Abstract
Purpose
To inform effective management strategies for severe asthma in China, this study aimed to comprehensively characterize clinical characteristics, treatment patterns, disease control status, and healthcare resource utilization among patients on GINA Step 4/5 therapies by analyzing data from the Adelphi Asthma Disease Specific Program conducted in China.
Patients and methods
All information was retrieved from medical records or collected from physicians and patients on the survey date (August–December 2018); no follow-up was conducted. Results were summarized descriptively for patients on GINA Step 4/5 therapies, who were pooled from a consecutive sample (comprising three or more consecutive patients with physician-diagnosed asthma from each participating physician) and an oversample (comprising the next two patients with physician-perceived severe asthma from each participating physician).
Results
Of the included patients (n=754), 51.5% had ever had a blood eosinophil measurement taken, 22.1% had available records for their most recent blood eosinophil measurements (68.9% of them had an elevated level ≥150 cells/µL), 39.9% had ever been tested for specific immunoglobulin E or radioallergosorbent, and 8.0% were prescribed maintenance oral corticosteroids. Asthma was not well controlled in 69.2% of patients. In the prior year, 27.1% experienced at least one severe exacerbation and 22.8% experienced at least one hospitalization (emergency visit or overnight stay) due to asthma.
Conclusion
In Chinese patients with asthma on GINA Step 4/5 therapies, biomarker testing was underutilized, asthma was not well controlled, and severe exacerbations were not infrequent. These findings highlight the urgent need for optimized asthma management for patients on GINA Step 4/5 therapies in China.
Keywords: severe asthma, type-2 high asthma, real-world, phenotype, exacerbations, health care resource utilization, eosinophils
Introduction
Asthma is a chronic respiratory disease characterized by airway hyperresponsiveness and reversible airflow obstruction, presenting with recurrent symptoms, such as wheezing, breathlessness, and coughing.1 It is estimated to affect over 262 million people (equivalent to a global prevalence of 3.4%) worldwide, including approximately 45.7 million adult patients (equivalent to a prevalence of 4.2%) from China.2,3 Severe asthma constitutes 3–10% of the asthma population,4,5 but disproportionately accounts for at least 50% of asthma-related healthcare costs.6,7 Therefore, effective management of severe asthma is essential for reducing the overall disease burden.
Severe asthma remains characterized by the need for high-intensity treatment, although its definition has evolved over the years and varies across guidelines. In the 2022 recommendations from the Global Initiative for Asthma (GINA), it is defined as asthma that is uncontrolled despite adherence with maximal optimized high dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) treatment, with or without add-on therapy, and management of contributory factors, that worsens when high-dose treatment is decreased.8 In the current Chinese asthma guidelines (published in 2020), severe asthma is defined as asthma that requires GINA Step 4/5 therapies to prevent the patient from becoming uncontrolled or remains uncontrolled despite this treatment.9 Type 2 (T2) high-inflammatory phenotype, characterized by eosinophilic inflammation, constitutes over 80% of the severe asthma population.10,11 A rapidly growing body of evidence demonstrates that T2-targeted biological therapies can substantially reduce the risk of exacerbations and improve symptom control and quality of life in patients with severe T2-high asthma.12–14 Considering this treatment landscape change, all patients with severe asthma are now recommended to be phenotyped and considered for add-on T2-targeted biological therapies if they have the T2-high phenotype.8
The commonly used T2 biomarkers for phenotyping include blood eosinophil count (BEC), sputum eosinophil count (SEC), serum immunoglobulin E (IgE), and fractional exhaled nitric oxide (FeNO).8 Among them, BEC has emerged as a valuable biomarker due to its accessibility and prognostic value.11,15 In China, three biological therapies have been approved for asthma: omalizumab for moderate-to-severe allergic asthma (approved in 2017), mepolizumab for severe asthma with an eosinophilic phenotype (approved in January 2024), and benralizumab for severe eosinophilic asthma (approved in August 2024),16–18 and several others, such as dupilumab and tezepelumab, are being evaluated in clinical development.19,20 Given the increasing availability of T2-targeted biological therapies in China, it is highly desirable to understand the landscape of BEC testing among the severe asthma population in China and optimize its use to fully realize the clinical benefits conferred by these therapies.
Asthma control is crucial to reducing disease burden in the asthma population;21–23 however, the level of control has been identified as being sub-optimal in China. For example, over a 12-month period, 15.5% and 7.2% of asthma patients in China were reported to experience exacerbations leading to emergency visit and hospitalization, respectively.3 To inform effective disease management strategies for patients with severe asthma in China, this study draws on the Adelphi Asthma Disease Specific Program (DSP) conducted in China in 2018 to provide a comprehensive description of clinical characteristics, including BEC and specific IgE testing status and levels, treatment patterns, disease control status, and healthcare resource utilization (HCRU) among patients on GINA Step 4/5 therapies.
Methods
Study Design and Participants
Detailed methodology for the DSP, a large, cross-sectional physician and patient survey, has been published previously.24 This study analyzed secondary data from Chinese adult patients with physician-confirmed asthma in the Asthma DSP (2018 version). This survey was performed from August to December 2018 in a consecutive sample representative of the asthma patient population receiving routine hospital-based care as well as in a separate oversample of patients with physician-perceived severe asthma. The DSP was carried out by Adelphi without any set hypothesis to provide unbiased observations of patient and disease characteristics, clinical practices, and disease burden in the real-world setting.
Hospital-based respiratory physicians (chief doctors, vice chief doctors, or doctors in charge) were eligible to participate in the survey if they worked with at least three asthma patients who were aged 18 years or older per week. Each participating physician was asked to complete a patient record form (PRF) for their next three or more consecutive patients aged 18 years or older with physician-diagnosed asthma (to form a consecutive asthma sample) and for two additional patients aged 18 years or older with physician-perceived severe asthma (to form a severe asthma oversample). The severity of asthma was assessed by physicians without guidance from Adelphi or their fieldwork partners.
Hospitals in China are classified into three tiers, based on their sizes and levels of care. Class III hospitals are those with over 500 inpatient beds, which offer high-level specialist medical treatment at the city, provincial, or national level and lead medical education and scientific research programs. Class II hospitals have an inpatient bed capacity between 101 and 500, providing comprehensive medical treatment to medium-sized cities, counties, or districts. Community health centers (CHCs), each of which has 100 and fewer inpatient beds, directly provide primary care to township communities. According to the sampling frame list held by Adelphi’s fieldwork partners, cities in China are classified into Tiers 1–4 based on administrative level, city size, population, and economic development level. Within the pragmatically pre-determined sample size of physicians and distribution of hospital classes, cities/provinces were selected across the north, south, west, and east regions of China to ensure geographic representation. Participating physicians and patients were recruited across three tiers of hospitals in eight cities/provinces (Beijing, Shanghai, Guangdong, Sichuan, Hubei, Shaanxi, Jiangsu, and Liaoning). The included cities and their tiers are provided in Supplementary Table 1.
