Abstract
Background
The opioid epidemic has severely impacted the US over the last 15 years. Buprenorphine is a partial opioid agonist indicated for the treatment of opioid use disorder (OUD) and is recognized as an effective treatment when taken as prescribed. However, adherence rates have been low in real-world settings. Blister-packaging has been shown to promote medication adherence across a variety of disease states, although it has never been studied in OUD.
Methods
An economic analysis was conducted to assess the impact of increased adherence of blister-packaged buprenorphine on health care resource utilization (HCRU) and health care costs for 10,000 patients initiating therapy for OUD. The model analyzed a commercially insured population within the US over a one-year time horizon. Medication adherence was defined in the model as proportion of days covered (PDC) of at least 80%. Literature-based references were used to inform both the impact of blister-packaging on the number of patients who became adherent as well as the impact of medication adherence on HCRU and health care costs. Model input uncertainty was assessed in one-way sensitivity analyses.
Results
With the implementation of blister-packaging buprenorphine, adherence rates increased from 37.1% of patients in the pre-intervention period to 45.3%, resulting in an additional 818 patients becoming adherent post-intervention. The increase in adherence led to a reduction of medical costs of $12,138,757 (-$1,214 per-patient (PP)). Specifically, inpatient costs decreased by $7,127,073 (-$713 PP) while outpatient costs decreased by $5,013,319 (-$501 PP). Pharmacy costs increased by $3,432,705 ($343 PP). Despite the increase in pharmacy costs, total health care costs saw a reduction of $8,559,684 (-$856 PP).
Conclusion
Blister-packaging buprenorphine for treatment of OUD has potential to improve medication adherence and health outcomes while reducing HCRU and health care costs. Future studies are necessary to assess the real-world application and impact of blister-packaging buprenorphine for OUD across various patient populations and health care settings.
Keywords: blister-packaging, blister-packing, medication adherence, buprenorphine, health care costs, medication adherence packaging, opioid use disorder
Plain Language Summary
The opioid epidemic has taken its toll in the United States for the last several decades. Several medications are available in the treatment of opioid use disorder (OUD), including buprenorphine. Buprenorphine has shown to be an effective treatment when taken as prescribed, however, in real-world settings, the occurrence of patients taking buprenorphine as prescribed is low. Blister-packaging medications has been shown to help promote medication adherence across a variety of disease states, although it has never been studied in OUD. As a result, we attempted to model the potential impact that blister-packaging buprenorphine for 10,000 commercially insured patients initiating treatment for OUD would have on health care resource utilization and health care costs using the best available peer-reviewed literature. This analysis saw adherence rates increased from 37.1% of patients in the pre-blister-packaging period to 45.3% post-blister-packaging period, resulting in an additional 818 patients becoming adherent. The increase in adherence led to reductions of medical costs of $12,138,757 (-$1214 per-patient (PP)). Specifically, inpatient costs decreased by $7,127,073 (-$713 PP) while outpatient costs decreased by $5,013,319 (-$501 PP). Pharmacy costs increased by $3,432,705 ($343 PP). Despite the increase in pharmacy costs, total health care costs saw a reduction of $8,559,684 (-$856 PP). Future studies are necessary to assess the real-world application and impact of blister-packaging buprenorphine for OUD across various patient populations and health care settings.
Introduction
The opioid epidemic has severely impacted the United States (US) over the last 15 years. Fatalities from opioid overdoses in the US have increased from 21,089 in 2010 to 47,600 in 2017.1 While fatal opioid overdoses decreased slightly in 2018 for the first time since 1990,2 they subsequently increased to record highs of over 50,000 in 2019, 68,630 in 2020, and 80,411 in 2021.1 Consequently, opioid overdose deaths in 2021 were more than 8 times higher than in 1990.2 From 2011 through 2021, approximately 17 million years of life were lost due to these opioid overdoses.3 Despite extensive measures implemented at state and national levels to mitigate the epidemic – such as reclassifying hydrocodone,4 restricting prescribing of long-acting opioids to patients not classified as opioid-naïve,5,6 limiting prescription quantity and day supply of initial opioid prescriptions for opioid-naïve patients,7 adding black-box warnings to all opioids and central nervous system depressants cautioning about their concurrent use,8 making naloxone available over-the-counter,9 and the increased utilization of prescription drug monitoring programs (PDMP),10,11 along with many others – fatal opioid overdoses have continued to increase annually due in part to nonprescribed opioids being contaminated with more potent substances including fentanyl and carfentanyl.2
Buprenorphine is a partial opioid agonist that is indicated for both the treatment of opioid use disorder (OUD) and the treatment of chronic pain severe enough to require opioid therapy.12,13 Additionally, buprenorphine is classified as a Schedule III controlled substance by the US Drug Enforcement Administration (DEA). While certain buprenorphine formulations are indicated only for OUD and others are indicated only for treatment of chronic pain, these formulations are commonly prescribed off-label for either condition.14,15 Up through 2023, the only prescribers who could prescribe patients buprenorphine products were those who were state licensed and registered with the DEA and received a waiver from the Substance Abuse and Mental Health Services Administration (SAMHSA) after completing specific certification training and requirements.16 Additionally, prescribers were limited to treating a maximum number of patients.16 These policies have restricted patient access to buprenorphine leading to its underutilization in the treatment of OUD.17–20 However, as part of the Consolidated Appropriations Act of 2023, the federal requirement for practitioners needing a special waiver to prescribe buprenorphine has been eliminated, expanding its potential prescribing and access.21 Despite these changes, barriers still exist limiting initiation of medications for OUD (MOUD) for patients in need.22
Additional MOUD are available and include methadone and naltrexone.23–26 Although these additional treatments are potential options and have been shown to be effective, they have some administration limitations that prevent them from being utilized on a large population level in the US. Methadone when used to treat OUD can only be dispensed in oral formulations by opioid treatment programs that are certified by the SAMHSA.26 Patients are typically required to go to their clinic every day to receive their methadone dosage under direct supervision of the staff at the clinic,27 which can be disruptive to patients’ lives leading to low uptake rates.28 However, in some instances, patients can receive take home supplies of methadone for treatment of OUD, with the settings and supplies ranging from 7 to 28 unsupervised days of treatment depending on the state.29 While naltrexone does have an oral formulation, it is only recommended in limited circumstances, and its extended-release injectable formulation is preferred over the oral formulation due to improved adherence.30 Studies indicate that extended-release injectable naltrexone has lower rates of adherence compared to buprenorphine.31
MOUD, and specifically buprenorphine, have been demonstrated as an effective treatment for OUD when taken as prescribed.32–35 However, adherence to these therapies have been low in real-world settings, with literature estimates ranging from 32% to 49% of patients.32–35 Reasons for low adherence in this population include social and structural factors including homelessness and mental illness, refraining to take medication in order to use illicit drugs, and forgetting to take their medication.36 Moreover, greater than 50% of patients who discontinue buprenorphine therapy resume opioids within one month of discontinuation.37 These patients often turn to nonprescribed opioids38 which can be contaminated with fentanyl or carfentyl, increasing the risk of opioid overdose.39 An analysis of fatal opioid overdoses in Rhode Island (RI) from 2018 through 2020 found that 7.5% of overdose deaths were among patients with OUD who discontinued MOUD therapy within 30 days of their death.40
Given the high risk of adverse outcomes with poor adherence of MOUD, assessing initiatives to enhance medication adherence is critical. Although various interventions have proven effective in improving medication adherence,41,42 medication adherence packaging, specifically blister-packaging, has been utilized for decades as a method to help promote medication adherence. While specific studies on the impact of blister-packaging on medication adherence and health outcomes for buprenorphine are lacking, research has shown that blister-packaging has increased medication adherence for other chronic conditions, including in behavioral health.43 Consequently, this study aims to model the potential impact of blister-packaging buprenorphine for the treatment of OUD to evaluate its potential use in this population.
