocal plane, these structures appeared as dots or tubular invaginations of the nuclear envelope in optical sections (Fig. 2P to 2R). These structures were also observed in uninfected cells stained with nucleoporin p62 (Fig. 2B) and have been previously shown to be extensions of the nuclear envelope by Fricker et al. (17).
At 20 h.p.i., UL34 protein no longer localized smoothly to the nuclear envelope, as was observed at 10 h (compare Fig. 2P to 2S). Rather, UL34 protein surrounded the nucleus in a heterogeneous fashion. At 20 h.p.i., anti-UL34 antibody also stained structures at and around the nuclear periphery. Many of these structures were tightly apposed to the nucleus, appeared membranous, and contained nucleoporin p62, suggesting a nuclear envelope origin (Fig. 2V to 2X).
UL31 protein localizes to the nuclear rim of HSV-1(F)-infected cells.
As the UL31 and UL34 proteins have been reported to interact, it was of interest to determine the localization of UL31 protein in infected cells (46, 47). Therefore, HEp-2 cells were infected with wild-type virus [HSV-1(F)] and then fixed and permeabilized in methanol at 0, 4, 8, 12, 16, and 20 h.p.i. Cells were subsequently incubated with rabbit polyclonal antiserum directed against the UL31-GST fusion protein and monoclonal antibody directed against ICP4, a known marker for central intranuclear domains of infected cells termed replication compartments (24). Localization of bound primary antibody was revealed by reaction with Texas. Red-conjugated donkey anti-rabbit Ig antibody and FITC-conjugated donkey anti-mouse Ig antibody. The stained cells were analyzed by confocal microscopy (Fig. 3).
Although the intensity of UL31 protein-specific staining increased over time, the distribution did not change during the course of infection. UL31 protein-specific staining was not detected at 0 and 4 h.p.i. By 8 h.p.i., UL31 protein was readily detectable at the nuclear rim and remained localized primarily at the nuclear rim until at least 20 h.p.i. At no time point was UL31 protein-specific staining detected in the cytoplasm of cells infected with wild-type HSV. The observed nuclear rim association of UL31 protein is in marked contrast to the interpretation of results derived from conventional microscopy, in which UL31 protein was described as being localized in an even pattern overlying the entire nucleus (8). This is likely a consequence of the superimposition of nuclear rim-associated UL31 protein over the nucleoplasm in conventional microscopic images.
UL31 protein colocalizes with UL34 protein at the nuclear rim of wild-type-infected cells.
To determine if UL31 and UL34 proteins colocalized in wild-type-infected cells, HEp-2 cells were infected with wild-type HSV-1(F) and fixed at 16 h.p.i. Cells were incubated with the antisera directed against UL31 and UL34 proteins and a mouse monoclonal antibody directed against ICP4, a previously characterized marker for replication compartments (24). Localization of bound primary antibody was revealed by reaction with FITC-conjugated donkey anti-rabbit Ig antibody, Texas Red-conjugated donkey anti-chicken Ig antibody, and Cy5 goat anti-mouse Ig antibody. The stained cells were analyzed by confocal microscopy. As shown in Fig. 4 (row labeled WT), UL31 and UL34 proteins colocalized at the nuclear rim in a region distinct from ICP4 staining. We conclude that UL31 protein colocalizes at the nuclear rim with UL34 protein in wild-type virus-infected cells.
UL34 protein is necessary for targeting UL31 protein to the nuclear rim of infected cells.
As UL31 protein and UL34 protein colocalized at the nuclear rim and have previously been shown to interact, it was of interest to determine if localization of these proteins was interdependent. To determine the localization of UL31 protein in the absence of UL34 protein, HEp-2 cells were infected with the UL34 null mutant virus vRR1072(tk+) or vRR1072Rep, a virus derived from the UL34 mutant virus but bearing a restored UL34 gene. The cells were fixed in ice-cold methanol at 0, 4, 8, 12, 16, and 20 h.p.i. It should be noted that while cells infected with the UL34 null mutant virus express GFP, fluorescence from GFP was eliminated by the methanol fixative (data not shown). Cells were incubated with UL31 protein-specific rabbit polyclonal antiserum UL34 protein-specific chicken polyclonal antiserum and ICP4 mouse monoclonal antiserum. The cells were fixed, reacted with the aforementioned conjugates, and subjected to confocal microscopy. As the pattern of UL31 protein distribution did not change markedly from 8 until 20 h.p.i., only the 16-h.p.i. sample is shown in Fig. 4 (row labeled 34−).