For the current study, patients from both the consecutive sample and the oversample were included for analysis if they had no co-diagnosis of chronic obstructive pulmonary disease (COPD) and were receiving Step 4/5 therapies as specified in the 2018 GINA treatment recommendations, the latest version at the time of the survey, on the survey date (the date when the questionnaires were completed).25 These patients were considered to have severe asthma according to both the current Chinese asthma guidelines and the 2018 GINA treatment recommendations.9,25 The current Chinese asthma guidelines, published in 2020, remain more aligned with the 2018 GINA recommendations than with the GINA recommendations from 2021 onwards due to significant changes the GINA recommendations underwent in 2021. Supplementary Table 2 provides a comparison between 2018 and 2022 GINA treatment recommendations for adult patients with asthma and the definitions of severe asthma in each version.
Data Collection
For the DSP, all information was either retrieved from medical records or collected from participants at the survey date by local fieldwork partners; no follow-up information was collected. Both physician and patient data were de-identified prior to receipt by Adelphi. Through completion of PRFs, physicians provided patients’ demographics, clinical characteristics, treatment regimens, comorbidities, disease burden, and symptoms. Eligible patients were invited by their physicians to complete non-mandatory patient self-completion (PSC) form about asthma control, disease burden, and the impact of asthma on work and overall health status.
The current study assessed asthma control based on a definition adapted from that by the American Thoracic Society (ATS)/European Respiratory Society (ERS).26 Asthma was deemed as uncontrolled if at least one of the following criteria was fulfilled: a) Asthma Control Questionnaire (ACQ) consistently ≥1.5 or Asthma Control Test (ACT) <20 (or “not well controlled” by National Asthma Education and Prevention Program and/or GINA 2018 treatment recommendations for this study),25,27 b) ≥2 bursts of oral corticosteroids (OCS) (≥3 days each) in the previous year, c) ≥1 hospitalization, intensive care unit stay or mechanical ventilation in the previous year, and d) after appropriate bronchodilator withhold forced expiratory volume in 1 s (FEV1) <80% predicted (in the presence of reduced FEV1/forced vital capacity [FVC] defined as less than the lower limit of normal).
Ethics Approval
The DSP was conducted as a market research survey adhering to the International Chamber of Commerce (ICC)/European Society for Opinion and Market Research (ESOMAR) International guidelines on observational research and performed in full accordance with the code of conduct outlined in the European Pharmaceutical Market Research Association (EphMRA) International guidelines. For this reason, Institutional Review Board (IRB) approval was neither necessary nor sought. In the DSP survey, all participating physicians provided consent; patient consent was not required to complete the PRF given the level of anonymization of data but was obtained from those who agreed to complete the PSC questionnaire. This study utilized the existing data from the DSP for disease understanding research and complies with the Declaration of Helsinki.
Objectives
This study describes patient characteristics, treatment patterns, disease control status, and HCRU of Chinese asthma patients receiving GINA Step 4/5 therapies. The analysis was additionally stratified by BEC testing status (BEC-tested and non-BEC-tested) and by BEC level (BEC-elevated [≥150 cells/µL], non-BEC-elevated [<150 cells/µL], and BEC-unknown).
Statistical Analysis
The DSP was a descriptive, non-interventional market research disease understanding survey, so no priori hypothesis was set. In this study, all results were summarized descriptively for patients receiving GINA Step 4/5 therapies as well as in subgroups stratified by BEC testing status and level. A sensitivity analysis was performed to assess the validity of pooling the GINA Step 4/5 subgroups from the two samples. All analyses were conducted using the Unicom Intelligence Reporter, version 7.5.28 This article presents the physician-reported results.
Results
Study Population
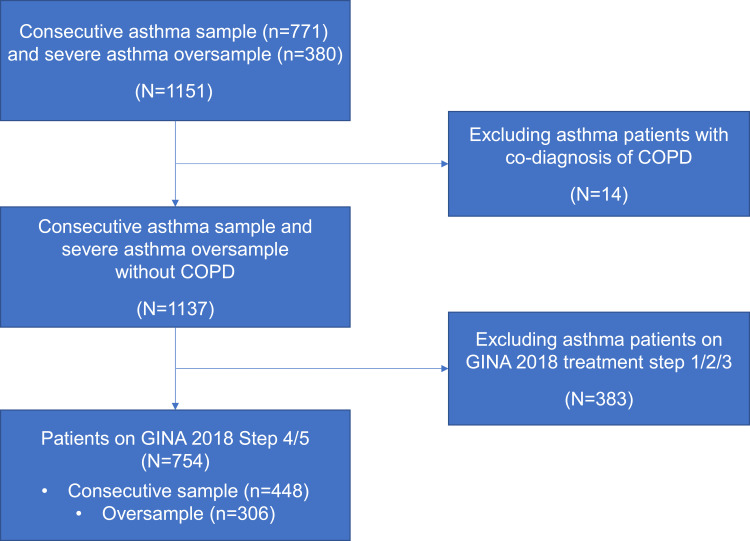
The DSP survey enrolled 230 hospital-based physicians (26 chief doctors, 94 vice chief doctors, 110 doctors in charge) across three hospital settings (125 Class III hospitals, 65 Class II hospitals, and 40 CHCs). Out of the 1,151 patients from the two asthma patient samples, 754 patients on GINA 2018 Step 4/5 therapies were included in the current study, with 448 from the consecutive sample and 306 from the oversample (Figure 1).
Figure 1.
Study design patient flow chart. The study population included patients prescribed GINA 2018 Step 4/5 treatment at the index date from both the consecutive asthma sample and the severe asthma oversample.
Abbreviations: COPD, chronic obstructive pulmonary disease; GINA, Global Initiative for Asthma.
Patient Demographics and Clinical Characteristics
In the included patients (n=754), mean age (standard deviation [SD]) was 45.6 (14.2) years, 51.9% (391) were female, 36.2% (273) were from Tier-2 cities, and 56.4% (425) were seeking medical help from Class III hospitals. Most patients were never or past smokers (91.8%, 691/753). The physician-perceived severity of asthma was mild, moderate, and severe in 31.4% (236/752), 32.2% (242/752), and 36.4% (274/752) of patients, respectively (Table 1).