Methods
Study Design
An economic and epidemiologic analysis was conducted to assess the impact of increased adherence of blister-packaged buprenorphine on health care resource utilization (HCRU) and health care costs for patients initiating therapy for OUD. The model analyzed a commercially insured population within the United States health care system over a one-year time horizon (Table 1). Medication adherence was defined in the model as a proportion of days covered (PDC) of at least 80%. The model utilized data on the impact of adherence to buprenorphine on HCRU and health care costs from a retrospective claims analysis of the MarketScan Commercial Claims database by Ronquest et al.32 This model followed best practices for building and reporting on economic models.44–47
Table 1.
Model Inputs and Parameters
| Model Parameters | Inputs | |
|---|---|---|
| Time-Horizon | 1 Year | |
| Number of Patients | 10,000 | |
| Percent Adherent at Baseline | 37.1% | |
| Increase in Patients Adherent due to Blister-Packaging | 13% | |
| Relapse-specific HCRU: | Adherent | Non-Adherent |
| OUD-HCRU | ||
| Continuous episodic dependence | 2.7% | 1.9% |
| Inpatient admission, primary diagnosis OUD | 3.8% | 15.9% |
| Detox, any diagnosis opioid use | 4.8% | 15.9% |
| ED visit, any diagnosis opioid use | 3.6% | 9.7% |
| Composite relapse | 11.4% | 26.3% |
| All-Cause HCRU | ||
| Inpatient admission | 17.9% | 40.1% |
| ED visit | 10.0% | 51.9% |
| Mean outpatient office visits | 13.2 | 10.0 |
| Mean Rx Claims | 42.3 | 35.3 |
| All-Cause Healthcare Costs | ||
| Inpatient Costs | $3025 | $11,741 |
| Outpatient Costs | $10,697 | $16,828 |
| --ED Costs | $713 | $1,703 |
| --Outpatient office visit costs | $1783 | $1423 |
| --Other Outpatient costs | $8202 | $13,703 |
| *Medical Costs | $13,724 | $28,569 |
| Pharmacy Costs | $9446 | $5248 |
| Total Healthcare Costs | $23,351 | $33,819 |
Notes: --Included in outpatient costs (ED costs, outpatient office visit costs, other outpatient costs). *Medical costs include both inpatient costs and outpatient costs.
Abbreviations: ED, emergency department; HCRU, healthcare resource utilization; OUD, opioid use disorder; Rx, prescription.
Population
The analysis focused on commercially insured patients newly initiating oral buprenorphine for treatment for OUD in the outpatient setting. The base case model assessed a population size of 10,000 patients and assumed that 37.1% of patients were adherent in the pre-implementation period, based on the adherence rate of commercially insured patients initiating buprenorphine in the Ronquest et al study, with the patient demographics from this study available in Supplemental Table 1. 32 The Ronquest et al study also assessed patients in a Medicaid population initiating buprenorphine.32 However, they found contradictory results on impact of adherence to HCRU and health care costs likely due to differences in comorbid conditions and confounding factors.32 Consequently, this model focused solely on the commercial population to minimize variability and to have a clearly representative population. While Medicaid represents the largest payer of patients with OUD in the U.S.48 (44%), which is greater than 1.5 times that of commercial insurance (24%),49 commercial insurance still represents a significantly large population of patients with OUD. In 2022, there were an estimated 9.4 million adults who needed OUD treatment in the US, with 2.4 million receiving MOUD.50 This would equate to commercial insurance covering 2.2 million adults needing OUD treatment, and 564,720 receiving MOUD in 2022.
Intervention
The primary focus of this analysis was the use of blister-packaging oral buprenorphine for patients initiating therapy for OUD, compared to traditional oral buprenorphine vial-filled packaging. The impact of blister-packaging on patients becoming adherent was derived from a retrospective pharmacoepidemiologic analysis of data from a national pharmacy chain from December 2006 through July 2009.51 This study evaluated the impact of patients receiving blister-packaged angiotensin-converting enzyme inhibitors (ACEi) on the likelihood of patients becoming adherent across different definitions of adherence, utilizing PDC greater than or equal to 80%.51 Their propensity-score matched analysis revealed that patients who initiated therapy with blister-packs had an adjusted odds ratio (aOR) of 1.13 (95% confidence interval (CI): 1.05–1.21) of becoming adherent with a definition of at least 80% PDC.51 The base-case analysis therefore assumed that blister-packaging would result in a 13% increase in patients being adherent to oral buprenorphine for treatment of OUD. Although this source study assessed ACEi and not buprenorphine directly, it is the best available evidence on the impact of blister-packaging medications on patients becoming adherent and provides an input in this hypothesis-generating economic model. The forthcoming one-way sensitivity analysis explored the potential variability of these results when applied to this population.
Outcomes
This analysis aimed to model the impact of increased medication adherence from blister-packaging therapy on disease-specific and all-cause HCRU and all-cause health care costs based on the data available in the Ronquest et al study.32
Health Care Resource Utilization
HCRU included both relapse-specific HCRU and all-cause HCRU. Relapse-specific HCRU encompassed continuous episodic dependence, inpatient admission with a primary OUD diagnosis, detoxification with any diagnosis related to opioid use, emergency department (ED) visit with any diagnosis related to opioid use, and a composite endpoint of any of the above-mentioned relapse-specific outcomes. All-cause HCRU consisted of inpatient admissions, ED visits, number of physician office visits, and number of outpatient prescription claims.