In contrast to the appearance of UL31 protein-specific staining in cells infected with wild-type virus, cells infected with the UL34 deletion mutant contained aberrantly localized UL31 protein staining predominantly within central regions of the nucleoplasm. The compartments bearing UL31 protein also contained ICP4, a replication compartment marker (24), within a similarly shaped pattern, although intranuclear UL31 protein staining extended slightly more peripherally than did ICP4 staining. Very small amounts of UL31 protein were localized diffusely in the cytoplasm beginning at approximately 8 h.p.i. In addition, brightly staining punctate aggregates containing UL31 protein, first detectable at 8 h.p.i., accumulated in regions of the nucleus peripheral to replication compartments. These aggregates did not stain appreciably with nucleic acid-specific stains such as DAPI (4′,6′-diamidino-2-phenylindole) (data not shown). The number of such foci varied from 0 to 14 per cell, with an average of 5.5 foci per cell (100 cells counted). Examples of these foci are marked with arrowheads in Fig. 4. The size of the aggregates and the UL31 protein-specific staining intensity within them increased between 16 and 20 h.p.i. In contrast to the appearance of cells infected with the UL34 deletion mutant, cells infected with a virus bearing a restored UL34 gene (vRR1072Rep) demonstrated UL31 protein staining exclusively at the nuclear rim. This distribution resembled that in cells infected with wild-type virus and is shown in Fig. 4 (row labeled 34R). From these data, we conclude that the UL34 gene product is necessary for exclusive localization of the UL31 gene product at the nuclear rim of infected cells.
UL31 protein is necessary for exclusive localization of UL34 protein at the nuclear rim.
HEp-2 cells were infected with R5132, a UL31 null mutant virus, or R5132Rep, a virus containing a restored UL31 locus. Fixed cells were immunostained with antibodies against UL31 protein, UL34 protein, and the replication compartment marker ICP4 (24). The results at 16 h.p.i. are shown in Fig. 4 (rows labeled 31- and 31R, for cells infected with the UL31 mutant and restored viruses, respectively). In UL31 mutant virus-infected cells, UL34 protein was detectable at the nuclear rim, in replication compartments coincident with ICP4, and in a perinuclear distribution in the cytoplasm. In contrast, UL34 protein localized exclusively at the nuclear rim of cells infected with a virus bearing a restored UL31 locus in a pattern resembling that of wild-type-infected cells. Negligible background staining was detectable in UL31 null mutant-infected cells with UL31 antiserum. From these data, we conclude that UL31 protein is necessary for UL34 protein to localize exclusively at the nuclear rim of infected cells.
UL31 and UL34 proteins are cotargeted to the nuclear rim in uninfected cells.
Thus far, the results indicate that the UL34 gene product is necessary for exclusive localization of UL31 protein at the nuclear rim and vice versa. The following series of experiments were performed to test the possibility that UL31 and UL34 are sufficient for mutual nuclear rim targeting (i.e., in the absence of other viral proteins). In the first part of the experiment, HEp-2 cells were transfected with either UL31 or UL34 expression constructs as described in Materials and Methods, and cells were probed with UL31 or UL34 protein-specific antiserum. Representative results are shown in Fig. 5. In contrast to the appearance of UL31 and UL34 proteins in cells infected with wild type viruses, transiently expressed UL31 protein was detected diffusely in the nucleoplasm in optical sections derived from the center of the transfected cell, and transiently expressed UL34 protein localized within aggregates (Fig. 5D to 5F) that localized mostly in the cytoplasm and, to a lesser extent, at the nuclear rim.
In the second part of the experiment, HEp-2 cells were cotransfected with UL31 and UL34 constructs and fixed at 18 h posttransfection, followed by costaining for the proteins. In contrast to the appearance of cells singly expressing UL31 or UL34 proteins, coexpression of UL31 and UL34 proteins resulted in localization of both proteins to the nuclear rim (Fig. 5G to 5I). UL31 and UL34 proteins were not detectable in the cytoplasm of cotransfected cells. At the nuclear rim, some areas exhibited colocalization of the two proteins, whereas some regions contained only UL31 protein or UL34 protein. In the majority of the cells at 18 h posttransfection, neither of the proteins was distributed smoothly and evenly around the nuclear rim, as was typically observed in wild-type-infected cells. Instead, the proteins appeared as fine, punctate structures on the nuclear rim. Most areas exhibited colocalization of the two proteins, whereas a minor portion of the nuclear rim contained only UL31 protein or UL34 protein. At later times posttransfection, UL31 protein staining appeared smoother and more evenly distributed, closely resembling that in wild-type-infected cells. In the case of UL34 protein, the punctate aggregates appeared slightly larger and more closely distributed around the nuclear rim, but did not demonstrate the smooth distribution pattern seen in infected cells. UL31 and UL34 proteins colocalized at the nuclear rim. Taken together, these data indicate that coexpression of UL31 and UL34 proteins is necessary and sufficient for exclusive targeting of the two proteins to the nuclear rim.
UL31 and UL34 proteins interact directly in vitro.