Table 1.
Physician-Reported Patient Demographics and Clinical Characteristics Among Patients with Asthma on GINA Step 4/5 Therapies
| Characteristic | BEC Tested | BEC not Tested (N=366) | Total (N=754) | |||
|---|---|---|---|---|---|---|
| BEC <150 Cells/µL (N=52) | BEC≥150 Cells/µL (N=115) | BEC Unknown (N=221) | Subtotal (N=388) | |||
| Mean age (SD), years | 41.3 (16.1) | 43.6 (15.0) | 47.9 (13.2) | 45.7 (14.4) | 45.4 (14.1) | 45.6 (14.2) |
| Sex, n (%) | ||||||
| Male | 32 (61.5) | 56 (48.7) | 110 (49.8) | 198 (51.0) | 165 (45.1) | 363 (48.1) |
| Female | 20 (38.5) | 59 (51.3) | 111 (50.2) | 190 (49.0) | 201 (54.9) | 391 (51.9) |
| Smoking history, n (%) | n=52 | n=115 | n=221 | n=388 | n=365 | n=753 |
| Current smoker | 2 (3.8) | 15 (13.0) | 13 (5.9) | 30 (7.7) | 9 (2.5) | 39 (5.2) |
| Ex-smoker | 8 (15.4) | 26 (22.6) | 56 (25.3) | 90 (23.2) | 83 (22.7) | 173 (23.0) |
| Never smoked | 41 (78.8) | 68 (59.1) | 142 (64.3) | 251 (64.7) | 267 (73.2) | 518 (68.8) |
| Do not know | 1 (1.9) | 6 (5.2) | 10 (4.5) | 17 (4.4) | 6 (1.6) | 23 (3.1) |
| City tier, n (%) | ||||||
| Tier 1 | 14 (26.9) | 29 (25.2) | 65 (29.4) | 108 (27.8) | 63 (17.2) | 171 (22.7) |
| Tier 2 | 29 (55.8) | 69 (60.0) | 90 (40.7) | 188 (48.4) | 85 (23.2) | 273 (36.2) |
| Tier 3 | 1 (1.9) | 6 (5.2) | 36 (16.3) | 43 (11.1) | 121 (33.1) | 164 (21.8) |
| Tier 4 | 8 (15.4) | 11 (9.6) | 30 (13.6) | 49 (12.6) | 97 (26.5) | 146 (19.4) |
| Hospital type, n (%) | ||||||
| CHC | 5 (9.6) | 5 (4.3) | 27 (12.2) | 37 (9.5) | 67 (18.3) | 104 (13.8) |
| Class II hospital | 9 (17.3) | 35 (30.4) | 61 (27.6) | 105 (27.1) | 120 (32.8) | 225 (29.8) |
| Class III hospital | 38 (73.1) | 75 (65.2) | 133 (60.2) | 246 (63.4) | 179 (48.9) | 425 (56.4) |
| Physician-perceived severity of asthma, n (%) | n=52 | n=115 | n=220 | n=387 | n=365 | n=752 |
| Mild | 16 (30.8) | 30 (26.1) | 39 (17.7) | 85 (22.0) | 151 (41.4) | 236 (31.4) |
| Moderate | 14 (26.9) | 40 (34.8) | 69 (31.4) | 123 (31.8) | 119 (32.6) | 242 (32.2) |
| Severe | 22 (42.3) | 45 (39.1) | 112 (50.9) | 179 (46.3) | 95 (26.0) | 274 (36.4) |
| Frequency of select comorbidities, n (%) | ||||||
| Allergic Rhinitis | 27 (51.9) | 65 (56.5) | 99 (44.8) | 191 (49.2) | 205 (56.0) | 396 (52.5) |
| Hypertension | 9 (17.3) | 23 (20.0) | 68 (30.8) | 100 (25.8) | 59 (16.1) | 159 (21.1) |
| CRSsNP | 13 (25.0) | 19 (16.5) | 27 (12.2) | 59 (15.2) | 50 (13.7) | 109 (14.5) |
| Diabetes | 8 (15.4) | 15 (13.0) | 24 (10.9) | 47 (12.1) | 30 (8.2) | 77 (10.2) |
| Elevated cholesterol/ hyperlipidemia | 4 (7.7) | 7 (6.1) | 19 (8.6) | 30 (7.7) | 23 (6.3) | 53 (7.0) |
| CRSwNP | 2 (3.8) | 4 (3.5) | 11 (5.0) | 17 (4.4) | 11 (3.0) | 28 (3.7) |
| Most recent pre-bronchodilator FEV1 predicted score in the last 12 months, n (%) | n=25 | n=46 | n=63 | n=134 | n=68 | n=202 |
| <30% | 0 | 0 | 3 (4.8) | 3 (2.2) | 0 | 3 (1.5) |
| 30–49% | 2 (8.0) | 11 (23.9) | 14 (22.2) | 27 (20.1) | 17 (25.0) | 44 (21.8) |
| 50–79% | 22 (88.0) | 28 (60.9) | 45 (71.4) | 95 (70.9) | 50 (73.5) | 145 (71.8) |
| ≥80% | 1 (4.0) | 7 (15.2) | 1 (1.6) | 9 (6.7) | 1 (1.5) | 10 (5.0) |
| Most recent FEV₁/FVC ratio | n=31 | n=50 | n=82 | n=163 | n=179 | n=342 |
| Mean ± SD, % | 68.3±12.7 | 63.1±20.2 | 64.9±16.0 | 65.0±17.0 | 65.1±17.4 | 65.0±17.1 |
| BEC taken in the last 12 months, n (%a) | 47 (90.4) | 101 (87.8) | 175 (79.2) | 323 (83.3) | 0 | 323 (83.3) |
| Most recent BEC | n=52 | n=115 | N/A | n=167 | N/A | n=167 |
| Geometric mean (log SD), cells/µL | 21 (1.0) | 530 (0.5) | N/A | 195 (1.7) | N/A | 195 (1.7) |
| Specific IgE/RAST test taken, n (%) | 40 (76.9) | 61 (53.0) | 132 (59.7) | 233 (60.1) | 68 (18.6) | 301 (39.9) |
| Most recent test result available, n (%) | 38 (95.0) | 59 (96.7) | 132 (100.0) | 229 (98.3) | 57 (83.8) | 286 (95.0) |
| Normal IgE, n (%) | 4 (10.5) | 8 (13.6) | 14 (10.6) | 26 (11.4) | 7 (12.3) | 33 (11.5) |
| Elevated IgE, n (%) | 34 (89.5) | 51 (86.4) | 118 (89.4) | 203 (88.6) | 50 (87.7) | 253 (88.5) |
Note: aThe proportion in each group was calculated using the number of patients in each group who had ever taken an BEC test as the denominator (n=388 for the total population).