Costs
Health care costs included all-cause medical costs, all-cause outpatient prescription costs, and all-cause total health care costs. Medical costs consisted of inpatient costs and outpatient (ED costs, outpatient physician office visit costs, and other outpatient costs) costs. The HCRU outcomes were evaluated both on the aggregate level and by calculating the percentage of patients with utilization. The health care costs were assessed on the aggregate level, the mean cost per patient (aggregate cost divided by all patients in the model), as well as per-patient per-month (PPPM) impact. PPPM was calculated by dividing the total aggregate cost by the number of patients in the model and then dividing that result by 12 months. Costs were adjusted to 2024 US dollars using the US medical consumer price index (CPI).52
Sensitivity Analysis
To address model uncertainty, one-way sensitivity analyses were conducted for every input parameter in the model to show the potential variation of said outcome in the model. Upper and lower bounds for inputs were derived from peer-reviewed literature-based references where available (Supplemental Table 2).34,35,53 When inadequate data was available in the literature to inform the upper and lower bounds, the remaining parameter values were varied by ± 20%.53
Results
The economic model analyzed 10,000 patients newly initiating buprenorphine for the treatment of OUD. With the implementation of blister-packaging buprenorphine, adherence rates increased from 37.1% of patients in the pre-intervention period to 45.3%, resulting in an additional 818 patients becoming adherent post-intervention.
In terms of relapse-specific HCRU, the intervention slightly increased continuous episodic dependence from 2.2% to 2.3% (220 to 226) (Table 2). However, all other relapse-specific HCRU measures saw reductions. It decreased the percentage of patients who had an inpatient admission with a primary diagnosis of OUD from 11.4% to 10.4%. Detox events with any diagnosis of opioid decreased from 11.8% of patients pre-intervention to 10.9% of patients post-intervention. Emergency department visits with any diagnosis of opioid use saw a reduction from 7.4% to 6.9%. The composite endpoint of relapse decreased by 1.2 percentage points from 20.8% to 19.6% of patients.
Table 2.
Impact of Blister-Packing on Medication Adherence, Healthcare Resource Utilization, and Healthcare Costs
| Pre-Blister Packaging | Post-Blister Packaging | Difference Post-Pre | ||||
|---|---|---|---|---|---|---|
| Aggregate | Percent/Mean | Aggregate | Percent/Mean | Aggregate | Percent/Mean | |
| Number of patients adherent | 3710 | 37.1% | 4528 | 45.3% | 818 | 8.2 percentage points |
| Relapse-Specific Healthcare Resource Utilization | ||||||
| Continuous episodic dependence | 220 | 2.2% | 226 | 2.3% | 7 | 0.1% |
| Inpatient admission, primary diagnosis OUD | 1141 | 11.4% | 1042 | 10.4% | −99 | −1.0% |
| Detox, any diagnosis opioid use | 1178 | 11.8% | 1087 | 10.9% | −91 | −0.9% |
| ED visit, any diagnosis opioid use | 744 | 7.4% | 694 | 6.9% | −50 | −0.5% |
| Composite relapse | 2077 | 20.8% | 1955 | 19.6% | −122 | −1.2% |
| All-Cause Healthcare Resource Utilization | ||||||
| Inpatient admission | 3186 | 31.9% | 3005 | 30.0% | −182 | −1.8% |
| ED visit | 3894 | 38.9% | 3293 | 32.9% | −601 | −6.0% |
| Mean outpatient office visits | 111,872 | 11.19 | 114,489 | 11.45 | 2617 | 0.26 |
| Mean prescription claims | 378,970 | 37.90 | 384,694 | 38.47 | 5724 | 0.57 |
| All-Cause Healthcare Costs | ||||||
| Inpatient Costs | $85,073,640 | $8507 | $77,946,567 | $7795 | -$7,127,073 | -$713 |
| ^Outpatient Costs | $145,533,990 | $14,553 | $140,520,671 | $14,052 | -$5,013,319 | -$501 |
| --ED Costs | $13,357,100 | $1336 | $12,547,577 | $1255 | -$809,523 | $81 |
| --Outpatient office visit cost | $15,565,600 | $1557 | $15,859,972 | $1586 | $294,372 | $29 |
| --Other Outpatient costs | $116,621,290 | $11,662 | $112,123,122 | $11,212 | $ (4,498,168) | -$450 |
| *Medical Costs | $230,615,050 | $23,062 | $218,476,294 | $21,848 | $(12,138,757) | -$1214 |
| Pharmacy Costs | $68,054,580 | $6,805 | $71,487,285 | $7,149 | $ 3,432,705 | $343 |
| Total Healthcare Costs | $299,353,720 | $29,935 | $290,794,036 | $29,079 | $ (8,559,684) | -$856 |
Notes: ^Outpatient costs include ED costs, outpatient office visit costs, and other outpatient costs. *Medical costs include inpatient costs and outpatient costs.
Abbreviations: ED, emergency department; HCRU: healthcare resource utilization; OUD, opioid use disorder.
When assessing all-cause HCRU, both inpatient admissions and ED visits showed significant shifts, with inpatient admissions having a 1.8 percentage point decrease (31.9% to 30.0%) and ED visits having a 6.0 percentage point decrease (38.9% to 32.9%). While inpatient admissions and ED visits saw reductions, the mean number of outpatient office visits increased from 11.19 to 11.45 while the mean number of prescription claims increased from 37.90 to 38.47.
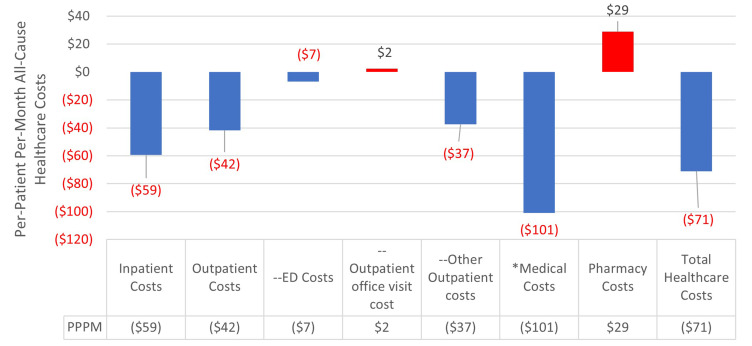
The increase in patient adherence due to blister-packaging led to reductions of medical costs of $12,138,757 (-$1214 per patient (PP)) from $230,615,050 pre-implementation to $218,476,294 post implementation of blister-packaging buprenorphine. Specifically, inpatient costs decreased by $7,127,073 (-$713 PP) while outpatient costs decreased by $5,013,319 (-$501 PP). Pharmacy costs increased by $3,432,705 ($343 PP) from $68,054,580 to $71,487,285. Despite the increase in pharmacy costs, total health care costs saw a reduction of $8,559,684 (-$856 PP; -$71 PPPM) from $299,353,720 pre-blister-pack to $290,794,036 post-blister-pack (Figure 1).
Figure 1.
Impact on Per-Patient Per-Month All-Cause Healthcare Costs Post-Implementation of Blister-Packaging Buprenorphine for the Treatment of Opioid Use Disorder.
Notes: --Included in outpatient costs (ED costs, outpatient office visit costs, other outpatient costs). *Medical costs include both inpatient costs and outpatient costs. ^Red text signifies cost savings.
Abbreviation: ED: emergency department.
