Abstract
Infectious diseases may lead to ocular complications including uveitis, an ocular inflammatory condition with potentially sight-threatening sequelae, and conjunctivitis, inflammation of the conjunctiva. Emerging infectious pathogens with known ocular findings include Ebola virus, Zika virus, Avian influenza virus, Nipah virus, severe acute respiratory syndrome coronaviruses, and Dengue virus. Re-emerging pathogens with ocular findings include Toxoplasma gondii and Plasmodium species that lead to malaria. The concept of One Health involves a collaborative and interdisciplinary approach to achieve optimal health outcomes by combining human, animal, and environmental health factors. This approach examines the interconnected and often complex human-pathogen-intermediate host interactions in infectious diseases that may also result in ocular disease, including uveitis and conjunctivitis. Through a comprehensive review of the literature, we review the ophthalmic findings of emerging infectious diseases, pathogenesis, and One Health perspectives that provide further insight into the disease state. While eye care providers and vision researchers may often focus on key local aspects of disease process and management, additional perspective on host-pathogen-reservoir life cycles and transmission considerations, including environmental factors, may offer greater insight to improve outcomes for affected individuals and stakeholders.
Key Words: emerging infectious disease, One Health, uveitis, ophthalmology, public health, Ebola virus disease, highly pathogenic avian influenza (HPAI), conjunctivitis
INTRODUCTION
One Health is a collaborative, multisectoral approach that aims to optimize health outcomes by recognizing the close connection of human health to the health of animals and our shared environment.1 The One Health concept was first associated with animal-human transmission during the emergence of severe acute respiratory disease (SARS) in 2003 and demonstrated the significance of animal reservoirs with the potential to cause high mortality in urban populations.2
Recently, highly pathogenic avian influenza (HPAI) presented as isolated, bilateral conjunctivitis in the absence of systemic symptoms in humans who were exposed to dairy cows and poultry infected with H5N1. These unusual findings highlight the extreme relevance of human-animal interactions, as well as the ocular system in clinical disease detection and management.3–6 Zoonoses account for a large majority of emerging infectious diseases, and as interactions between humans, animals, and the environment continue to expand, monitoring these interactions to better control disease outbreaks is paramount.7
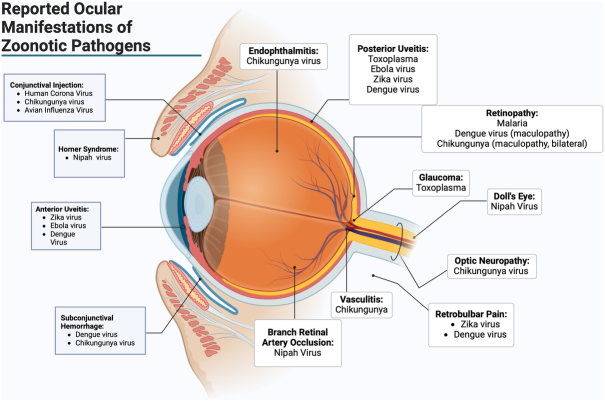
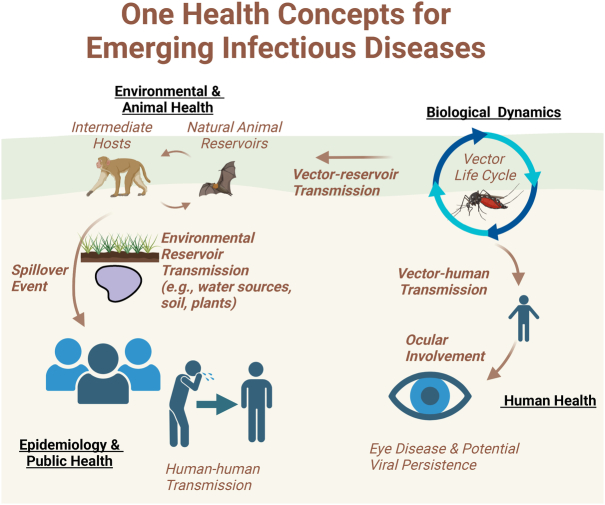
The eye is uniquely suited to harbor persistent pathogens, acting as a long-term reservoir for multiple pathogens that may lead to chronic indolent inflammation (eg, anterior uveitis due to rubella or cytomegalovirus) or aggressive, sight-threatening uveitis [eg, panuveitis due to Ebola virus disease (EVD)] (Fig. 1). Besides viruses, parasites such as Toxoplasma gondii that may lead to chorioretinitis further highlight these relevant human-animal relationships and the eye. While ophthalmologists often focus on the known host risk factors, ophthalmic disease findings, and targeted therapies for patients with eye disease, a more comprehensive understanding of the animal reservoir-host-environment relationships and One Health concepts can broaden the view of disease from an eye care provider’s standpoint (Fig. 2). Beyond an improved understanding of disease life cycle, pathogenesis and interactions, these insights provide opportunities for therapeutic considerations that extend beyond the patient’s visual system.
FIGURE 1.
Reported ocular manifestations of zoonotic pathogens. Created in BioRender. Blyden, K. (2024). Biorender.com/e49s563
FIGURE 2.