Abbreviations: BEC, blood eosinophil count; CHC, community health center; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GINA, Global Initiative for Asthma; IgE, allergen-specific immunoglobulin E; N/A, not available; RAST, radioallergosorbent test; SD, standard deviation.
Among patients with a pre-bronchodilator FEV₁ measure in the last 12 months, 71.8% (145/202) had predicted values between 50% and 79%. In those with ≥1 recorded FEV1/FVC ratio (n=342), obtained anytime between diagnosis and the survey date, the mean for the most recently recorded value was 65.0% (SD, 17.1%) (Table 1).
Biomarker Measurements
Of the included patients, 51.5% (388/754) had ever undergone a BEC test, of which, 83.2% (323/388) had a test in the last 12 months. In patients with available records for their most recent BEC tests, the geometric mean was 195 (log SD, 1.7) cells/μL for the most recent BEC, 31.1% (52/167) had a value <150 cells/µL, and 68.9% (115/167) had a value ≥150 cells/µL (Table 1).
The proportion of BEC-tested patients decreased with city tier, from 66.7% (296/444) in those treated in Tier 1/2 cities to 29.7% (92/310) in those treated in Tier 3/4 cities. The proportion of BEC-tested patients increased with hospital class. In addition, the proportion of BEC-tested patients increased with physician-perceived asthma severity, from 36.0% (85/236) for mild asthma, to 50.8% (123/242) for moderate asthma, and to 65.3% (179/274) for severe asthma (Table 1).
In BEC-tested patients (n=388), 76.3% (296) were from Tier 1/2 cities and 63.4% (246) were seeking medical help from Class III hospitals. In non-BEC-tested patients (n=366), 40.4% (148) were from Tier 1/2 cities and 48.9% (179) were seeking medical help from Class III hospitals. Physicians perceived 46.3% (179/387) of BEC-tested patients and 26.0% (95/365) of non-BEC-tested patients to have severe disease (Table 1).
Only 39.9% (301/754) of patients had ever been tested for specific IgE/radioallergosorbent (RAST). Of those with their most recent test results available, 88.5% (253/286) had an elevated specific IgE level according to physicians’ judgement (Table 1). Specific IgE/RAST testing increased with increasing hospital class.
Symptomatic Burden
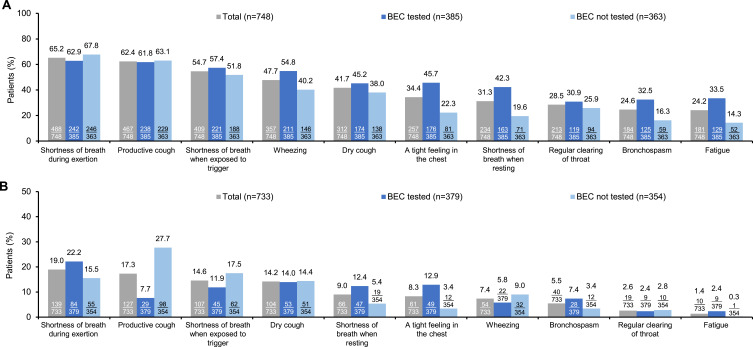
As reported by physicians, the symptoms that were most frequently experienced by patients (n=748) in the last four weeks included shortness of breath during exertion (65.2%), productive cough (62.4%), shortness of breath when exposed to trigger (54.7%), wheezing (47.7%), and dry cough (41.7%) (Figure 2A); and the most troublesome symptom experienced by patients (n=733) in the last 12 months was shortness of breath during exertion, productive cough, shortness of breath when exposed to trigger, dry cough, and shortness of breath when resting in 19.0%, 17.3%, 14.6%, 14.2%, and 9.0% of patients, respectively (Figure 2B).
Figure 2.
Physician-reported symptomatic burden of asthma. (A) Most common symptoms in the last four weeks (multiple choices available, n=748) and (B) Most troublesome symptom in the last 12 months (single choice, n=733) a.
Note: aY-axis of Figure 2b has been adjusted to fit the range of data.
Abbreviation: BEC, blood eosinophil count.
Treatment Patterns
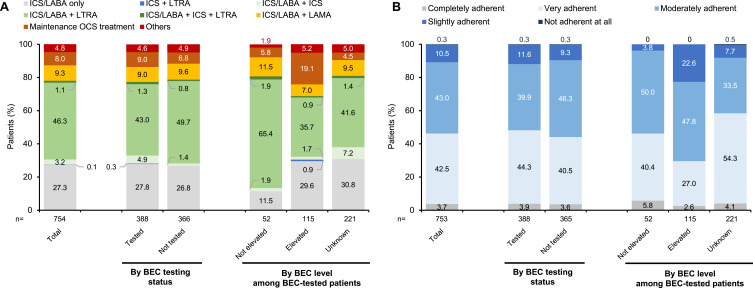
Of the included patients (n=754), 92.0% (694) were prescribed Step 4 treatment and 8.0% (60) were prescribed Step 5 treatment (Table 2). For currently prescribed maintenance treatment, ICS/LABA, whether used with other treatments or not, was prescribed for 87.1% (657) of patients (Figure 3A). Of those who had an ICS total daily dose recorded (n=537), 19.7% (106), 59.4% (319), and 20.9% (112) received a low, medium, and high total daily dose (Table 2), respectively, according to the criteria in the 2018 GINA recommendations (Supplementary Table 3).
Table 2.