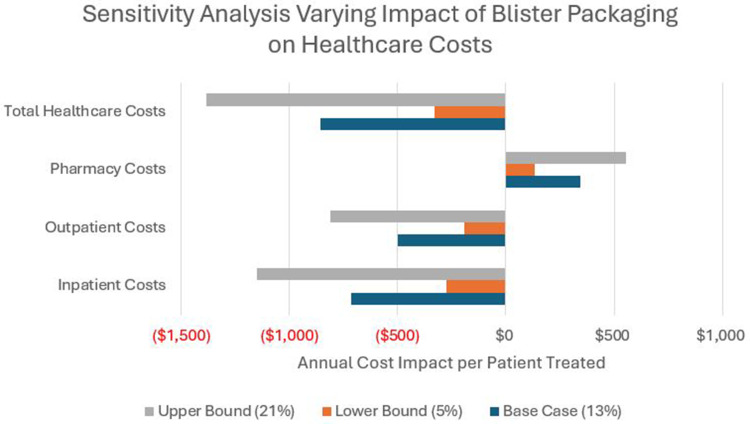
Sensitivity analyses indicated potential variation in outcomes in the model (Table 3). Inpatient admissions in the model ranged from a reduction of 153 to a reduction of 207, whereas ED visits varied from a reduction of 566 to a reduction of 866. Varying total health care costs reflected a range from a reduction of $4,977,340 (-$498 PP) to -$11,585,174 (-$1,159 PP). The impact of varying the percentage of patients who became adherent on total health care costs showed variability ranging from -$3,292,186 (-$329) to -$13,827,181 (-$1,383 PP) (Figure 2).
Table 3.
Sensitivity Analysis Results
| Base Case Difference Post-Pre | Lower Bounds Difference Post-Pre | Upper Bounds Difference Post-Pre | ||||
|---|---|---|---|---|---|---|
| Aggregate | Percent/ Mean | Aggregate | Percent/ Mean | Aggregate | Percent/ Mean | |
| Number of patients adherent | 818 | 8.2 percentage points | 883 | 8.8 percentage points | 671 | 6.7 percentage points |
| Relapse-Specific Healthcare Resource Utilization | ||||||
| Continuous episodic dependence | 7 | 0.1% | 3 | <0.1% | 11 | 0.1% |
| Inpatient admission, primary diagnosis OUD | −99 | −1.0% | −87 | −0.8% | −105 | −1.0% |
| Detox, any diagnosis opioid use | −91 | −0.9% | 124 | 1.2% | −99 | −1.0% |
| ED visit, any diagnosis opioid use | −50 | −0.5% | −46 | −0.4% | −56 | −0.6% |
| Composite relapse | −122 | −1.2% | −103 | −1.0% | −141 | −1.4% |
| All-Cause Healthcare Resource Utilization | ||||||
| Inpatient admission | −182 | −1.8% | −153 | −1.6% | −207 | −2.1% |
| ED visit | −601 | −6.0% | −866 | −8.6% | −566 | −5.7% |
| All-Cause Healthcare Costs | ||||||
| Inpatient Costs | -$7,127,073 | -$713 | -$5,617,599 | -$562 | -$7,621,782 | -$762 |
| Outpatient Costs | -$5,013,319 | -$501 | -$4,988,788 | -$499 | -$7,000,330 | -$700 |
| Pharmacy Costs | $3,432,705 | $343 | $3,090,088 | $309 | $4,977,340 | $498 |
| Total Healthcare Costs | -$8,559,684 | -$856 | -$4,977,340 | -$498 | -$11,585,174 | -$1159 |
Abbreviations: ED, emergency department; OUD, opioid use disorder.
Figure 2.
Results of Sensitivity Analysis Varying the Impact of Blister-Packaging Buprenorphine on the Percentage of Patients Becoming Adherence and the Impact on Annual Healthcare Costs per Patient Treated.
Notes: ^Red text signifies cost savings.
Discussion
This economic and epidemiologic model evaluated the potential impact of increasing medication adherence to buprenorphine for the treatment of OUD through blister-packaging on HCRU, health outcomes, and health care costs in a commercially insured population. For a hypothetical 10,000 patients receiving buprenorphine for OUD, the model showed blister-packaging buprenorphine provided increases in prescription fills and outpatient provider visits, while also highlighting significant reductions in inpatient and ER visits. These changes collectively led to a decrease in total health care costs of greater than $8.5 million.
In addition to the source reference used in this economic analysis, further studies have also shown the impact of medication adherence on HCRU, health outcomes, and health care costs for patients utilizing buprenorphine and MOUD in the treatment of OUD. A propensity-scored claims analysis of the Truven Health Marketscan Research Database was conducted to assess the impact of buprenorphine adherence (defined as PDC of at least 80%) on HCRU and costs in a commercially insured population of patients newly initiating buprenorphine/naloxone for the treatment of OUD.34 This analysis revealed that patients who were adherent to buprenorphine had significantly lower adjusted odds of all-cause health care events (aOR: 0.62, 95% CI: 0.52–0.74), all-cause ED visits (aOR: 0.70, 95% CI: 0.58–0.84), all-cause inpatient admissions (aOR: 0.43, 95% CI: 0.34–0.54), OUD health care events (aOR: 0.44, 95% CI: 0.31–0.63), OUD ED visits (aOR: 0.42, 95% CI: 0.19–0.97) and OUD inpatient admissions (aOR: 0.33, 95% CI: 0.22–0.51).34 Additionally, patients who were adherent had significantly lower mean PPPM inpatient costs ($334.59 vs $759.10, p<0.001) and outpatient costs ($627.11 vs $1,189.85, p<0.001).34 A retrospective claims analysis of Aetna pharmacy and medical claims data of fully insured HMO members from January 2007 through September 2012, further validated these findings, showing that buprenorphine-adherent patients incurred significantly lower total health care charges.35 After adjusting for covariates, they found that patients who were adherent had significantly lower mean inpatient hospitalizations (0.52 vs 1.41, p<0.001), inpatient days (3.7 vs 10.0), ED visits (0.78 vs 1.61), and total health care charges ($38,458 vs $49,051, p<0.001).35 Moreover, a retrospective claims analysis by Kinsky et al of UPMC Medicaid data for the calendar years 2015 through 2016 evaluated predictors of medication adherence in patients initiating OUD treatment with methadone and buprenorphine as well as the relationship between nonadherence (defined has medication coverage gap more than 10 consecutive days over course of 6 months) and health outcomes.33 They found that patients who were adherent to MOUD for 6 months had a significantly lower adjusted hazard ratio (aHR) for time to nonfatal drug overdose (aHR: 0.290, 95% CI: 0.176–0.477).33 When assessing the impact of medication adherence on cost, they found nonsignificant differences in PPPM costs for patients utilizing buprenorphine (adherent: $553; nonadherent: $476), but significantly higher PPPM costs for nonadherent methadone patients ($1174) compared to adherent methadone patients ($13).33
While MOUD have been shown to be effective in treating OUD, leading to improved quality of life and a reduction in opioid relapse, HCRU, costs, and overdose,54 utilization of MOUD remains relatively low, with just under 28% of patients with OUD in 2019 receiving MOUD55 and 22.3% of patients in 2021.56 However, given the high prevalence of OUD in the United States and the increased access potential for buprenorphine following the removal of the prescribing waiver, there is potential for an increase in patients initiating therapy for OUD in the coming years. Health-system and statewide policies and strategies have been implemented in the last several years to try to initiate OUD therapy sooner, including as part of ED discharges for opioid overdose.57–59 With more patients set to receive MOUD, it is imperative to utilize different strategies to help promote medication adherence to ensure the best chance of treatment success.