One Health concepts for emerging infectious diseases and the eye. Created in BioRender. Yeh, S. (2024). BioRender.com/n53n924
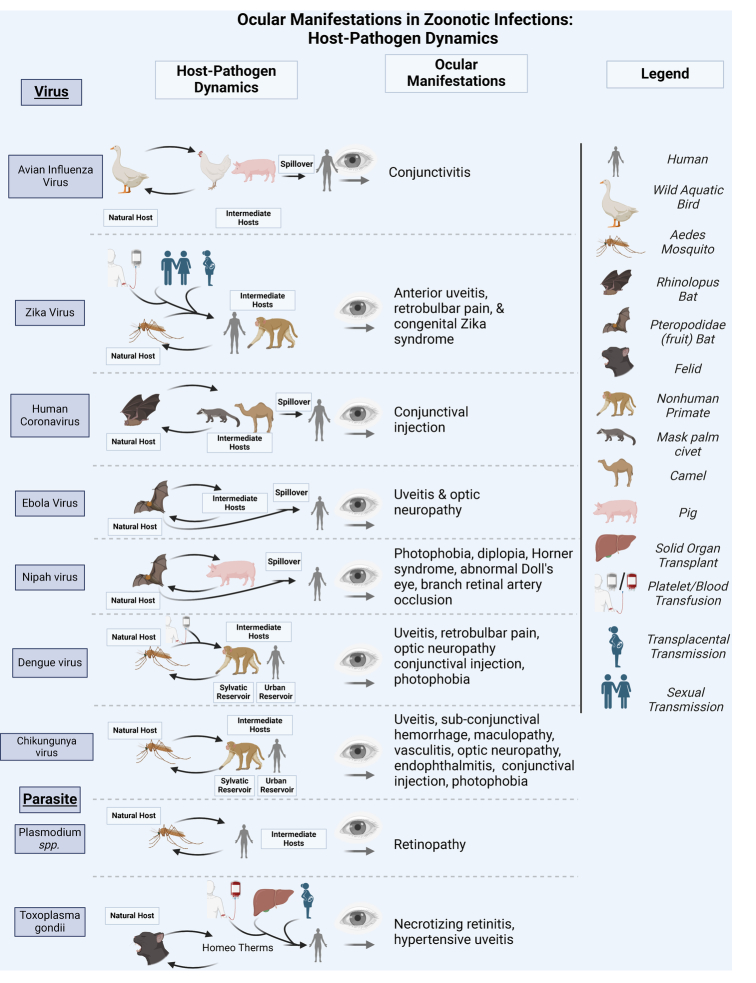
This review aims to highlight One Health concepts that clinicians may also consider when dealing with emerging infectious diseases that affect the eye. This review focuses on clinically relevant risk factors, including pathogen-reservoir-host life cycle interactions, the environmental aspects of disease transmission, and ocular pathogenesis (Fig. 3). Utilizing this approach offers insights that inform methods of mitigating transmission and disease management that may eventually lead to improved clinical outcomes.8 Given the breadth and multifaceted nature of the One Health approach, this review is not meant to be exhaustive. Additional details related to implementation frameworks and One Health challenges, and other specialized reviews are available.9,10
FIGURE 3.
Ocular manifestations in zoonotic infections: host-pathogen dynamics. Created in BioRender. Blyden, K. (2024). Biorender.com/v80m899
Ebola Virus Disease
Ebola virus disease (EVD) is a deadly viral hemorrhagic fever caused by Ebolavirus (EBOV). EBOV is a negative sense, single-stranded RNA virus which belongs to the Filoviridae family and is endemic to regions of west and equatorial Africa.11 EVD was first discovered in 1976 when 2 simultaneous outbreaks occurred in South Sudan and the Democratic Republic of Congo (DRC).12 Since its discovery, over 30 outbreaks of Ebola disease have been recorded in sub-Saharan Africa, with a high case fatality rate exceeding 50%.13 Between 2014 and 2016, the largest EVD outbreak occurred in West Africa that ultimately exceeded 28,000 cases and over 11,300 deaths. Thousands of EVD survivors predominantly within Sierra Leone, Liberia, and Guinea were found to be at-risk for sight-threatening ocular disease and systemic sequelae.14,15
Life Cycle and Animal Reservoir
Ebolavirus enters cells via a viral surface fusion glycoprotein (GP)-mediated binding to a variety of potential host cell attachment factors, followed by micropinocytosis, a nonspecific cellular engulfing process. This viral endosome enters the endosomal/lysosomal pathway, where the proteases cathepsin B and L process GP, allowing for the interaction of Niemann-Pick C1 (NPC-1), subsequent fusion with the endosomal membrane and release of viral particle into the cytoplasm, allowing for viral replication, assembly, and budding.16,17 Here, it is proposed that the ribonucleoprotein (RNP) complex associated protein, VP24, is released, allowing for transcription by RNP complex proteins NP, VP35, VP30, and L. Host ribosomes translate viral mRNA, and virion assembly is coordinated by VP40 at the plasma membrane, with eventual release of the virus to the extracellular space.18
A review of literature suggests that the single natural reservoir of EBOV is yet to be identified, but suspected incidental animal reservoirs have tested positive for EBOV RNA and include duikers, gorillas, chimpanzees, and rodents.11,19–21 Multiple studies suggest that bats, specifically fruit bats that belong to the Pteropodidae family, are a natural reservoir of EBOV.22 These conclusions have been made based on the probable index cases of EVD outbreaks, which involve a history of contact with wild animals that lead to the transmission event.11
Transmission
Multiple outbreaks have begun by EBOV being introduced to humans through transmission from an unidentified, likely animal reservoir with subsequent human-to-human transmission through direct contact or contact with body fluids, infected tissues, or contaminated fomites.23,24 EBOV has been identified in multiple bodily fluids including breast milk, saliva, urine, semen, cerebrospinal fluid, and blood. During survivorship, EBOV has also been detected in the aqueous humor, cerebrospinal fluid, and semen.25–27 While uncommon, sexual transmission of EBOV by survivors has been reported, leading to novel transmission chains.28