To determine if the UL31 and UL34 gene products could directly interact in the absence of other viral proteins, radiolabeled UL31 and UL34 proteins were produced in a rabbit reticulocyte lysate expression system and subjected to pull-down reactions with Sepharose beads bound to GST fused to either full-length UL31 or UL34 protein. As controls, reactions were also performed with Sepharose beads bound to GST alone. The reactions were electrophoretically separated on denaturing polyacrylamide gels, and the gels were immersed in sodium salicylate, dried, and subjected to fluorography at −80°C. Radioactive counts immobilized on the dried gels were also quantitated with a Molecular Dynamics PhosphorImager. The results of this study are shown in Fig. 6.
FIG. 6.
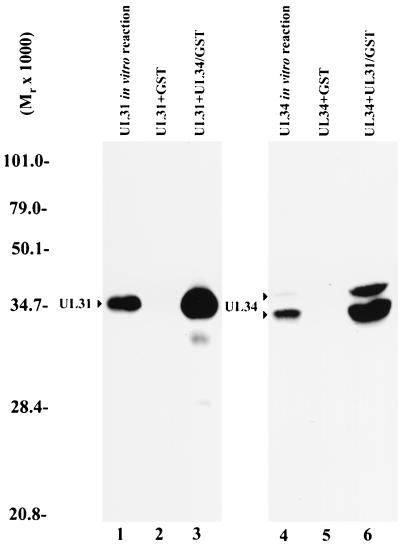
Digitized fluorographic image of GST pull-down reactions using equal amounts of GST or GST fusion proteins reacted with either in vitro-expressed radiolabeled UL31 protein (left panel) or radiolabeled UL34 protein (right panel). GST pull-down reactions in lanes 2, 3, 5, and 6 were performed similarly. Radiolabeled protein was added to Sepharose beads bearing either GST or GST fused to the putative interaction partner. After incubation, the beads were washed extensively, followed by elution in SDS sample buffer and electrophoretic separation on a denaturing polyacrylmide gel. The gel was subjected to analysis on a Molecular Dynamics PhosphorImager and fluorography. Lane 1, product of UL31 transcribed and translated in vitro. The amount of radiolabeled UL31 protein loaded in lane 1 was approximately 20% of that used in the reactions shown in lanes 2 and 3. Lane 2, radiolabeled UL31 protein bound to GST immobolized on Sepharose beads. Lane 3, radiolabeled UL31 protein bound to Sepharose beads containing UL34-GST. Lane 4, radiolabeled UL34 protein produced in an in vitro transcription-translation reaction. The amount of UL34 protein loaded in lane 4 was approximately 20% of that used in reactions shown in lanes 5 and 6. Lane 5, radiolabeled UL34 protein bound to immobilized GST. Lane 6, radiolabeled UL34 protein bound to immobilized UL31-GST. Molecular weights are indicated in thousands to the left of the figure.
Lane 1 demonstrates the electrophoretic mobility of UL31 protein expressed in the reticulocyte lysate system as resolved on a 12% denaturing polyacrylamide gel. The amount of protein loaded in lane 1 represents approximately 20% of that used in the GST pull-down reactions shown in lanes 2 and 3. Equal amounts of GST and GST fusion proteins were used for the pull-down reactions. PhosphorImager analysis indicated that the amount of UL31 protein bound to Sepharose beads bearing the UL34-GST fusion protein (lane 3) was 43-fold greater than the amount of UL31 protein bound to beads containing GST alone (lane 2). Lane 4 demonstrates the electrophoretic mobility of UL34 protein expressed in the reticulocyte lysate system and contains approximately 20% of that used in the GST pull-down reactions shown in lanes 5 and 6. As determined by PhosphorImager analysis, the amount of UL34 protein bound to the immobilized UL31-GST fusion protein (lane 6) was 22-fold greater than the amount of UL34 protein bound to beads containing GST alone (lane 5). From these data, we conclude that the UL31 and UL34 proteins can interact in the absence of other viral proteins.
Normal distribution of UL34 protein at the nuclear rim requires the US3-encoded protein kinase.
The HSV-1 UL34 gene product is a phosphoprotein and a known substrate of the HSV-1 US3 protein kinase (34). To test the hypothesis that US3 might influence UL34 protein localization, confocal analysis was performed on cells infected with US3 null HSV-1(F) mutants.
Parallel cultures of HEp-2 cells were infected with two independently derived US3 null mutants, one lacking the N-terminal portion of US3 (R7039) and one lacking the C-terminal portion of US3 (R7037). The use of two independent mutants controls for the possibility of second-site mutations that might complicate interpretation of the observed effects of US3 protein on localization of the UL34 protein.