Physician-Reported Asthma Treatment Among Patients with Asthma on GINA Step 4/5 Therapies
| Characteristic | BEC Tested | BEC not Tested (N=366) | Total (N=754) | |||
|---|---|---|---|---|---|---|
| BEC<150 Cells/µL (N=52) | BEC≥150 Cells/µL (N=115) | BEC Unknown (N=221) | Subtotal (N=388) | |||
| GINA treatment step | ||||||
| Step 4 | 49 (94.2) | 93 (80.9) | 211 (95.5) | 353 (91.0) | 341 (93.2) | 694 (92.0) |
| Step 5 | 3 (5.8) | 22 (19.1) | 10 (4.5) | 35 (9.0) | 25 (6.8) | 60 (8.0) |
| ICS total daily dosagea, n (%) | n=26 | n=103 | n=184 | n=313 | n=224 | n=537 |
| Low | 6 (23.1) | 22 (21.4) | 42 (22.8) | 70 (22.4) | 36 (16.1) | 106 (19.7) |
| Medium | 15 (57.7) | 43 (41.7) | 120 (65.2) | 178 (56.9) | 141 (62.9) | 319 (59.4) |
| High | 5 (19.2) | 38 (36.9) | 22 (12.0) | 65 (20.8) | 47 (21.0) | 112 (20.9) |
| Number of OCS bursts in the last 12 months | n=52 | n=115 | n=221 | n=388 | n=365 | n=753 |
| Mean ± SD | 1.4±2.7 | 0.5±1.0 | 0.6±1.6 | 0.7±1.7 | 0.9±2.1 | 0.8±1.9 |
Notes: a Data on daily dosage were retrieved from medical records and categorized based on cut-offs defined by the 2018 GINA treatment recommendations (Supplementary Table 3).
Abbreviations: BEC, blood eosinophil count; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; OCS, oral corticosteroid; SD, standard deviation.
Figure 3.
Physician-reported asthma treatment patterns.a (A) Currently prescribed treatment and (B) Adherence to prescribed treatment.
Note: aThe proportion of patients on one currently prescribed treatment was calculated with the total population of each group as the denominator, stratified by BEC testing status and level.
Abbreviations: BEC, blood eosinophil count; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid.
Maintenance OCS treatment was prescribed for 8.0% (60/754) of patients and was more frequently prescribed among BEC-tested patients than those with no test: 9.0% (35/388) vs 6.8% (25/366) (Table 2). In BEC-tested patients on maintenance OCS treatment, 62.9% (22/35) of patients had elevated BEC levels (≥150 cells/μL) whilst 8.6% (3/35) did not. The mean number (SD) of OCS bursts in the last 12 months was 0.8 (1.9) for the overall population, 0.7 (1.7) for BEC-tested patients, 0.9 (2.1) for non-BEC-tested patients, 0.5 (1.0) for BEC-elevated patients, and 1.4 (2.7) for non-BEC-elevated patients (Table 2).
Physician perceived 46.2% (348/753) of the overall patients, 48.2% (187/388) of BEC-tested patients, and 44.1% (161/366) of none-BEC-tested patients to be very or completely adherent to prescribed treatments. In BEC-tested patients, 29.6% (34/115) of BEC-elevated patients and 46.2% (24/52) of non-BEC-elevated patients were perceived to be very or completely adherent to prescribed treatments (Figure 3B).
Asthma Control, Exacerbation, and HCRU
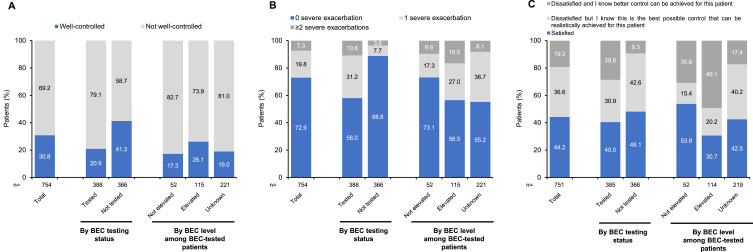
According to the adapted ATS/ERS definition, asthma was well controlled on the survey date in 30.8% (232/754) of patients; 20.9% (81/388) of BEC-tested patients and 41.3% (151/366) of non-BEC-tested patients (Figure 4A). In BEC-tested patients, asthma was well controlled in 26.1% (30/115) of BEC-elevated patients and 17.3% (9/52) of non-BEC-elevated patients (Figure 4A). By IgE testing status, 23.3% (70/301) of IgE-tested patients and 35.8% (162/453) of non-IgE-tested patients had well-controlled asthma. Additionally, asthma was well controlled in 21.5% (59/274) of patients with physician-perceived severe asthma and 36.0% (172/478) of patients with physician-perceived mild/moderate asthma.
Figure 4.
Physician-reported asthma control and exacerbation history. (A) Asthma control based on the adapted ATS/ERS taskforce definition; (B) Exacerbations leading to OCS course and/or ER visit and/or hospitalization in the last 12 months; and (C) Physician’s satisfaction with current asthma control.
Abbreviations: ATS, American Thoracic Society; BEC, blood eosinophil count; ER, emergency room; ERS, European Respiratory Society; GINA, Global Initiative for Asthma; OCS, oral corticosteroid; SD, standard deviation.
Physicians were dissatisfied with asthma control status in 55.8% (419/751) of patients, including 59.5% (229/385) of BEC-tested patients and 51.9% (190/366) of non-BEC-tested patients. In BEC-tested patients, asthma control status was deemed sub-optimal by physicians in 69.3% (79/114) of BEC-elevated patients and 46.2% (24/52) of non-BEC-elevated patients (Figure 4C).
In the last 12 months, 27.1% (204/754) of patients, including 42.0% (163/388) of BEC-tested patients and 11.2% (41/366) of non-BEC-tested patients, experienced ≥1 exacerbation that led to acute OCS treatment, emergency room (ER) visit, or overnight hospitalization (hereafter referred to as severe exacerbation) (Figure 4B). In BEC-tested patients, 43.5% (50/115) of BEC-elevated patients, compared to only 26.9% (14/52) of non-BEC-elevated patients, experienced ≥1 severe exacerbation in the last 12 months. More results on exacerbations are provided in Supplementary Table 4.
In the previous 12 months, patients visited healthcare professionals (HCPs) for a mean of 5.0 (SD, 3.6) times due to asthma. Furthermore, 22.8% (172/754) of patients experienced ≥1 hospitalization (including ER visit or overnight hospitalization) due to asthma. By BEC test at any point in history, patients with a test reported were more likely to have a hospitalization in the last 12 months (38.1%, 148/388) than those without (6.6%, 24/366). In BEC-tested patients, 41.7% (48/115) of BEC-elevated patients and only 21.2% (11/52) of non-BEC-elevated patients were hospitalized at least once due to asthma in the last 12 months (Table 3).
Table 3.