This analysis is based on the results of the commercially insured population of the Ronquest et al study.32 While patients with Medicaid insurance who had lower PDC rates had higher rates of relapse (adjusted odds of relapse compared to PDC≥80% ranging from aOR 1.48 to 1.90 depending on the adherence threshold), the impact on health care costs were not statistically different.32 Some reasons the authors give for the differences seen between the Medicaid and Commercial groups were that the Medicaid population was more heterogeneous in nature with more preexisting comorbid conditions requiring a personalized approach to OUD management. Additionally, Medicaid patients sometimes struggle with restricted provider and care continuum networks and have limited access to treatment. These results are similar to those in the above-mentioned study by Kinsky et al, which found that in a Medicaid population, while patients adherent to buprenorphine had significantly lower time to ED nonfatal drug overdose (aHR: 0.290, 95% CI: 0.176–0.477), there was no significant difference in health care costs between the two groups.33 Therefore, it is important to note that this analysis assessed the impact in a commercial population and the results would not be generalizable to a Medicaid population.
There are numerous factors that contribute to poor adherence in treating OUD, including a high rate of comorbid severe mental health conditions.60 An analysis of the National Epidemiologic Survey on Alcohol and Related Conditions indicated that patients with OUD had higher rates of anxiety disorders (36.3%) than the general population (16.2%).61 Patients with mental health conditions as well as patients with concurrent chronic conditions have historically shown lower medication adherence compared to other chronic conditions.62 A retrospective analysis of MarketScan data from 2012 through 2014 of patients utilizing buprenorphine found high prevalence rates of comorbid non-specific mental health disorders (32.5%), anxiety (22.0%), major-depressive disorder or bipolar disorder (MDDBP) (15.9%), and schizophrenia or other psychosis (2.4%).63 This study also found that patients with MDDBP had significantly lower adjusted odds of being adherent to buprenorphine (defined as medication possession ratio (MPR) of at least 80%) with an aOR of 0.805 (95% CI: 0.651–0.994).63 Patients with comorbid anxiety disorder or nonspecific mental health conditions had slightly decreased odds of being adherent, although the results were not statistically significant.63 Additionally, certain patient demographics have been shown to influence adherence to buprenorphine. An analysis of the RI PDMP from July 2017 through June 2020 found that among patients newly initiating treatment, those aged 35 years and older has significantly higher adjusted odds of 12-month sustained buprenorphine compared to those aged 18 through 34.64 Moreover, females had significantly higher odds of sustained treatment compared to males (aOR: 1.137, 95% CI: 1.001–1.292), and patients with Medicaid insurance had significantly higher odds compared to those with private insurance (aOR: 1.235, 95% CI: 1.076–1.419), while patients who lived 5 miles or more away from their pharmacy had significantly lower odds (aOR: 0.719, 95% CI: 0.617–0.838).64 Similar findings were seen in the analysis by Kinsky et al which found that patients who were at least 40 years of age as well as females in general had significantly lower risk of non-adherence (age 40+ aHR: 0.822, 95% CI: 0.691–0.978; female aHR: 0.856, 95% CI: 0.735–0.998).33 In another analysis of the RI PDMP from October 2016 through September 2020, among 6499 patients who initiated buprenorphine for OUD, 58% of patients discontinued treatment within 180 days of initiating therapy, with daily doses of 16 mg having a greater risk of treatment discontinuation than a daily dose of 24 mg (adjusted hazard ratio (aHR): 1.20, 95% CI: 1.06–1.37).65
One significant barrier to adherence for buprenorphine is the out-of-pocket (OOP) costs incurred by patients. While analyses of IQVIA Longitudinal Prescription Data have shown that the OOP buprenorphine costs for patients in the US have decreased significantly from 2015 through 2020 by nearly 60% across all payer types,66,67 these costs remain substantial for patients. Even with the reduction, in 2020 the mean OOP costs for a 30-day supply of a buprenorphine prescription equated to $57.30 for all patients, $3.00 for patients with Medicaid insurance, $13.80 for patients with Medicare insurance, $54.60 for patients with private or commercial insurance, and $253.20 for patients who self-paid for their prescriptions.66 Patients who self-paid for prescriptions, who had the highest OOP costs, had a significantly lower mean day-supply per prescription filled (12.62 days, 95% CI: 12.60–12.65) compared to private insurance (21.37 days, 95% CI: 21.36–21.38), Medicare (20.60 days, 95% CI: 20.59–20.62), and Medicaid (15.59 days, 95% CI: 15.58–15.59).66 Furthermore, an analysis of IBM MarketScan Commercial Claims and Encounters database from January 2016 through June 2017 found that a one-dollar increase in OOP costs for buprenorphine led to a 12% reduction in treatment retention at 180 days (aOR: 0.88, 95% CI: 0.86–0.90), a 14% reduction in treatment retention at 360 days (aOR: 0.86, 95% CI: 0.84–0.89), and a 13% reduction in treatment retention at 540 days (aOR: 0.87, 95% CI: 0.84–0.89).68 Since formulary placement of medications such as buprenorphine, co-pay structures, patient deductibles and OOP maximums vary across health plans, it is difficult to determine the impact of OOP costs on cost-related nonadherence in the source data, as well as how much this impact will differ between plans in real-world settings.
A limitation of this analysis is that the input used in the model for the impact of blister-packaging on medication adherence is from a study that assessed blister-packaging ACEi and not buprenorphine or other MOUDs.51 Although OUD and hypertension are both chronic conditions, the patient populations are heterogeneous, with different factors potentially influencing medication nonadherence. Although no studies were identified that specifically assessed the impact of blister-packaging MOUD, several have been conducted for behavioral health conditions and have shown that in these populations blister-packaging medications can improve adherence.43 Notably, a pragmatic randomized controlled trial (pRCT) was conducted in a Veterans Affairs (VA) Medical Center revealed that blister-packaging all prescribed medications significantly improved adherence among psychiatric patients.43 The study found that patients in the blister-pack group were 59% closer to perfect adherence (95% CI: 6.6–112.2) than the control group and reported less symptom distress.43 Of note, 38% of patients included in the study were characterized as being diagnosed with drug abuse, 25% with drug dependence, 37% with alcohol abuse, and 27% with alcohol dependence.43 A cost-utility analysis of this pRCT showed that blister-packaging behavioral health medications has the potential to be cost-effective or cost-neutral, as their analysis showed the blister-packaging group was dominant (lower cost, higher quality adjusted life years (QALY)), although the differences between the groups were not significantly different.69
This economic analysis suggests that blister-packaging buprenorphine could potentially enhance medication adherence and reduce HCRU and health care costs in the treatment of OUD. This model is hypothesis generating in nature, with future studies needed to assess the impact of blister-packaging buprenorphine for OUD on medication adherence, HCRU, costs, and health outcomes in real-world settings. Due to the potential improvement in patient outcomes and reduction in costs this analysis modeled, pharmacies and health systems may also consider pilot-testing this intervention as a way to promote patient adherence in this population.