At 6, 8, 10, and 20 h.p.i., infected cells were fixed, immunostained with anti-UL34 antibody (Texas Red detected), and anti-nucleoporin p62 antibody (FITC detected), and analyzed by confocal microscopy (Fig. 7). At 6 and 8 h.p.i., UL34 protein localized predominantly to pleiomorphic, perinuclear structures (Fig. 7A to 7F). Unlike the perinuclear UL34 protein structures observed in cells infected for 4 h with wild-type virus (Fig. 2I), these UL34 protein-containing structures also contained nucleoporin p62. This suggests that the lack of US3 kinase affects the organization of the infected-cell nuclear envelope. Similar to cells infected for 4 and 6 h with wild-type HSV-1(F), UL34 protein was also detected in small structures throughout the cytoplasm, and only a fraction of infected cells showed detectable levels of UL34 protein expression. Identical patterns of UL34 protein and nucleoporin p62 localization were observed in cells infected with R7037 (data not shown). To further examine the relationship of the observed perinuclear UL34 protein structures to the nuclear envelope, an LBR-GFP fusion protein was used as an alternative nuclear envelope marker (14). Unlike nucleoporin p62, LBR-GFP was not detected with UL34 protein in perinuclear structures in cells infected for 8 h with R7039 (Fig. 7I). This indicates that the apparent nuclear envelope reorganization does not affect all nuclear envelope proteins. Identical results were observed in cells infected with R7037 (data not shown).
By 10 h.p.i., virtually every cell infected with a US3 null virus showed preferential nuclear envelope UL34 protein localization and a lack of the nucleoporin p62-containing perinuclear bodies observed at earlier times (Fig. 7J to 7L). In contrast to UL34 protein localization in cells infected for 10 h with wild-type virus, which showed relatively smooth. uniform nuclear envelope localization, 10 h of infection with a US3 null virus yielded a punctate pattern of UL34 protein localization (compare Fig. 2P and 7J). This difference was more pronounced in a three-dimensional reconstruction of a series of confocal sections (Z-stack). Z-stack analysis of cells infected with HSV-1(F) for 10 h revealed that UL34 protein and nucleoporin p62 are smoothly distributed at the nuclear envelope (Fig. 7S to 7U). In the absence of the US3-encoded kinase, UL34 protein accumulated in speckles at the nuclear envelope, whereas nucleoporin p62 remained evenly distributed (Fig. 7V to 7X). Identical results were obtained with R7037 (data not shown).
As noted above, between 10 and 20 h.p.i., the distributions of UL34 protein and nucleoporin p62 in HSV-1(F)-infected cells changed from smooth nuclear envelope localization to heterogeneous localization at and around the nuclear periphery (Fig. 2P to 2X). Similar transition of UL34 protein and nucleoporin p62 was not observed in cells infected with R7039 (compare Fig. 7J to 7L with Fig. 7M and 7O) or R7037 (data not shown). At 20 h.p.i., UL34 protein in R7039-infected cells localized tightly to the nuclear envelope in a punctate pattern while nucleoporin p62 staining remained smoothly distributed (Fig. 7M to 7R).
US3-encoded protein kinase is necessary but not sufficient for proper localization of UL34 protein.
When independently expressed, as described in Fig. 5, UL34 protein (Texas Red detected) localized to discrete structures throughout the cytoplasm and at the nuclear envelope (Fig. 8A to 8C), and US3 protein (FITC detected) localized diffusely throughout the cytoplasm and was excluded from the nucleus (Fig. 8D to 8F). When coexpressed, UL34 protein and US3 appeared to influence each other's localization and colocalized extensively (Fig. 8G and 8H), as indicated by the yellow color upon merging of the two images. In the presence of US3, UL34 protein localized more smoothly at the nuclear envelope and throughout the cytoplasm, suggesting that in both infected and transfected cells, US3 reduces UL34 aggregation either in the cytoplasm or in the nuclear envelope. Coexpression of UL34 and US3 did not, however, result in the exclusive nuclear rim localization observed in wild-type virus-infected cells. From these data, we conclude that the US3-encoded kinase is necessary but not sufficient for proper UL34 protein localization.
Normal distribution of UL31 protein at the nuclear rim requires the US3-encoded protein kinase.
As the localization of UL34 protein was altered from an even distribution pattern around the nuclear rim to punctate aggregates in the absence of US3, it was hypothesized that the US3-encoded protein kinase might also affect the localization of UL31 protein. To test this possibility, cells were infected with US3 mutant virus R7037, fixed at various times after infection, and stained with antibodies directed against UL31 protein and UL34 protein. The distribution of the respective proteins at 16 h.p.i. is shown in Fig. 9. Identical results were obtained using US3 mutant virus R7039 (data not shown).
FIG. 9.