Physician-Reported Healthcare Resource Utilization per Patient Due to Asthma in the Last 12 Months Among Patients with Asthma on GINA Step 4/5 Therapies
| Characteristic | BEC Tested | BEC not Tested (N=366) | Total (N=754) | |||
|---|---|---|---|---|---|---|
| BEC<150 Cells/µL (N=52) | BEC≥150 Cells/µL (N=115) | BEC Unknown (N=221) | Subtotal (N=388) | |||
| HCP visits, mean ± SD | 4.0±2.2 | 4.2±4.3 | 6.1±3.5 | 5.3±3.8 | 4.7±3.3 | 5.0±3.6 |
| Hospitalizations (including ER visits and overnight hospitalizations), n (%) | ||||||
| 0 | 41 (78.8) | 67 (58.3) | 132 (59.7) | 240 (61.9) | 342 (93.4) | 582 (77.2) |
| 1 | 10 (19.2) | 33 (28.7) | 77 (34.8) | 120 (30.9) | 15 (4.1) | 135 (17.9) |
| ≥2 | 1 (1.9) | 15 (13.0) | 12 (5.4) | 28 (7.2) | 9 (2.5) | 37 (4.9) |
| ER visits (no overnight), mean ± SD | 0.12±0.32 | 0.16±0.51 | 0.09±0.29 | 0.11±0.37 | 0.03±0.19 | 0.07±0.30 |
| Overnight hospitalizations, mean ± SD | 0.12±0.32 | 0.44±0.65 | 0.37±0.51 | 0.36±0.54 | 0.08±0.37 | 0.22±0.49 |
| Number of in-patient nights at hospital, mean ± SD | ||||||
| Sample size | 9 | 41 | 80 | 130 | 20 | 150 |
| Mean ± SD | 10.7±8.0 | 11.7± 9.8 | 8.9±4.3 | 9.9±6.8 | 12.4±10.8 | 10.3±7.5 |
Abbreviations: BEC, blood eosinophil count; ER, emergency room; HCP, healthcare professional; SD, standard deviation.
Sensitivity Analysis
In the included patients, the distributions of city or hospital settings were similar for patients from the consecutive sample and the oversample. No major directional demographic differences were identified between the two subgroups except that the oversample subgroup was slightly older (mean age [SD], 48.7 [14.1] versus 43.4 [13.9] years). Of note, the oversample subgroup had a higher proportion of patients who had undergone a BEC test (62.7% [192/306] versus 43.8% [196/448]) or a specific IgE/RAST test (49.7% [152/306] versus 33.3% [149/448]). More patients in the oversample subgroup were prescribed maintenance OCS treatment (11.8% [36/306] versus 5.4% [24/448]), experienced severe exacerbations (51.0% [156/306] versus 10.7% [48/448]), or were hospitalized due to asthma (44.8% [137/306] versus 7.8% [35/448]) in the last 12 months, compared with the consecutive subgroup.
Discussion
Although severe asthma accounts for a significant proportion of asthma-related healthcare costs, prior studies on asthma from China have rarely focused on this population. Those which did either lacked geographic representation or focused solely on risk factors for severe asthma.29–31 To our knowledge, our study is the first to comprehensively investigate patient characteristics, treatment patterns, disease control status, and HCRU in patients on GINA Step 4/5 therapies, closely resembling the severe asthma population in China, from Chinese real-world clinical practice. Based on data from the Asthma DSP, our study revealed a low BEC and specific IgE/RAST testing frequency and data availability, an unsatisfactory asthma control level, and high HCRU burden in this patient population, highlighting the urgent need for further improvement in phenotyping, individual risk assessment, symptom control, and disease management.
Phenotyping with biomarkers is recommended for all patients with severe asthma to guide add-on treatment.8,25 However, up to 48.5% and 60.1% of patients in the current study had not received a BEC or specific IgE/RAST test, respectively, demonstrating a wide gap between clinical practice and treatment recommendations. This underutilization of biomarker testing might be largely attributed to a lack of incentive due to the limited availability of biological therapies at that time. At the conduct of the survey, omalizumab was the only biologic therapy approved for asthma in China and had been launched to the market only several months prior. Furthermore, the decreasing proportions of BEC-tested and specific IgE/RAST-tested patients from Class III hospitals to Class II hospitals and CHCs indicate that physicians from lower-ranking hospitals may be less likely to request biomarker tests and consequently also less likely to use them to guide clinical decisions. This may be attributed to the fact that physicians from lower-ranking hospitals are not fully aware of guideline recommendations and/or had limited access to some of the biomarker tests.32–34 This decreasing trend may, however, also relate to the spectrum of the severe asthma population seeking help at the different hospital levels and the proportion of patients with a reported BEC test was found to increase with the physician-perceived severity in our study.
BEC is a valuable biomarker for the management of asthma because elevated BEC levels, which are driven by interleukin-5, are associated with more frequent exacerbations, poorer disease control, and better response to T2-targeted biological therapies.11,15 The ease of acquiring BEC levels through a simple blood test further underscores its usefulness in clinical practice. Markedly, BEC results were unknown in a substantial proportion (57.0%) of BEC-tested patients, but the exact reasons for this were unavailable due to the retrospective nature of the current study. Despite the possibility that physicians who completed the PRF had no access to detailed medical records for some patients, such a high level of missing data suggests that some physicians might consider BEC not important in making clinical decisions for asthma and thus had not retained these records although patients underwent BEC testing. Additionally, asthma control level was lower in BEC-tested versus non-BEC tested patients, and in IgE-tested versus non-IgE-tested patients, indicating that some physicians may reserve biomarker testing for those with poorer disease control. With the improved availability of T2-targeted biological therapies in China, efforts should be made to increase physicians’ awareness of and adherence to the recommendations on the use of biomarkers, including BEC and IgE, so that patients could benefit from optimized asthma management.
Our study identified a high rate (69.2%) of not well controlled asthma, a considerable proportion (27.1%) of patients experiencing severe exacerbations, and a heavy HCRU burden (22.8% hospitalized due to asthma) in patients on GINA Step 4/5 therapies from China. This likely reflects the severe disease burden in this patient population, which can be attributed to several factors. Firstly, poor adherence may have contributed to the unsatisfactory disease control status, since fewer than half (46.2%) of patients in this study were perceived by physicians to adhere very well or completely to prescribed treatments. Meanwhile, the unoptimized use of available treatment options could also have played a significant role, as indicated by the rather low proportion (8.0%) of patients on GINA Step 5 therapies despite the high rate of not well-controlled asthma. Furthermore, the failure of standard therapies, in the absence of biologic therapies, likely contributed to the inadequate disease control, too. On a separate note, despite the long-term adverse effects of OCS,35 a considerable proportion of patients (8.0% overall and up to 19.1% in BEC-elevated patients) were prescribed maintenance OCS treatment, which was an add-on to consider in Step 5 in the 2018 GINA recommendations but reserved as a last resort in the 2022 GINA recommendations.8,25 Finally, inaccurate assessment of disease severity may also contribute to poor asthma control in the GINA Step 4/5 population. While all patients had severe disease according to the then GINA recommendations, up to 63.6% of them were not perceived as such by physicians and may not have received sufficient interventions. This reflects physicians’ unfamiliarity with the GINA recommendations, indicating a low probability of their adherence to GINA recommendations.