Ocular Manifestations
During the 2014-2016 EVD outbreak, many survivors experienced sight-threatening ophthalmic complications ranging from anterior uveitis to panuveitis, which developed during convalescence. The range of inflammatory response can be mild to severe, resulting in secondary structural complications (ie, cataract, vitreous opacity) and resultant severe vision impairment or blindness.29,30 The PREVAIL longitudinal study of survivors of EVD demonstrated an increase in the prevalence and incidence of uveitis during the first year of follow-up.31 EBOV detection from an aqueous humor sample obtained from an EVD survivor with severe, sight-threatening panuveitis highlights the contribution of EBOV persistence to disease pathogenesis.
One Health Mitigation of Ebola Virus Disease
Factors that influence EVD spillover and transmission include ecological as well as socioeconomic challenges and civil conflicts as demonstrated in the Democratic Republic of Congo, where numerous deadly EVD outbreaks have occurred.32 While the specific, unique reservoir for EBOV has not been identified, it is speculated to originate in the rainforest,33 and the likelihood of EVD outbreaks is also related to the incidence of recent deforestation.34 EBOV spillover into African great apes has also been documented, threatening their populations and providing an additional potential source of human infection via bushmeat hunting and consumption. Great apes may serve as sentinels in EBOV-endemic areas, and consideration of veterinary interventions such as vaccination could provide reciprocal benefits to human and nonhuman health.35 Understanding the environmental and mammalian distribution of EBOV allows for the identification of possible zones that serve as an interface between susceptible mammalian subtypes, ecosystems, and humans.36 Robust monitoring, reduction of deforestation, health interventions for affected animal populations, and community education are some strategies that could be considered in a coordinated One Health approach to prevent EVD spillover and transmission among human populations and reduce the burden of vision impairment in resource-limited settings.34,37,38
Zika Virus Disease
Zika virus disease, caused by the Zika virus (ZIKV), is transmitted by Aedes mosquitoes. ZIKV is positive-sense, single-stranded RNA arthropod-borne virus (arbovirus) in the genus Flavivirus and the family Flaviviridae. ZIKV was first identified in the Zika forest of Uganda in 1947 in a febrile rhesus macaque monkey, and was subsequently identified in humans in 1954 in Nigeria.39 Outbreaks of Zika virus disease have been recorded in Africa, the Americas, Asia and the Pacific. The first outbreak of Zika virus disease was reported in the Island of Yap (Federated States of Micronesia) in 2007, infecting over 70% of the population.40 This was followed by a larger outbreak in French Polynesia and other countries and territories in the Pacific in 2013. In March 2015, Brazil reported a large outbreak of Zika virus disease, where a surge in associated Guillain-Barré syndrome cases was observed.41
Life Cycle and Animal Reservoir
The viral life cycle of ZIKV begins when an infected mosquito bites the host organism, from which the virus can then infect different cells, including skin cells (fibroblasts, keratinocytes).42 ZIKV also has tropism for retinal pericytes, retinal microvascular endothelial cells, and neural progenitor cells, as many of these cells contain cell surface receptors (Tyro3, DC-SIGN, AXL, and TIM01) that facilitate viral entry.43 ZIKV attaches to these host cell receptors using its envelope (E) protein and is then endocytosed by clathrin proteins.44 Mature virions exit the infected cell via exocytosis, where they can be picked up by another feeding Aedes mosquito.