Digital confocal images of HEp-2 cells infected with US3 null mutant virus R7037 at an MOI of 5 that were fixed and permeabilized in ice-cold methanol at 16 h.p.i. Cells were immunostained with antisera directed against UL31 and UL34 proteins. UL31 protein-specific binding (green) was detected with FITC-conjugated donkey anti-rabbit Ig antibody, and UL34 protein-specific binding (red) was detected with Texas Red-conjugated donkey anti-chicken Ig antibody. Areas of colocalization of UL31 and UL34 proteins are indicated by a yellow color upon merging of the images. Confocal Z-sections were acquired in 0.4-μm slices through the entire thickness of the cell. Digital slices from the top (row labeled Top) of the cell or the middle of the cell (row labeled Middle) are shown. A confocal Z-stacked image derived from the Z-sections is shown in the row labeled Stacked. Original magnification, X600.
The distribution of UL31 protein was significantly altered in US3 null virus-infected cells compared with wild-type-infected cells examined by acquiring Z-sections of infected cells with subsequent three-dimensional reconstruction of Z-series images to generate a Z-stacked image. Sections derived from the top and the middle of the infected cell as well as the Z-stacked image are shown in Fig. 9 (rows labeled top, middle, and stacked, respectively). In contrast to the smooth, even-appearing distribution of protein-specific staining at the nuclear rim observed in cells infected with wild-type virus, UL31 protein localized in discrete punctate sites at the nuclear rim of cells infected with the US3 mutant. The punctate regions staining positively for UL31 protein also contained UL34 protein, as revealed by the yellow color produced upon merging of the images. From these data, we conclude that the US3-encoded protein kinase is required for the even distribution of UL31 and UL34 proteins at the nuclear rim, but in the absence of US3, interaction of UL31 protein and UL34 protein occurs at discrete sites within the nuclear rim.
DISCUSSION
Although there are other possibilities, data presented here and elsewhere suggest a model in which UL34 protein is produced in the endoplasmic reticulum, diffuses laterally into the outer nuclear membrane, and migrates past the nuclear pore to the inner nuclear membrane. If the UL31 protein is present, such as at late times after infection in cells infected with wild-type virus, the UL34 protein is retained at the inner nuclear membrane by interaction of the nucleoplasmic domain of UL34 protein, with UL31 protein transiently associated with the nuclear lamina. Stable association of UL31 protein with the nuclear lamina must also be enabled by the association with UL34 protein. The complex is retained at the nuclear rim during infection and somehow facilitates envelopment, possibly by engagement of nucleocapsids destined to become enveloped. The following considerations support this model.
(i) Cellular proteins targeted to the inner nuclear membrane are believed to undergo transport to the inner nuclear membrane in a similar fashion (40, 42). Specifically, both LBR and emerin (also type II integral membrane proteins) move to the inner nuclear membrane and are retained there by interaction with lamin B or lamins A and C, respectively (14, 30, 41). Diffusion past the nuclear pore is apparently restricted if the nucleoplasmic domain is greater than 60 to 70 kDa (42). Interestingly, all herpesvirus proteins are predicted to contain nucleoplasmic domains under this limit; thus, all HSV integral membrane proteins might be expected to at least transiently associate with the inner nuclear membrane. One caveat is that, while the PRV UL34 protein has been shown to be localized at the inner nuclear membrane, our results do not distinguish between localization at the inner and localization at the outer nuclear membrane for the HSV-1 protein.
(ii) UL34 protein accumulates primarily in the cytoplasm in the absence of its proposed nucleoplasmic ligand, UL31 protein. Thus, UL34 protein expressed either (i) early in infection, before UL31 protein is expressed at corresponding levels; (ii) transiently, in the absence of other viral proteins (Fig. 5); or, (iii) in cells infected with the UL31 null mutant (Fig. 4), accumulates primarily in the cytoplasm.
(iii) Transiently expressed UL31 protein is targeted to and selectively retained within the nucleus in these studies and those of Zhu et al. (48). Thus, the UL31 protein is properly targeted to act as a ligand for the nucleoplasmic domain of UL34 protein. It is of interest that the bulk of UL31 protein in infected cells is resistant to extraction with detergent, DNase, and high concentrations of salt, suggesting that the protein associates with the nuclear matrix (5, 8, 43). These observations, coupled with localization of UL31 protein at the nuclear rim in infected cells, are most consistent with association of UL31 protein with components of the nuclear lamina, a major component of the nuclear matrix located at the nuclear rim (18, 19). As a component of the nuclear lamina, UL31 protein would therefore provide a stable anchoring point for UL34 protein.
(iv) The UL31 protein is mistargeted in the absence of UL34 protein. Although the UL31 protein is targeted to the nucleus of transfected cells, nuclear rim retention at late times after transfection requires the presence of UL34 protein (Fig. 5). One possible explanation for these observations is that the nucleoplasmic domain of UL34 protein enables stable association of UL31 with the nuclear lamina.