One important note is that while this model assessed the impact of blister-packaging oral buprenorphine therapies, various other routes of administration are available for buprenorphine therapy as well as non-buprenorphine MOUD therapies that would not be able to be blister-packed. In addition to buprenorphine being available as oral tablets (both as alone and in combination with naloxone), there is also buprenorphine sublingual film (both alone and in combination with naloxone), subcutaneous extended-release injection, and subdermal implant.70 While buprenorphine tablets can be blister-packed, the sublingual films are individually wrapped and would not be able to utilize blister-packaging. The same can be said about the parental formulations of buprenorphine. As mentioned previously, while buprenorphine is one treatment option for OUD, other treatment options are available such as methadone and naltrexone, however, neither would be a candidate for blister-packaging. Methadone when used for OUD can only be dispensed by certified opioid treatment programs which typically requires patients to go to the clinic every day and receive their dosage under direct supervision,26,27 although there are exceptions.29 Methadone, however, may be a preferred treatment option over buprenorphine for patients who are at high risk of buprenorphine treatment drop and fentanyl overdose.71 While naltrexone does have an oral formulation, it is recommended only in limited circumstances.29 The formulation of highest utilization for naltrexone is its extended-release injection, which would not be able to utilize blister-packaging.
Limitations
In addition to the limitations already discussed, the most important limitation is the impact of blister-packaging on medication adherence in the model coming from a population of patients utilizing ACEi. An additional limitation of the analysis was that it was only conducted for commercially insured patients limiting its generalizability to Medicaid or uninsured populations. This is particularly important when interpreting the results, as Medicaid is the largest payer type in the treatment of OUD48 and represents 44% of nonelderly adults with OUD – a proportion nearly double that of patients with private or commercial insurance (24%, p<0.05).49 Another limitation is that the source data from Ronquest et al is from 2014, and while there is more recent data and studies with consistent results,34 most of these studies precede the increased uptake of fentanyl and carfentanyl in nonprescribed opioids. Additionally, the removal of the waiver required to prescribe MOUD may allow more patients access to these treatments, changing the landscape of treatment initiation and maintenance. Thus, it is uncertain how the uptick of patients receiving treatment affects the impact of medication adherence on HCRU and costs. Moreover, this study focused only on patients initiating buprenorphine for OUD and did not assess those already receiving treatment, limiting the understanding of the broader impact of blister-packaging on long-term adherence in OUD treatment. An additional limitation is that we were unable to identify any publicly available information regarding self-reports of patient satisfaction utilizing blister-packaging of buprenorphine for OUD, and we do not know how this will ultimately affect treatment success and medication adherence.
Conclusion
Effective adherence to MOUD is critical for successful treatment outcomes and improved patient well-being. Blister-packaging buprenorphine for treatment of OUD has significant potential to improve medication adherence and health outcomes while reducing HCRU and lower health care costs. Future studies are necessary to assess the real-world application and impact of blister-packaging buprenorphine for OUD across various patient populations and health care settings.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
ACEi, Angiotensin-converting enzyme inhibitors; aHR, Adjusted hazards ratio; aOR, Adjusted odds ratio; CI, Confidence interval; CPI, Consumer price index; DEA, Drug Enforcement Administration; ED, Emergency department; HCRU, Health care resource utilization; MDDBP, Major-depressive disorder or bipolar disorder; MOUD, Medications for opioid use disorder; MPR, Medication possession ratio; OOP, Out-of-pocket; OUD, Opioid use disorder; PDC, Proportion of days covered; PDMP, Prescription drug monitoring program; PP, Per-patient; PPPM, Per-patient per-month; pRCT, Pragmatic randomized controlled trial; QALY, Quality adjusted life year; SAMHSA, Substance Abuse and Mental Health Services Administration; US, United States; VA, Veterans Affairs.
Disclosure
Saad, Dumitru, Nelkin, and Lucaci are employees and shareholders of Becton, Dickinson and Company. Borrelli and Barnes are employees of Becton, Dickinson and Company. Barnes is a shareholder of BioMérieux. The authors report no other conflicts of interest in this work.
References
- 1.National Institute on Drug Abuse. Drug Overdose Death Rates. National Institute on Drug Abuse. 2023. Available from: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed March 21, 2024.
- 2.Jalal H, Burke DS. Carfentanil and the rise and fall of overdose deaths in the United States. Addiction. 2021;116(6):1593–1599. doi: 10.1111/add.15260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes T, Ledlie S, Tadrous M, Mamdani M, Paterson JM, Juurlink DN. Trends in opioid toxicity-related deaths in the US before and after the start of the COVID-19 pandemic, 2011-2021. JAMA Network Open. 2023;6(7):e2322303. doi: 10.1001/jamanetworkopen.2023.22303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Federal Register. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. United States Federal Register. 2014;79(163):49661–49682. Available from https://www.govinfo.gov/content/pkg/FR-2014-08-22/pdf/2014-19922.pdf. [PubMed] [Google Scholar]
- 5.Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 7.American College of Emergency Physicians. Opioid Regulations: state by State Guide. American College of Emergency Physicians. 2021. Available from: https://www.acep.org/siteassets/sites/acep/media/by-medical-focus/opioids/opioid-guide-state-by-state.pdf. Accessed April 22, 2024.
- 8.United States Food and Drug Administration. FDA drug safety communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. United States Food and Drug Administration. 2017. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-serious-risks-and-death-when-combining-opioid-pain-or. Accessed April, 12, 2024.
- 9.United States Food and Drug Administration. FDA approves first over-the-counter naloxone nasal spray: agency continues to take critical steps to reduce drug overdose deaths being driven primarily by illicit opioids. 2023. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray. Accessed May 1, 2024.
- 10.Centers for Disease Control and Prevention. Prescription Drug Monitoring Programs (PDMPs). Centers for Disease Control and Prevention. 2024. Available from: https://www.cdc.gov/overdose-prevention/hcp/clinical-guidance/prescription-drug-monitoring-programs.html. Accessed May 2, 2024.
- 11.Substance Abuse and Mental Health Services Administration. Prescription drug monitoring programs: a guide for healthcare providers. Substance Abuse and Mental Health Services Administration. 2017;10(1):1–12. Available from https://store.samhsa.gov/sites/default/files/sma16-4997.pdf. [Google Scholar]
- 12.Zubsolv (buprenorphine-naloxone) [package insert]. (2023). Orexo US, Inc. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5f5cfcfe-d52b-49e6-8fe4-550477332dd2. Accessed March, 28, 2024.