While ZIKV was first isolated from a febrile rhesus macaque monkey, the precise animal reservoir has yet to be identified.45 ZIKV is an arbovirus, maintained in nature through biological transmission between a susceptible vertebrate host and a hematophagous arthropod such as a mosquito. Anti-ZIKV antibodies have been observed in many nonhuman primates and other wild and domestic animals such as rodents, domestic sheep, goats, and orangutans, suggesting multiple animal reservoirs.44
Transmission
ZIKV is usually transmitted when an infected female Aedes mosquito bites a human or a nonhuman primate and has distinct human and nonhuman primate transmission cycles.44 The most common mode of biological transmission is horizontal via a mosquito vector that has taken a viremic blood meal. ZIKV has been associated with intrauterine vertical transmission and congenital infections, resulting in severe microcephaly and cerebral atrophy with coarse subcortical calcifications. In mothers with clinical signs of ZIKV, studies have demonstrated intrauterine transmission with ZIKV RNA via reverse transcription PCR (RT-PCR) in the brain and placental tissues of fetuses and mothers, respectively.46,47 In addition, there have been reports of successfully cultured ZIKV particles from breast milk isolates, suggesting that breastfeeding may be a potential route of transmission.48 Sexual transmission has also been implicated. While ZIKV infections related to blood transfusions have not been reported, there is one case report of probable transmission of ZIKV through a platelet transfusion.49,50
Ocular Manifestations
ZIKV-infected patients experience a range of ophthalmic manifestations, from common (eg, mild conjunctivitis, retrobulbar pain) to rarely reported findings (eg, chorioretinal lesions and uveitis). These findings are thought to develop as ZIKV infects blood-retinal barrier cells and cause an inflammatory response mediated by TNFα, IFNα, and interleukins.43 Up to 50% of infants with congenital Zika syndrome (CZS) present with ophthalmic manifestations.51 CZS presents with retinal findings including pigment mottling of the macula and well-circumscribed chorioretinal atrophy.52 In patients in whom anterior uveitis develops, patients may present with ocular hypertension, which may be treated with a combination of corticosteroid and anti-inflammatory medications applied topically.53,54 Cases of posterior uveitis have had varying presentations including bilateral neuroretinitis, optic disc edema, chorioretinal lesions, and outer retinal and retinal pigment epithelial architecture disruption.55
Studies in mice have confirmed the detection of ZIKV in tears, and ZIKV in conjunctival swab samples.56,57 ZIKV RNA can be detected in conjunctival swabs in the late convalescent phase as well, suggesting that the ocular surface may be a reservoir for the virus with a potential public health risk of transmission even after recovery.58
One Health Mitigation of Zika Virus Disease
Arthropod-borne viruses remain a continuing threat to humans living in endemic regions. One Health approaches to ZIKV prevention and control may utilize vector control strategies including physical alteration of environments to reduce mosquito breeding, mechanical traps that lure and kill mosquitoes, and chemical and biological measures. Environmental health interventions that focus on water-based viral surveillance and outbreak control may allow for early detection, vector control, and reduction in outbreak potential.59 The integrated vector management approach is a promising vector control strategy that focuses on making vector control more economically advantageous and ecologically sustainable.60 The use of plain language public health messaging regarding disease transmission is an additional consideration in the One Health approach to reducing the burden of ZIKV disease.61
Human Coronavirus Infection
The human coronavirus (CoV) was first documented in 1965 and is known for its ability to cause acute upper respiratory tract infections in adults. Besides the recent COVID-19 pandemic caused by SARS-CoV-2, CoV outbreaks of significant public health impact include severe acute respiratory syndrome (SARS), caused by SARS-CoV-1 and Middle East respiratory syndrome (MERS), caused by MERS-CoV. These resulted from cross-species infections and spill-over events due to increased human-animal interactions.62,63 CoV are single-stranded, positive-sense RNA viruses that belong to the Coronaviridae family.64 To date, there are 7 coronaviruses that infect humans, with SARS-CoV-1 and MERS-CoV associated with higher mortality.
Life Cycle and Animal Reservoir
The coronavirus family is well-known for its spike protein, which facilitates viral entry into target cells by binding to an angiotensin-converting enzyme 2 receptor. This interaction allows viral attachment to the surface of the target cell and subsequent fusion of viral and cellular membranes.65
Animal coronaviruses, including bovine coronavirus and feline infectious peritonitis virus, have been recognized since as early as the 1930s.66 The first CoV isolated was an avian infectious bronchitis virus in 1937. Bats are reservoir hosts for all CoVs, serving as gene pools and playing a crucial role in their evolutionary pathway.67,68
Transmission
Human coronaviruses are typically transmitted via respiratory droplets, aerosol, fecal-oral route, and contact with contaminated surfaces.69 The precise mechanism of zoonotic transmission from the reservoir host to humans remains unclear, and it is speculated that intermediate hosts play an important role in viral transmission. The prevalence and genetic diversity of bat coronaviruses, along with the frequent recombination events within bats provide an ideal milieu for spillover to humans. In SARS-CoV-1, intermediate hosts, including civets and other mammals, allow for mutation acquisition with resultant spillover.70
Human coronavirus 229E (HCoV-229E), the first human CoV isolated, is closely related with CoVs that existed in African bats suggesting transmission to humans from these reservoir hosts.71 MERS-CoV strains responsible for MERS outbreaks have been associated with contact with dromedary camels.67 Even then, there is a phylogenetic gap between bat MERS-related CoV and human and camel MERS-CoV, indicating that viruses that have not yet been identified contributed to the emergence of MERS-CoV in humans and camels.