(v) Transiently expressed UL31 protein is sufficient to retain the UL34 protein at the nuclear rim in the absence of other viral proteins (Fig. 5). This further supports the hypothesis that UL31 acts to anchor UL34 protein at the nuclear membrane.
(vi) Transiently expressed UL34 protein is sufficient to retain UL31 protein at the nuclear rim in the absence of other viral proteins (Fig. 5). This supports the hypothesis that UL34 enables stable association of UL31 protein with the nuclear lamina.
(vii) The tight nuclear envelope localization of the UL31-UL34 protein complex starting at 10 h.p.i. is consistent with a role in primary envelopment of nucleocapsids. Absence of detectable fluorescence staining from other parts of the cytoplasm suggests that the proteins either are not incorporated into virions or are lost during subsequent maturation steps, as suggested by Klupp et al. in studies of PRV (23). Purves et al. reported detection of small amounts of UL34 in HSV-1 virions purified from cellular homogenates (35), and Ye et al. showed that UL34 can interact with dynein and with the major capsid protein VP5 and suggested that virion-incorporated UL34 mediates capsid transport to the nucleus following entry (46). As there are many examples of multifunctional HSV proteins, it is possible that the UL34 gene product functions in both entry and egress of HSV-1. The issue of HSV UL34 incorporation into virus particles deserves careful further study, since its retention in virus particles might suggest that the virus retains the envelope acquired in primary envelopment, whereas loss of the protein during egress would support a deenvelopment-reenvelopment model.
(viii) The UL31 and UL34 proteins have been shown to directly or indirectly interact in lysates of infected cells (46) and in rabbit reticulocyte lysates (Fig. 6). Thus, the data are consistent with the possibility that the proteins interact at the nuclear membrane of infected cells.
Also relevant to these studies are the following considerations. Purves et al. have reported that HSV-1 UL34 protein is present on the surface of infected cells (35). Using the arguably more sensitive localization technique of confocal microscopy with a different antibody, we detect UL34 protein only at the nuclear envelope and in perinuclear and cytoplasmic structures, depending on the stage of infection. Another salient conclusion of these studies is when infected cells are analyzed by confocal microscopy, UL31 protein localizes primarily at the nuclear rim of infected cells, whereas previous studies indicated that the UL31 protein distributed diffusely throughout the nucleoplasm (8). As noted in the Results section, we favor the idea that the apparently disparate results are simply a consequence of the use of conventional versus confocal microscopy in the two studies.
Previously published immunoblot analysis indicated that at late times postinfection (i.e., 18 h.p.i.), amounts of UL31 protein were reduced in lysates of cells infected with a UL34 mutant virus (47). The reduction in UL31 protein was dependent on an intact proteosomal degradation pathway. These data, coupled with our immunofluorescence experiments demonstrating that UL31 protein is partially mislocalized in the absence of UL34 protein, suggest that some UL31 protein is mistargeted and subsequently degraded in the absence of UL34 protein.
The experiments detailed in this study also indicate that while localization of the UL34 and UL31 proteins at the nuclear rim is interdependent, the normal distributions of UL31 protein and UL34 protein also requires functions of the protein kinase encoded by US3. Thus, in the absence of US3, the UL31 and UL34 proteins still colocalize but within punctate aggregates that accumulate predominantly at the nuclear rim. Furthermore, in the absence of US3, the appearance of UL34 at the nuclear rim is delayed by at least 2 h. We do not yet know whether this delay results in a corresponding delay in production of infectious virus, nor are there any data yet that quantitatively address the effect of a US3 deletion on single-step growth of HSV-1 in HEp-2 cells. Our observations suggest, however, that phosphorylation of UL34 protein or other functions of US3, although required for proper targeting of the UL34-UL31 protein complex, are dispensable for the interaction between the two proteins. Since US3 protein has been shown to be dispensable for replication of HSV in some cells, the aggregates containing the UL31 and UL34 proteins must be sufficient to mediate some nucleocapsid envelopment (33). These issues will require further study to clarify the functional relevance of the effect of US3 on targeting of UL31 and UL31.
Herpesvirus infection has been shown to alter nuclear architecture (6, 20). Consistent with these observations, we report that UL34 protein localization is related to several potential nuclear envelope modifications. UL34 protein is found in discrete, nucleoporin p62-containing perinuclear structures in cells infected with a US3-deficient virus for 6 and 8 h. These structures are not observed in cells infected with wild-type virus. Therefore, mislocalization of UL34 protein may be related to modification of nuclear envelope organization. Consistent with this idea, Ye et al. have reported that the inner and outer nuclear membranes separate from each other in Vero cells expressing UL34 protein from a baculovirus expression vector (46). It has also been observed that electron-dense aggregates accumulate in the nuclei of cells infected with the UL34 null virus at late times postinfection (37). These structures appeared to be surrounded by nuclear membrane and likely correspond to the UL31 protein-containing nuclear aggregates reported in the present study. Taken together, these results suggest that UL34 protein may directly or indirectly affect the structure of the nuclear envelope.
