Abstract
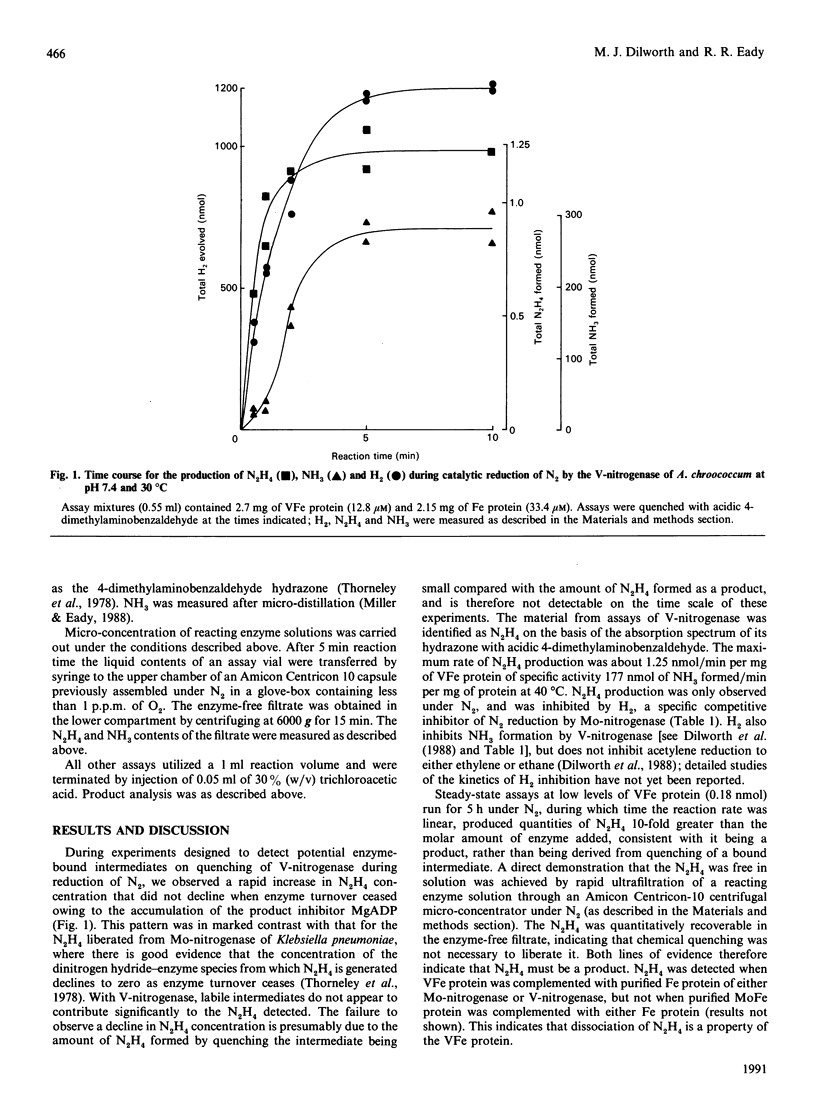
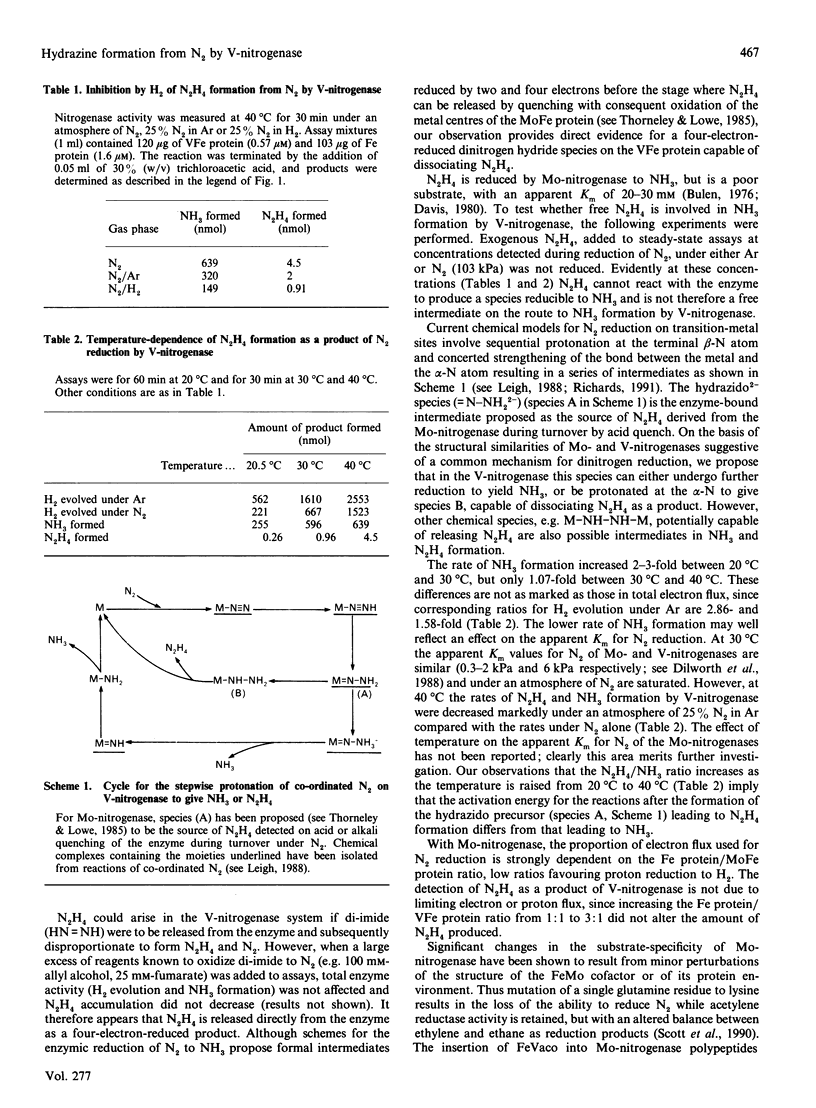
During the enzymic reduction of N2 to NH3 by Mo-nitrogenase, free hydrazine (N2H4) is not detectable, but an enzyme-bound intermediate can be made to yield N2H4 by quenching the enzyme during turnover [Thorneley, Eady & Lowe (1978) Nature (London) 272, 557-558]. In contrast, we show here that the V-nitrogenase of Azotobacter chroococcum produces a small but significant amount of free N2H4 (up to 0.5% of the electron flux resulting in N2 reduction) as a product of the reduction of N2. The amount of N2H4 formed increased 15-fold on increasing the assay temperature from 20 degrees C to 40 degrees C. Activity cross-reactions between nitrogenase components of Mo- and V-nitrogenases showed that the formation of free N2H4 was associated with the VFe protein. These data provide the first direct evidence for an enzyme intermediate at the four-electron-reduced level during the reduction of N2 by V-nitrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH M. K. Hydrazine and biological nitrogen fixation. Biochim Biophys Acta. 1957 Oct;26(1):104–113. doi: 10.1016/0006-3002(57)90060-4. [DOI] [PubMed] [Google Scholar]
- Davis L. C. Hydrazine as a substrate and inhibitor of Azotobacter vinelandii nitrogenase. Arch Biochem Biophys. 1980 Oct 1;204(1):270–276. doi: 10.1016/0003-9861(80)90033-8. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J., Eady R. R., Eldridge M. E. The vanadium nitrogenase of Azotobacter chroococcum. Reduction of acetylene and ethylene to ethane. Biochem J. 1988 Feb 1;249(3):745–751. doi: 10.1042/bj2490745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Richardson T. H., Miller R. W., Hawkins M., Lowe D. J. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the Fe protein. Biochem J. 1988 Nov 15;256(1):189–196. doi: 10.1042/bj2560189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rivera J., Burris R. H. Hydrazine and hydroxylamine as possible intermediates in the biological fixation of nitrogen. Arch Biochem Biophys. 1967 Mar;119(1):167–172. doi: 10.1016/0003-9861(67)90443-2. [DOI] [PubMed] [Google Scholar]
- Hales B. J., Case E. E., Morningstar J. E., Dzeda M. F., Mauterer L. A. Isolation of a new vanadium-containing nitrogenase from Azotobacter vinelandii. Biochemistry. 1986 Nov 18;25(23):7251–7255. doi: 10.1021/bi00371a001. [DOI] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., Eady R. R. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase. Biochem J. 1988 Dec 1;256(2):429–432. doi: 10.1042/bj2560429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. J., May H. D., Newton W. E., Brigle K. E., Dean D. R. Role for the nitrogenase MoFe protein alpha-subunit in FeMo-cofactor binding and catalysis. Nature. 1990 Jan 11;343(6254):188–190. doi: 10.1038/343188a0. [DOI] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]