Further measures should be taken to optimize disease management in patients on GINA Step 4/5 therapies in China. Patient education is warranted to strengthen medication adherence, with additional attention to ensure optimal inhaler technique. Asthma treatment should also be fully optimized. For example, GINA step change may be considered for patients with uncontrolled asthma after assessment on adherence, symptom control, and disease severity, and alternative options like biological therapies should be considered for patients on maintenance OCS. Specifically, as per the latest guidelines, phenotype-guided use of biological therapies should be considered as an add-on for patients whose asthma cannot be well controlled with optimized standard therapies.8,9 Hence, it is advisable to assess patients’ eligibility for available biological therapies before turning to maintenance OCS treatment as last resort. Finally, the identification of limited BEC and specific IgE/RAST measurements would suggest that continuing medical education efforts are required to ensure that physicians are aware of and adhere to the latest guideline recommendations, particularly among those from lower-ranking hospitals, about the relevance of biomarker measures in asthma management.
Our study has provided a comprehensive overview of patient characteristics, treatment patterns, disease control status, and HCRU in patients on GINA Step 4/5 therapies, based on a large real-world patient sample, and added valuable insights to the understanding of the management landscape of severe asthma in China. However, this study has some limitations. Firstly, paper-based questionnaires were used for the DSP survey, which led to some missing data due to missed questions. Additionally, patients included for the current analysis might not be representative of the general GINA Step 4/5 population because they were recruited from patients who visited hospitals rather than randomly selected from the population and therefore may have more severe disease than the general GINA Step 4/5 population. Furthermore, the concentration of recruitment in major cities may limit the generalizability of study findings, especially given the substantial regional differences in economic and medical resources across China. Another limitation is the heterogeneity between the consecutive subgroup and the oversample subgroup, which may limit the generalizability of the study findings. Recall bias might also have affected responses from physicians, which is an inherent limitation of surveys. Moreover, the study did not capture how asthma diagnoses were made, so it was likely that some patients were included although they did not have asthma as defined by GINA or local guidelines. Lastly, the survey was conducted in 2018, and the temporal aspect of the data should be considered when interpreting the results; however, patient management has still remained largely suboptimal and biological therapies have not been widely used in China since then,33,36 and thus the identified gaps in the management of asthma likely still persist.
Conclusions
Although this descriptive, observational study was conducted across eight major cities and provinces in 2018, its findings may extend to the current patient population with severe asthma in China today given the continued suboptimal management of asthma since then. Significant unmet needs among patients are identified, as indicated by the high proportion of patients with poorly controlled asthma and the frequent occurrence of severe exacerbations. HCPs continue to face challenges in managing severe asthma, which is evident from their dissatisfaction with control achieved. The healthcare system still bears a heavy HCRU burden (with the associated costs incurred), given the considerable proportion of hospitalized patients. These findings highlight the need for prompt action to support improving the management of severe asthma in China. Our study also reveals that BEC testing was underutilized, and among those who underwent BEC testing, more than half did not have available BEC records. Furthermore, BEC testing was most likely conducted for those with physician-perceived severe disease or seeking medical help from Class III hospitals. Given the potential of this simple test in guiding treatment, it is recommended that education be enhanced to include BEC testing as part of the clinical and biomarker-based phenotyping of patients with severe asthma in China.
Acknowledgments
The authors would like to thank medical writing support for the development of this manuscript (in the form of manuscript development, collating author comments, and grammatical editing), under the direction of the authors, provided by Yunshu Zhang and Jinlong Guo (Costello Medical). The medical writing support was funded by GSK. Part of the results of this paper were presented at the European Respiratory Society (ERS) International Congress 2022, Barcelona, Spain, 4–6 September 2022, the 2022 Annual Congress of Chinese Thoracic Society (CTS), virtual, China, 8–11 December 2022, and the ERS International Congress 2023, Milan, Italy, 9–13 September 2023, as poster presentations with interim findings. The poster abstract for the 2022 ERS congress was published in “ERS International Congress 2022 abstracts” in European Respiratory Journal, Volume 60, Issue suppl 66: 2131; DOI: 10.1183/13993003.congress-2022.2131. The poster abstract for the 2022 CTS congress (PU-1175) was published on Pages 1505–1506 in the CTS 2022 abstract book, which can be accessed via https://cts2022.sciconf.cn/cn/web/jump/13474?mid=1348472. The poster abstract for the 2023 ERS congress was published in “ERS International Congress 2023 abstracts” in European Respiratory Journal, Volume 62, Issue suppl 67: PA2062; DOI: 10.1183/13993003.congress-2022.2131.
Funding Statement
This study was funded by GSK (study identifier: 214911). The sponsor was involved in the analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Abbreviations
ATS, American Thoracic Society; BEC, blood eosinophil count; CHC, community health centers; COPD, Chronic Obstructive Airways Disease; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; DSP, Disease-Specific Program; EphMRA, European Pharmaceutical Market Research Association; ER, emergency room; ERS, European Respiratory Society; ESOMAR, European Society for Opinion and Market Research; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GINA, Global Initiative for Asthma; HCP, healthcare professional; HCRU, healthcare resource utilization; ICC, International Chamber of Commerce; ICS, inhaled corticosteroid; ICU, intensive care unit; IgE, immunoglobulin E; IRB, Institutional Review Board; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; PRF, patient record form; PSC, patient self-completion; RAST, radioallergosorbent test; SABA, short-acting beta-agonist; SEC, sputum eosinophil count; SD, standard deviation.
Data Sharing Statement
The datasets used and/or analyzed during the current study were made fully available to all authors but are not publicly available and remain the intellectual property of Adelphi Real World. Requests to access to the data can be made by contacting the corresponding author.
Disclosure
VB, ZT, TY, PH, AR, and RAC are employees of GSK. VB, ZT, PH, AR, and RAC hold stocks and shares of GSK. JS was an employee of Adelphi Real World at the time of the study conduct, and AH and MS are employees of Adelphi Real World. The authors report no other conflicts of interest in this work.