The nuclear envelope modification observed in the absence of US3 kinase does not, however, appear to involve the lamina network since the perinuclear UL34-nucleoporin p62-containing bodies do not also contain LBR (Fig. 7I). Since LBR is found almost exclusively on the inner nuclear membrane (14), it seems likely that the observed modification involves only the outer nuclear membrane. Alternatively, the observed modification could be specific for nucleoporin p62, with little or no effect on the nuclear envelope as a whole. In either case, it is not yet clear whether the apparent nuclear envelope modification is due to mislocalization of UL31 and UL34 proteins or to lack of US3 kinase.
Nuclear envelope alteration may also occur in later stages of infection with wild-type HSV-1(F). In wild-type-infected cells at 20 h.p.i., UL34 protein is localized to structures at the nuclear periphery. These structures appear membranous, are often convoluted, and contain nucleoporin p62. Thus, it appears that UL34 protein continues to associate with the nuclear envelope as it is disrupted at late stages in infection. Interestingly, in the absence of US3 protein, neither UL34 protein nor nucleoporin p62 is found in similar perinuclear structures (Fig. 7). Therefore, it appears that US3 kinase has some effect on nuclear envelope organization late in infection, possibly by way of its effect on UL34 protein.
Although the two proteins may perform other functions as well, the phenotypes attributed to the UL31 and UL34 mutant viruses exhibit strikingly similar defects in production of extranuclear particles. This observation, coupled with localization of the two proteins primarily at the site of initial nucleocapsid envelopment, strongly suggests that the UL31-UL34 protein complex plays an essential role in nucleocapsid envelopment or exit from the nucleus (9, 37).
ACKNOWLEDGMENTS
A.E.R. and B.J.R. contributed equally to this work.
We thank Bernard Roizman for the UL31 and US3 deletion viruses and Jan Ellenberg for providing the LBR-GFP fusion protein. We are grateful to Jarek Okulicz-Kozaryn (Cornell University) and Jean Ross (Central Microscopy Research Facility, University of Iowa) for expert technical assistance.
These studies were supported by the University of Iowa and Public Health Service Awards AI 41478 (R.J.R.) and GM 50740 (J.D.B.), National Research Service Award F32 GM20448 (A.E.R.), training grant AI07533 to University of Iowa (B.J.R.), and equipment grant RR12847 to Cornell University.
REFERENCES
- 1.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines J D, Koyama A H, Huang T, Roizman B. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J Virol. 1994;68:2929–2936. doi: 10.1128/jvi.68.5.2929-2936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Chee M S, Hutchison III C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. The DNA sequence of the human cytomegalovirus genome. DNA Seq. 1991;2:1–12. doi: 10.3109/10425179109008433. [DOI] [PubMed] [Google Scholar]
- 4.Barth W, Chatterje S, Stochaj U. Targeting of the mammalian nucleoporin p62 to the nuclear envelope in the yeast Saccharomyces cerevisiae and HeLa cells. Biochem Cell Biol. 1999;77:355–365. [PubMed] [Google Scholar]
- 5.Berezney R, Mortillaro M J, Ma H, Wei X, Samarabandu J. The nuclear matrix: a structural milieu for genomic function. In: Berezney R, Jeon K W, editors. Nuclear matrix—structure and function. 1st ed. San Diego, Calif: Academic Press; 1995. pp. 1–66. [DOI] [PubMed] [Google Scholar]
- 6.Bibor-Hardy V, Suh M, Pouchelet M, Simard R. Modifications of the nuclear envelope of BHK cells after infection with herpes simplex virus type 1. J Gen Virol. 1982;63:81–94. doi: 10.1099/0022-1317-63-1-81. [DOI] [PubMed] [Google Scholar]
- 7.Blaho J, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. [PubMed] [Google Scholar]
- 8.Chang Y E, Roizman B. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein, which partitions with the nuclear matrix. J Virol. 1993;67:6348–6356. doi: 10.1128/jvi.67.11.6348-6356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y E, Van Sant C, Krug P W, Sears A E, Roizman B. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlington R W, Moss L H. Herpesvirus envelopment. J Virol. 1968;2:48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 12.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 14.Ellenberg J, Siggia E D, Moreria J E, Smith C L, Presley J F, Worman H J, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farina A, Santarelli R, Gonnella R, Bei R, Muraro R, Cardinali G, Uccini S, Ragona G, Frati L, Faggioni A, Angeloni A. The BFRF1 gene of Epstein-Barr virus encodes a novel protein. J Virol. 2000;74:3235–3244. doi: 10.1128/jvi.74.7.3235-3244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frame M C, Purves F C, McGeoch D J, Marsden H S, Leader D P. Identification of the herpes simplex virus protein kinase as the product of the viral gene US3. J Gen Virol. 1987;68:2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- 17.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136:531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gant T M, Wilson K L. Nuclear assembly. Annu Rev Cell Dev Biol. 1997;13:669–695. doi: 10.1146/annurev.cellbio.13.1.669. [DOI] [PubMed] [Google Scholar]