Ocular Manifestations
The eye is not a commonly known entry route for human CoVs and is rarely involved in infection despite the conjunctiva being exposed to pathogens and communicating with the upper respiratory tract via the nasolacrimal duct. While the prevalence of SARS-CoV-2 RNA in the tear film of patients with COVID-19 has been low in several studies, one hospital-based evaluation showed that 25% of hospitalized patients with acute illness tested positive for SARS-CoV-2.72–74
In animal populations, CoV may have unique and important ophthalmic findings.75 Feline CoV is a coronavirus that affects domestic and wild cats and has been associated with pyogranulomatous anterior uveitis, choroiditis with retinal detachment, and retinal vasculitis. Murine CoV mouse hepatitis virus is associated with a virus-induced retinal degeneration and is considered neurovirulent.14 While some of these specific ophthalmic findings associated with coronavirus have not been observed in humans, understanding the ocular manifestations of coronavirus and related pathogenesis in animals may help in identifying parallel mechanisms to understand and manage human ophthalmic disease in the future.
One Health Mitigation of Human Coronavirus Disease
One Health actions to target human CoV disease include the development of diagnosis and treatment capacity, food safety measures, and surveillance strategies.63,76 Most of these One Health approaches to preventing human CoV disease address the animal-human interface, with relatively fewer approaches addressing the human-animal-environment interface despite having relevance to outbreak prevention and control. Further research is needed to evaluate the environmental drivers of spillover, the ecology of the viruses in the wildlife, and the effects of habitat loss and destruction.63,77
Avian Influenza Virus Disease
Avian influenza viruses (AIVs) are type A influenza viruses and members of the Orthomyoxoviridae family infecting birds and mammals. Influenza A viruses are single-stranded, segmented RNA viruses with genes encoding 2 particular transmembrane proteins, hemagglutinin (HA) and neuraminidase (NA), which are key players in infectivity as HA and NA facilitate viral entry and release, respectively.78 Among AIVs, 16 HA and 9 NA subtypes have been identified, creating a source of antigenic diversity and increasing outbreak potential.79
There are reports as early as 1878 of a nonbacterial disease of high mortality in domestic chickens and poultry, described as the “fowl plague.”80 It was not until 1955 that the type A influenza virus was identified as the causative pathogen of what is now known as avian influenza virus disease.81,82 Highly pathogenic AIVs (HPAI) consist of the H5 or H7 subtypes thought to arise from mutations in low pathogenic AIVs (LPAI) in poultry.83 The HPAI H5N1 virus strain found in geese in Guandong Province, China caused outbreaks in chicken farms in Hong Kong in 1997, with zoonic transmission resulting in human fatalities. Despite all poultry in Hong Kong being culled, H5N1 viruses re-emerged in 2001 and have since spread from Asia into Europe, Africa, the Mediterranean, Russia, and as of recently, Canada and the United States.3,81,82,84 Approaches to disease reduction and prevention in humans before the emergence of highly pathogenic disease outbreaks like H5N1 have either lapsed over time or were never implemented.81 Subsequent outbreaks of HPAI viruses from zoonotic transmission call for a One Health approach to bring awareness to this potentially fatal disease.81
Life Cycle and Animal Reservoir
The life cycle of AIVs involves host cell entry via HA binding to host cell sialic acid residues and receptor-mediated endocytosis into an endosome.85 The acidic environment of the endosome allows for viral ribonucleoproteins (vRNP) to be released and enter the host cell cytoplasm. The nuclear localization signals associated with vRNPs lead the proteins to the nucleus for transcription and eventual exit from the nucleus via Exportin 1.86,87 The viral particles form in the cytoplasm alongside its segmented genome, and NA removes sialic acid residues, allowing for viral release from the plasma membrane.85
Wild aquatic birds like wild waterfowl (Anseriformes) and shorebirds (Charadriiformes) are considered natural hosts to all influenza viruses.
Transmission
Type A influenza viruses are transmitted from bird to bird via the fecal-oral route. Transmission to other intermediate hosts can occur via direct contact with an infected bird’s saliva, mucus, or feces, or indirectly from environmental contamination or an intermediate host.88 While direct transmission of AIVs from its natural host to humans is documented and associated with the 1997 outbreak in Hong Kong,89–91 transmission to humans typically requires an intermediate host.91 Transmission of AIVs to domestic birds and mammals offers additional avenues for reassortment and human spillover.92–94 Although HPAI H5N1 is rarely transmissible among mammals, the outbreak of HPAI H5N1 in lactating dairy cattle across multiple US states in March 2024 marks an unprecedented and sustained mammal-to-mammal transmission. Furthermore, cases of conjunctivitis in humans exposed to infected poultry and dairy cows highlight the potential for H5N1 to spill over into the human population, raising concern for another respiratory pandemic.3,95–97
The HA of AIVs recognize α-2,3-linked sialic acid receptors on the conjunctiva and ciliated nasal epithelial cells and allow viral entry and propagation.98–100 Influenza A viruses are prone to antigenic shifts that increase transmissibility and risk of pandemics due to the segmented nature of the viral genome, the ability for influenza A virus to undergo reassortment, and the error-prone RNA polymerase.79,101 Such antigenic shifts have been the cause of major influenza A outbreaks, including the Asian flu of 1957 (H2N2), and the Hong Kong flu of 1968 (H3N2).102
Ocular Manifestations