References
- 1.Chen ZH, Wang PL, Shen HH. Asthma research in China: a five-year review. Respirology. 2013;18(Suppl 3):10–19. doi: 10.1111/resp.12196 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/s0140-6736(19)31147-x [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Dixey P, Abubakar-Waziri H, et al. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin Med J. 2022;135(10):1141–1155. doi: 10.1097/cm9.0000000000001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howraman M, Louise Lindhardt T, Pradeesh S, Truls Sylvan I, Jensen J-US. Recent developments in the management of severe asthma. Breathe. 2022;18(1):210178. doi: 10.1183/20734735.0178-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17(2):74–80. doi: 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi: 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention (updated 2022); 2022. Available from: https://ginasthma.org/gina-reports/. Accessed January 23, 2023.
- 9.Asthma Group of Chinese Throacic Society. Guidelines for bronchial asthma prevent and management (2020 edition) Asthma Group of Chinese Thoracic Society. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(12):1023–1048. doi: 10.3760/cma.j.cn112147-20200618-00721 [DOI] [PubMed] [Google Scholar]
- 10.Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi: 10.1016/j.chest.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 11.Azim A, Newell C, Barber C, et al. Clinical evaluation of type 2 disease status in a real-world population of difficult to manage asthma using historic electronic healthcare records of blood eosinophil counts. Clin Exp Allergy. 2021;51(6):811–820. doi: 10.1111/cea.13841 [DOI] [PubMed] [Google Scholar]
- 12.Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 13.Charles D, Shanley J, Temple SN, Rattu A, Khaleva E, Roberts G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: a systematic review and meta-analysis. Clin Exp Allergy. 2022;52(5):616–627. doi: 10.1111/cea.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagase H, Suzukawa M, Oishi K, Matsunaga K. Biologics for severe asthma: the real-world evidence, effectiveness of switching, and prediction factors for the efficacy. Allergol Int. 2023;72(1):11–23. doi: 10.1016/j.alit.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 15.Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634. doi: 10.1183/13993003.00634-2017 [DOI] [PubMed] [Google Scholar]
- 16.GSK. Nucala (mepolizumab) approved in China for use in severe asthma with an eosinophilic phenotype. Available from: https://www.gsk.com/en-gb/media/press-releases/nucala-mepolizumab-approved-in-china-for-use-in-severe-asthma-with-an-eosinophilic-phenotype/. Accessed January 19, 2024.
- 17.Su N, Zhi L, Liu F, et al. Real-world safety and effectiveness of omalizumab in moderate to severe allergic asthma patients in China: a post-authorization study. J Asthma Allergy. 2023;16:625–636. doi: 10.2147/jaa.S406628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AstraZeneca. Fasenra approved in China for the treatment of severe eosinophilic asthma. Available from: https://www.astrazeneca.com/media-centre/press-releases/2024/fasenra-approved-in-china-for-the-treatment-of-severe-eosinophilic-asthma.html. Accessed September 10, 2024.
- 19.Efficacy and safety study of dupilumab in patients with persistent asthma; 2022. Available from: https://www.clinicaltrials.gov/study/NCT03782532. Accessed October 13, 2023.
- 20.Study to evaluate tezepelumab in adults with severe uncontrolled asthma (DIRECTION); 2023. Available from: https://www.clinicaltrials.gov/study/NCT03927157. Accessed October 13, 2023.
- 21.Lee LK, Ramakrishnan K, Safioti G, Ariely R, Schatz M. Asthma control is associated with economic outcomes, work productivity and health-related quality of life in patients with asthma. BMJ Open Respir Res. 2020;7(1). doi: 10.1136/bmjresp-2019-000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilke T, Timmermann H, Mueller S, et al. Association between asthma control and healthcare costs: results from a German linked data study. Health Serv Manage Res. 2023;36(1):42–50. doi: 10.1177/09514848221100749 [DOI] [PubMed] [Google Scholar]
- 23.Doz M, Chouaid C, Com-Ruelle L, et al. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med. 2013;13:15. doi: 10.1186/1471-2466-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040 [DOI] [PubMed] [Google Scholar]
- 25.Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention (updated 2018); 2018. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf. Accessed January 23, 2023.
- 26.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 27.Cloutier MM, Baptist AP, Blake KV, et al. 2020 focused updates to the asthma management guidelines: a report from the national asthma education and prevention program coordinating committee expert panel working group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi: 10.1016/j.jaci.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNICOM® Intelligence 7.5; 2017. Available from: https://www.unicomsi.com/products/unicom-intelligence/. Accessed October 12, 2024.
- 29.Wang G, Wang F, Gibson PG, et al. Severe and uncontrolled asthma in China: a cross-sectional survey from the Australasian Severe Asthma Network. J Thorac Dis. 2017;9(5):1333–1344. doi: 10.21037/jtd.2017.04.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su N, Lin JT, Wang WY, et al. A cross-section study of severe asthma in eight provinces of China. Zhonghua Nei Ke Za Zhi. 2016;55(12):917–921. doi: 10.3760/cma.j.issn.0578-1426.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Wang WY, Lin JT, Zhou X, et al. A survey on clinical characteristics and risk factors of severe asthma in China. Zhonghua Yi Xue Za Zhi. 2020;100(14):1106–1111. doi: 10.3760/cma.j.cn112137-20191117-02497 [DOI] [PubMed] [Google Scholar]
- 32.Asthma Workgroup, Chinese Thoracic Society, and Chinese Society of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version). J Thorac Dis. 2013;5(5):667–677. doi: 10.3978/j.issn.2072-1439.2013.10.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Lai K. Enhancing standardized treatment of bronchial asthma in Chinese primary hospitals (in Chinese). Guo Ji Hu Xi Za Zhi. 2023;43(12):1378–1382. doi: 10.3760/cma.j.cn131368-20231119-00343 [DOI] [Google Scholar]
- 34.Qiu J-X, Li F, Liang X-J, Liu J-D, Lu C-Q, Lin M. Analysis of diagnosis and treatment of adults with bronchial asthma in primary hospital (in Chinese). J Clin Pulm Med. 2019;24(1):74–78. doi: 10.3969/j.issn.1009-6663.2019.01.019 [DOI] [Google Scholar]
- 35.Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi: 10.1183/13993003.00703-2018 [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, He X, Wu J. RWD177 treatment pattern and disease burden of severe asthma: a real-world study based on claims data in China. Value Health. 2024;27(6):S391. doi: 10.1016/j.jval.2024.03.2474 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study were made fully available to all authors but are not publicly available and remain the intellectual property of Adelphi Real World. Requests to access to the data can be made by contacting the corresponding author.