- 19.Gruenbaum Y, Wilson K L, Harel A, Goldberg M, Cohen M. Review: nuclear lamins—structural proteins with fundamental functions. J Struct Biol. 2000;129:313–323. doi: 10.1006/jsbi.2000.4216. [DOI] [PubMed] [Google Scholar]
- 20.Haines H, Baerwald R J. Nuclear membrane changes in herpes simplex virus-infected BHK-21 cells as seen by freeze-fracture. J Virol. 1976;17:1038–1042. doi: 10.1128/jvi.17.3.1038-1042.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson L, Johnson D C. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerome K R, Fox R, Chen Z, Sears A E, Lee H Y, Corey L. Herpes simplex virus inhibits apoptosis through the action of two genes, US5 and US3. J Virol. 1999;73:8950–8957. doi: 10.1128/jvi.73.11.8950-8957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klupp B G, Granzow H, Mettenleiter T C. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol. 2000;74:10063–10073. doi: 10.1128/jvi.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knipe D M, Senechek D, Rice S A, Smith J L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987;61:276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long E O, Rosen-Bronson S, Karp D R, Malnati M, Sekaly R P, Jaraquemada D. Efficient cDNA expression vectors for stable and transient expression of HLA-DR in transfected fibroblast and lymphoid cells. Hum Immunol. 1991;31:229–235. doi: 10.1016/0198-8859(91)90092-n. [DOI] [PubMed] [Google Scholar]
- 27.Longnecker R, Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987;236:573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- 28.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama Y, Yamada Y, Kurachi R, Daikoku T. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology. 1992;190:256–268. doi: 10.1016/0042-6822(92)91212-d. [DOI] [PubMed] [Google Scholar]
- 30.Ostlund C, Ellenberg J, Hallberg E, Lippincott-Schwatrz J, Worman H J. Intracellular trafficking of emerin, the Emery-Dreifuss muscular dystrophy protein. J Cell Sci. 1999;112:1709–1719. doi: 10.1242/jcs.112.11.1709. [DOI] [PubMed] [Google Scholar]
- 31.Parks G D, Lamb R A. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991;64:777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- 32.Post L E, Mackem S, Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 33.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 that is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purves F C, Spector D, Roizman B. The herpes simplex virus protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purves F C, Spector D, Roizman B. UL34, the target of the herpes simplex virus US3 protein kinase, is a membrane protein that in its unphosphorylated state associates with novel phosphoproteins. J Virol. 1992;66:4295–4303. doi: 10.1128/jvi.66.7.4295-4303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds A E, Fan Y, Baines J D. Characterization of the UL33 gene product of herpes simplex virus 1. Virology. 2000;266:310–318. doi: 10.1006/viro.1999.0090. [DOI] [PubMed] [Google Scholar]
- 37.Roller R J, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J Virol. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Shiba C, Daikoku T, Goshima F, Takakuwa H, Yamauchi Y, Koiwai O, Nishiyama Y. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J Gen Virol. 2000;81:2397–2405. doi: 10.1099/0022-1317-81-10-2397. [DOI] [PubMed] [Google Scholar]
- 40.Smith S, Blobel G. The first membrane-spanning region of the lamin B receptor is sufficient for sorting to the inner nuclear membrane. J Cell Biol. 1993;120:631–637. doi: 10.1083/jcb.120.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soullam B, Worman H J. The amino-terminal domain of the lamin B receptor is a nuclear envelope targeting signal. J Cell Biol. 1993;120:1093–1100. doi: 10.1083/jcb.120.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soullam B, Worman H J. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130:15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staufenbiel M, Deppert W. Preparation of nuclear matrices from cultured cells: subfractionation of nuclei in situ. J Cell Biol. 1984;98:1886–1894. doi: 10.1083/jcb.98.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taus N S, Salmon B, Baines J D. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–125. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 45.Telford E A, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 46.Ye G J, Vaughan K T, Vallee R B, Roizman B. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye G J, Roizman B. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proc Natl Acad Sci USA. 2000;97:11002–11007. doi: 10.1073/pnas.97.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H Y, Yamada H, Jiang Y M, Yamada M, Nishiyama Y. Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch Virol. 1999;144:1923–1935. doi: 10.1007/s007050050715. [DOI] [PubMed] [Google Scholar]