The ocular surface is likely a portal of entry for AIV infection, as the virus has demonstrated replication within the corneal epithelium as well as an ocular surface tropism.103 Ocular involvement of HPAI H5N1 appears to be limited to isolated conjunctivitis with at least one report of a subconjunctival hemorrhage.6,104 Viral presence has been confirmed through positive RT-PCR results in conjunctival swabs from infected patients.3,105 In addition, H5N1-inoculated domestic ducks developed corneal opacification with keratitis and edema.106 Treatment with the antiviral oseltamivir inhibits virus replication in ocularly inoculated animal models; however, the persistence of HPAI in ocular tissue after infection resolution has not been shown.107,108
One Health Mitigation of Avian Influenza Virus Disease
The One Health approach to AIV disease largely involves controlling outbreaks in poultry populations with large-scale culling operations during outbreaks, surveillance of human exposure, and antiviral prophylaxis of those exposed while conducting depopulation operations.109 However, mitigating the spread of AIV is complicated by the influence of climate change, which alters the AIV ecology and transmission cycle due to shifts in bird migration patterns. These migrational changes facilitate interactions between host species and intermediate hosts, potentially driving AIV evolution and enabling interspecies transmission.110 Interspecies transmission events have been associated with the recent spillover of HPAI to dairy cattle, efficient cow-cow transmission, and cow-human transmission.111 This bovine HPAI facilitates the risk of infection and transmission in mammals, as AIV RNA has also been isolated in other animals on affected farms (eg, raccoons, domestic cats). Transmission of H5N1 virus from bovine milk-inoculated lactating mice to offspring as well as mice in proximity to H5N1 infected mice has been documented.112 This important discovery of mammal-to-mammal transmission emphasizes that following the spill-over of AIV, the public health risk and pandemic potential of HPAI is increased. Moreover, these data call for increased AIV research, surveillance, and multidisciplinary collaboration among relevant authorities including public health departments, the United States Food and Drug Administration, Centers for Disease Control and Prevention, veterinary health organizations, etc.
AIV persists in aquatic environmental reservoirs like ice lakes and ponds, also facilitates viral transmission among other species and increasing the risk for spillover.113 Areas of potential mitigation include enhanced biosecurity, hygiene, contact precautions with birds and poultry, and surveillance of environmental reservoirs.114 Transmission of AIV can be prevented by treatment of agricultural water supplies and avian protein sources, in addition to separation of animals with known transmissibility.101 In addition, an “all-in and/or all-out” approach can be applied to birds removed from their farm and taken to the market, or any transmission-susceptible environment, and they should not be returned back to the farm.114 A combination of clinical and veterinary surveillance along with human and veterinary collaboration will ultimately be needed to significantly reduce the probability of transmission.114
Toxoplasmosis
Toxoplasmosis is a global zoonotic disease caused by the single-celled protozoan Toxoplasma gondii and is a member of the Apicomplexa group of parasitic protozoans. T. gondii was first isolated in 1908, where it was proposed to have originated in South American felids and spread to domestic animals including cats, rats and mice through the transatlantic slave trade.115 It is estimated that around 2 billion people are chronically infected by this parasite globally, and it is recognized as the leading cause of posterior infectious uveitis worldwide.116,117 Toxoplasmosis can develop in those who are immunocompromised or pregnant.118 The most notable disease manifestation in the immunocompromised is encephalitis. Pregnant women with acquired infection are at risk for transplacental migration of the parasite and subsequent systemic congenital infection that is characterized by premature birth, intrauterine growth retardation, fever, pneumonia, hepatosplenomegaly, thrombocytopenia, and fetal death.119,120 Congenital disease commonly affects the eyes and brain, resulting in chorioretinitis, microphthalmia, meningoencephalitis, seizures, microcephaly, cerebral calcifications, and/or deafness.
Life Cycle and Animal Reservoir
The life cycle of T. gondii begins in its definitive, obligate felid host with spread into intermediate hosts, including homeotherm (warm-blooded) vertebrates like sheep, goats, pigs, chickens.119,121 Once T. gondii reaches its intermediate host, it can only reproduce sexually, excreting massive numbers of oocysts into the external environment by way of feces.122 The oocytes sporulate infectious sporozoites, and these sporulated oocysts are ingested or inhaled by an intermediate host and invade host tissues like the heart, lungs, and CNS beginning asexual reproduction. The life cycle is completed when the tissues of intermediate hosts are consumed by the definitive hosts. T. gondii has been shown to use host manipulation to attract intermediate hosts (eg, rodents) to cat urine by causing rodents to lose their innate fear of cats, thereby completing their life cycle.123
Transmission
T. gondii is easily transmitted between organisms via the fecal-oral route. Inoculation of T. gondii is associated with consumption of raw or undercooked meat containing parasitic cysts, or ingestion of contaminated water or other substance contaminated with feces containing oocysts. It is estimated that 30 to 80 million feral domestic cats exist in the United States, allowing for easy transmissability.119 T. gondii infection is also known to be associated with blood transfusions and organ transplants.124 The more common of the 2 is due to the transplantation of an organ containing parasitic cysts. A patient who receives a contaminated organ may succumb to an active infection due to their immunocompromised status.124 This parasite is also known to be transmitted vertically via transplacental migration to the fetus.