- 13.Buprenorphine [package insert]. 2024. Actavis Pharma, Inc. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03819118-86f6-4329-adaf-4599e7b71f46. Accessed March, 28, 2024.
- 14.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace L, Kadakia A. Buprenorphine transdermal system utilization. Postgrad Med. 2017;129(1):81–86. doi: 10.1080/00325481.2017.1267537 [DOI] [PubMed] [Google Scholar]
- 16.United States Federal Register. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. United States Federal Register. 2021. Available from: https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder. Accessed April, 28, 2024.
- 17.Haffajee RL, Bohnert ASB, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med. 2018;54(6 Suppl 3):S230–S242. doi: 10.1016/j.amepre.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Deventer E, Antonson D, Soske J, Geary M, Vanjani R. Barriers to buprenorphine prescribing among attending physicians in an academic residency program - implications for increased buprenorphine usage. R I Med J. 2023;106(9):31–35. [PubMed] [Google Scholar]
- 19.Rowe CL, Ahern J, Hubbard A, Coffin PO. Evaluating buprenorphine prescribing and opioid-related health outcomes following the expansion the buprenorphine waiver program. J Subst Abuse Treat. 2022;132:108452. doi: 10.1016/j.jsat.2021.108452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silwal A, Talbert J, Bohler RM, et al. State alignment with federal regulations in 2022 to relax buprenorphine 30-patient waiver requirements. Drug Alcohol Depend Rep. 2023;7:100164. doi: 10.1016/j.dadr.2023.100164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Substance Abuse and Mental Health Services Administration. Waiver Elimination (MAT Act). Substance Abuse and Mental Health Services Administration. 2024. Available from: https://www.samhsa.gov/medications-substance-use-disorders/waiver-elimination-mat-act. Accessed April 28, 2024.
- 22.Paiva TJ, Wightman RS, St John K, Nitenson AZ, Onyejekwe C, Hallowell BD. Buprenorphine prescribing and treatment accessibility in response to regulation changes due to the COVID-19 public health emergency. J Subst Use Addict Treat. 2024;162:209382. doi: 10.1016/j.josat.2024.209382 [DOI] [PubMed] [Google Scholar]
- 23.Banken R, Otuonye I, Fazioli K, et al. Extended-release opioid agonists and antagonist Medications for Addiction Treatment (MAT) in patients with opioid use disorder: effectiveness and value. Institute for Clinical and Economic Review. 2018. Dec 3. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_OUD_Final_Evidence_Report_120318.pdf. Accessed April 4, 2024.
- 24.Otuonye IS, Banken R, Kumar VM, Pearson SD. A summary from the institute for clinical and economic review’s new England comparative effectiveness public advisory council. J Manag Care Spec Pharm. 2019;25(6):630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naltrexone hydrochloride tablet [package insert]. Accord Healthcare, Inc. 2024. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870. Accessed April, 27, 2024.
- 26.Methadone hydrochloride [package insert]. Hikma Pharmaceuticals USA. 2024. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e72841bf-364b-49b1-8e69-7e26ddcd2657. Accessed April 27, 2024.
- 27.Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Substance Abuse and Mental Health Services Administration. 2015. Available from: https://store.samhsa.gov/sites/default/files/guidelines-opioid-treatment-pep15-fedguideotp.pdf. Accessed April 27, 2024.
- 28.Frank D, Mateu-Gelabert P, Perlman DC, Walters SM, Curran L, Guarino H. ”It’s like ‘liquid handcuffs”: the effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm Reduct J. 2021;18(1):88. doi: 10.1186/s12954-021-00535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration. Methadone Take-Home Flexibilities Extension Guidance. Substance Abuse and Mental Health Services Administration. 2024. Available from: https://www.samhsa.gov/medications-substance-use-disorders/statutes-regulations-guidelines/methadone-guidance. Accessed September 12, 2024.
- 30.American Society of Addiction Medicine. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1–91. doi: 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- 31.Jarvis BP, Holtyn AF, Subramaniam S, et al. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113(7):1188–1209. doi: 10.1111/add.14180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronquest NA, Willson TM, Montejano LB, Nadipelli VR, Wollschlaeger BA. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78. doi: 10.2147/SAR.S150253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinsky S, Houck PR, Mayes K, Loveland D, Daley D, Schuster JM. A comparison of adherence, outcomes, and costs among opioid use disorder Medicaid patients treated with buprenorphine and methadone: a view from the payer perspective. J Subst Abuse Treat. 2019;104:15–21. doi: 10.1016/j.jsat.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 34.Liao S, Jang S, Tharp JA, Lester NA. Relationship between medication adherence for opioid use disorder and health care costs and health care events in a claims dataset. J Subst Use Addict Treat. 2023;154:209139. doi: 10.1016/j.josat.2023.209139 [DOI] [PubMed] [Google Scholar]
- 35.Tkacz J, Volpicelli J, Un H, Ruetsch C. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat. 2014;46(4):456–462. doi: 10.1016/j.jsat.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 36.Godersky ME, Saxon AJ, Merrill JO, Samet JH, Simoni JM, Tsui JI. Provider and patient perspectives on barriers to buprenorphine adherence and the acceptability of video directly observed therapy to enhance adherence. Addict Sci Clin Pract. 2019;14(1):11. doi: 10.1186/s13722-019-0139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentzley BS, Barth KS, Back SE, Book SW. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat. 2015;52:48–57. doi: 10.1016/j.jsat.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davstad I, Stenbacka M, Leifman A, Beck O, Korkmaz S, Romelsjö A. Patterns of illicit drug use and retention in a methadone program: a longitudinal study. J Opioid Manag. 2007;3(1):27–34. doi: 10.5055/jom.2007.0036 [DOI] [PubMed] [Google Scholar]
- 39.Weidele HR, Wightman R, St John K, Marchetti L, Bratberg J, Hallowell BD. Fentanyl and fentanyl analogs detected among unintentional opioid involved overdose deaths in Rhode island: January 2019-December 2021. R I Med J. 2022;105(10):64–66. [PubMed] [Google Scholar]
- 40.Hallowell BD, Weidele HR, Daly M, et al. History of methadone and buprenorphine opioid agonist therapy among people who died of an accidental opioid-involved overdose: Rhode island, January 1, 2018-June 30, 2020. Am J Public Health. 2021;111(9):1600–1603. doi: 10.2105/AJPH.2021.306395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conn VS, Ruppar TM, Chan KC, Dunbar-Jacob J, Pepper GA, De Geest S. Packaging interventions to increase medication adherence: systematic review and meta-analysis. Curr Med Res Opin. 2015;31(1):145–160. doi: 10.1185/03007995.2014.978939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ágh T, Hiligsmann M, Borah B, et al. Systematic review of outcomes for assessment of medication adherence enhancing interventions: an ISPOR special interest group report. Value Health. 2024;27(2):133–142. doi: 10.1016/j.jval.2023.10.016 [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez PM, Wortzel HS, Forster JE, Leitner RA, Hostetter TA, Brenner LA. Blister packaging medication increases treatment adherence in psychiatric patients. J Psychiatr Pract. 2017;23(5):320–327. doi: 10.1097/PRA.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 44.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford university press; 2015. [Google Scholar]
- 45.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a Report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31. doi: 10.1016/j.jval.2021.10.008 [DOI] [PubMed] [Google Scholar]
- 46.Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices - overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(5):796–803. doi: 10.1016/j.jval.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 47.McIntosh E, Clarke PM, Frew EJ, Louviere JJ. Applied Methods of Cost-Benefit Analysis in Health Care. 1sted ed. Oxford University Press; 2010. [Google Scholar]
- 48.Kim SJ, Medina M, Chang J. Healthcare utilization of patients with opioid use disorder in US Hospitals from 2016 to 2019: focusing on racial and regional variances. Clin Drug Investig. 2022;42(10):853–863. doi: 10.1007/s40261-022-01192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orgera K, Tolbert J The Opioid Epidemic and Medicaid’s Role in Facilitating Access to Treatment. Kaiser Family Foundation. 2019. Available from: https://www.kff.org/medicaid/issue-brief/the-opioid-epidemic-and-medicaids-role-in-facilitating-access-to-treatment/. Accessed April 19, 2024.