Ocular Manifestations
Infection with T. gondii can cause ocular toxoplasmosis, with around 2% of immunocompetent adults developing ocular manifestations.125 T. gondii invades target tissues using what is likened to a trojan horse approach by utilizing immune cells.126 Once it has achieved intracellular access, it secludes itself from the host cytoplasm by forming a vacuole that protects it from potentially toxic host molecules and the acidic host phagolysosomes.127 In fact, pH plays a significant role in T. gondii invasiveness and host defense. Tachyzoites are very susceptible to acidic environments, and phagosome acidification likely serves as a primary immune defense against T. gondii in ocular tissues.128,129 This is further supported by the higher risk of being diagnosed with ocular toxoplasmosis in patients exposed to protein pump inhibitors or histamine 2 blockers, which suggests a possible mechanism of ocular parasite persistence.130
Though the details of retinal invasion are still being studied, Toxoplasma has been shown to cross retinal pigment epithelial cells in its free form using intercellular adhesion molecule-1 (ICAM-1), and using vascular cell adhesion molecule-1 (VCAM-1) and activated leukocyte cell adhesion molecule (ALCAM) in dendritic cells.131 The largest indicator of progression or recurrence of ocular toxoplasmosis seems to be the quality of the immune response.131 Due to the lack of proper lymphatic drainage of the eye, the presentation of ocular-derived antigens to T cells is significantly reduced. The posterior pole of the eye provides an ideal environment for chronic Toxoplasma ocular persistence as it is an immunosuppressed area due to the excretion of suppressive cytokines like TGFB2, a-MSH, and vasoactive intestinal peptide (VIP).132
One Health Mitigation of Toxoplasmosis
Though the global seroprevalence of T. gondii is high, its seroprevalence appears to be dependent on various regional factors including climate, diet, and hygiene that call for a One Health approach.133–135 Despite the prevalence and disease burden of toxoplasmosis, additional work that focuses on implementing strategies that explore the transmission and life cycle of T. gondii is needed. One Health mitigation strategy could be aimed at environmental control by improving the understanding of oocyst resistance, survival in environmental and tissue reservoirs, and the reduction of contamination of these reservoirs while improving hygienic practices.133 In addition, understanding the prevalence in other animal reservoirs of different environments may offer a continued refinement of the One Health approach to understanding and reducing the burden of Toxoplasmosis.136–138
Malaria
Malaria is a mosquito-borne disease that is caused by parasites of the Plasmodium genus. There are 5 species known to infect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi. P. falciparum and vivax are the primary species responsible for the majority of malarial infections, with P. falciparum being the most prevalent in Africa and also the deadliest malaria species.139 Millenia of human exposure to many of these species have placed selection pressures that have led to a finely tuned human-parasite interaction.140
Life Cycle and Animal Reservoir
The malaria parasite’s life cycle begins as a sporozoite in Anopheles spp. mosquito’s salivary gland. When the mosquito feeds on a human, the sporozoites in the mosquito’s saliva are released into the blood stream and undergo an asexual reproductive process known as schizogony to form merozoites once they reach target organs, like the liver. These merozoites enter the bloodstream and bind to red blood cells (RBCs) and undergo the erythrocytic phase to form the trophozoite. These trophozoites mature to their final mitotic replicative developmental stage called the schizont, then differentiate into merozoites and eventually gametocytes and oocytes. These oocysts mature before rupturing and releasing infectious sporozoites that migrate to the salivary gland for further transmission.141
Transmission
Transmission from mosquito to human is influenced by many factors including age, gametocyte density and sex ratio, antimalarial treatment, and host immunity. Malaria’s pathogenesis is primarily driven by asexual stages. However, about 0.1% to 5% of asexual parasites develop into male and female gametocytes, and this gametocyte is the only stage that is transmitted to the mosquito vector.141 The fertilization of the gametes leads to the development of sporozoites in the mosquito, making them infectious to humans.
Ocular Manifestations
Cerebral malaria is a serious and deadly complication of malaria, characterized by an unarousable coma. A pattern of retinal changes secondary to cerebral malaria were first described in Malawian children.142 These retinal changes present as a symmetrical retinopathy in both eyes and include retinal whitening, vessel discoloration, retinal hemorrhages resembling Roth spots, and optic disc edema.142 The solitary finding of optic disc edema suggests severe illness and possible co-infection.143 The diagnosis of cerebral malaria can be confirmed by examination of the retina for signs of malaria retinopathy, with retinal hemorrhages being a significant prognostic sign.144,145 Pathogenesis of ocular malaria involves small vessel occlusion by sequestered red blood cells in the microvasculature of the retina leading to hypoxia and ischemia.146 Recent studies in murine models have shown that malaria parasites cross the blood-brain barrier and infiltrate the neuroretina and can cause irreversible retinal neurodegeneration.147
One Health Mitigation of Malaria
The One Health approach to malaria largely relies on vector control and includes insecticide-mediated interventions such as insecticide-treated nets and indoor residual spraying.148 Economic development during periods when malaria burden is low has added incentive to vector control efforts, thus leading to a considerable decline in malaria cases.149 A campaign to prioritize malaria eradication called for by the Bill and Melinda Gates Foundation in 2007 focused on reduction of transmission.32–34 Current mitigation strategies focus on controlling the vector through insecticide-treated nets and antimalarial combination therapy including a transmission-blocking drug.141 In addition, methods of larval source management aimed at controlling environmental reservoirs and decreasing mosquito survival and breeding are also a crucial role in the One Health approach to malaria. These methods include alterations of aquatic habitats, habitat modification, larvicide, and the introduction of predatorial biological controls into such environments.150 Despite the efforts made toward vector control, the increase in insecticide resistance in Anopheles mosquitos and the morbidity and mortality of the disease are concerning.151 The vaccine landscape continues to develop with favorable potential, as the MosquirixTM vaccine and other candidate vaccines like R21/Matrix-M and PfSPZ have proved efficacious in clinical trials.152 While hopeful, vaccine deployment in addition to continued vector control improvements might enhance the One Health approach to malaria.