- 50.Dowell D, Brown S, Gyawali S, et al. Treatment for Opioid Use Disorder: population Estimates - United States, 2022. MMWR Morb Mortal Wkly Rep. 2024;73(25):567–574. doi: 10.15585/mmwr.mm7325a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zedler BK, Joyce A, Murrelle L, Kakad P, Harpe SE. A pharmacoepidemiologic analysis of the impact of calendar packaging on adherence to self-administered medications for long-term use. Clin Ther. 2011;33(5):581–597. doi: 10.1016/j.clinthera.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 52.US Inflation Calculator. Health Care Inflation in the United States (1948-2024). US Inflation Calculator. Available from: https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/. Accessed March 29, 2024.
- 53.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. ISPOR-SMDM modeling good research practices task force. model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force--6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014 PMID: 22999133. [DOI] [PubMed] [Google Scholar]
- 54.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open. 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mauro PM, Gutkind S, Annunziato EM, Samples H. Use of medication for opioid use disorder among us adolescents and adults with need for opioid treatment, 2019. JAMA Network Open. 2022;5(3):e223821. doi: 10.1001/jamanetworkopen.2022.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones CM, Han B, Baldwin GT, Einstein EB, Compton WM. Use of medication for opioid use disorder among adults with past-year opioid use disorder in the US, 2021. JAMA Network Open. 2023;6(8):e2327488. doi: 10.1001/jamanetworkopen.2023.27488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers LC, Hallowell BD, Samuels EA, Daly M, Baird J, Beaudoin FL. An evaluation of the association between specific post-overdose care services in emergency departments and subsequent treatment engagement. J Am Coll Emerg Physicians Open. 2023;4(1):e12877. doi: 10.1002/emp2.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chambers LC, Hallowell BD, Samuels EA, Daly M, Baird J, Beaudoin FL. Evaluation of a statewide policy to improve post-overdose care in emergency departments and subsequent treatment engagement. R I Med J. 2023;106(2):34–39. [PMC free article] [PubMed] [Google Scholar]
- 59.Beaudoin FL, Jacka BP, Li Y, et al. Effect of a peer-led behavioral intervention for emergency department patients at high risk of fatal opioid overdose: a randomized clinical trial. JAMA Network Open. 2022;5(8):e2225582. doi: 10.1001/jamanetworkopen.2022.25582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institute on Drug Abuse. Common Comorbidities with Substance Use Disorders Research Report. National Institute on Drug Abuse. 2020. Apr. Available from: https://www.ncbi.nlm.nih.gov/books/NBK571451/pdf/Bookshelf_NBK571451.pdf. Accessed April 14, 2024. [PubMed]
- 61.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2006;67(2):247–257. doi: 10.4088/JCP.v67n0211 [DOI] [PubMed] [Google Scholar]
- 62.Semahegn A, Torpey K, Manu A, Assefa N, Tesfaye G, Ankomah A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Syst Rev. 2020;9(1):17. doi: 10.1186/s13643-020-1274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Litz M, Leslie D. The impact of mental health comorbidities on adherence to buprenorphine: a claims based analysis. Am J Addict. 2017;26(8):859–863. doi: 10.1111/ajad.12644 [DOI] [PubMed] [Google Scholar]
- 64.Hallowell BD, Chambers LC, Samuels EA, et al. Sociodemographic and prescribing characteristics that impact long-term retention in buprenorphine treatment for opioid use disorder among a statewide population. Drug Alcohol Depend. 2022;241:109680. doi: 10.1016/j.drugalcdep.2022.109680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chambers LC, Hallowell BD, Zullo AR, et al. Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl. JAMA Network Open. 2023;6(9):e2334540. doi: 10.1001/jamanetworkopen.2023.34540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strahan AE, Desai S, Zhang K, GP G Jr. Trends in out-of-pocket costs for and characteristics of pharmacy-dispensed buprenorphine medications for opioid use disorder treatment by type of payer, 2015 to 2020. JAMA Network Open. 2023;6(2):e2254590. doi: 10.1001/jamanetworkopen.2022.54590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terranella A, Guy G, Strahan A, Mikosz C. Out-of-pocket costs and payer types for buprenorphine among us youth aged 12 to 19 years. JAMA Pediatr. 2023;177(10):1096–1098. doi: 10.1001/jamapediatrics.2023.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunphy C, Peterson C, Zhang K, Jones CM. Do out-of-pocket costs influence retention and adherence to medications for opioid use disorder? Drug Alcohol Depend. 2021;225:108784. doi: 10.1016/j.drugalcdep.2021.108784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavigne JE, Falbo K, Gutierrez PM. Cost–utility analysis of blister packaging all outpatient medications for veterans with bipolar disorder, major affective disorder, post-traumatic stress disorder or schizophrenia. J Pharm Health Serv Res. 2019;10(4):401–406. doi: 10.1111/jphs.12324 [DOI] [Google Scholar]
- 70.Poliwoda S, Noor N, Jenkins JS, et al. Buprenorphine and its formulations: a comprehensive review. Health Psychol Res. 2022;10(3):37517. doi: 10.52965/001c.37517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bromley L, Kahan M, Regenstreif L, Srivastava A, Wyman J, Dabam F, Methadone Treatment for People Who Use Fentanyl: Recommendations. Toronto: META: PHI; 2021. Accessed September 12, 2024.:24. Available from https://www.metaphi.ca/wp-content/uploads/Guide_MethadoneForFentanyl.pdf. [Google Scholar]