Nipah Virus Disease
Nipah virus disease is caused by a virus from the family Paramyxoviridae and is a single stranded RNA virus.153 It was first discovered in 1998 in the village of Sungai Nipah in Malaysia after many pigs began dying from respiratory and neurological disease, accompanied by an outbreak of acute encephalitis in surrounding human populations.154 Nipah virus (NiV) then spread to Singapore in early 1999 after pigs were imported from Malaysia.153 Since then, there have been outbreaks in Malaysia, Bangladesh, and India, with nearly annual outbreaks in Bangladesh since 2001.153 In India, the first reported case was in West Bengal with more recent outbreaks in Kerala. An epidemiologic study from the 2018 Kerala outbreak showed that 87% of cases showed respiratory symptoms with a fatality of 91%.153,154
Life Cycle and Animal Reservoir
The life cycle of NiV starts when the virus attaches to the host cell. NiV infects host cells by 2 glycoproteins, G and F proteins. G protein aids with attachment to host cell receptors while the F protein allows fusion of virus to host.154 In addition, viruses can target the DNA-damage response pathway to increase Nipah virus production as well as encode accessory proteins to avoid immune detection.154,155 Pteropus fruit bats are the natural reservoir for the disease.153 These bats are endemic to tropical and subtropical areas of Asia, East Africa, and Australia. While the bats are asymptomatic carriers, they shed the virus in fluids such as saliva, urine, and excreta.153 Pigs can be an intermediate and amplifying source, allowing for zoonotic transmission.154
Transmission
Nipah virus disease can be transmitted in many ways including indirectly from the bat reservoir, by intermediate hosts, or, in some instances, human-to-human.154 In Malaysia, Nipah virus was transmitted to humans through infected pigs.153 In Bangladesh and India, NiV has been transmitted from consumption of raw date palm sap contaminated by bat urine, excreta, or saliva. There have also been reports of human-to-human transmission associated with close contact with an infected person, although cases have been limited.153 The transmission of NiV has changed with variations in resources and landscapes that have altered the natural habitat for fruit bats leading to more unseasonal spillover in areas such as Bangaldesh.154
Ocular Manifestations
Ocular manifestations are not well-studied in NiV disease, but some reports include blurry vision, photophobia, and double vision during the acute illness phase.156 There are also limited reports of transient blindness, Horner’s syndrome, and branch retinal artery occlusion (BRAO).157 In some patients, doll’s eye reflex was also found, and this brainstem involvement indicated a poor prognosis and higher rate of mortality.
One Health Mitigation of Nipah Virus Disease
The One Health approach to mitigating NiV disease outbreaks focuses on reducing transmission from bats to humans. While raw sap from fresh date palms is a popular delicacy in Southeast Asian countries, it is associated with the indirect transmission of NiV from bats to humans. Fruit bats also consume date palms, making it is easy for them to contaminate and spread NiV to humans who consume raw date palm sap. To reduce contamination, date palm juice can be boiled, fruits should be washed thoroughly before eating, and bat exposure to fruits and sap sources like bamboo sap skirts should be limited.158 Bat-human habitats continue to overlap due to deforestation and urbanization increasing the odds of spillover events with outbreak potential, emphasizing the effect that early detection systems can have on preparedness against future outbreaks. In addition, personal protection may decrease the risk of human-human and animal-human transmission, especially for those handling sick animals or humans.158,159 The limited availability of antiviral therapeutics and vaccines further emphasize the role of strengthening and integrating current One Health approaches that includes increased community awareness, early detection with the implementation of integrated data systems, and garnering resources to fund the comprehensive management of NiV disease.160,161
Dengue Virus Disease
Dengue virus is a member of the Flavivirus genus and is a single-stranded positive sense RNA virus that can be caused by 4 serotypes (DENV 1-4).162 Dengue was first reported in the late 1700s in Asia, Africa, and North America; however, a disease with similar symptoms was also reported during the Chin Dynasty in 265-420 ad.162 In the last 50 years, this virus has increased 30-fold in incidence,163 with outbreaks reported in tropical and subtropical areas with an increased spread with urbanization, global travel, and poor mosquito control measures. The epicenter of DENV is India although dengue is endemic to over 100 countries.163
Life Cycle and Animal Reservoir
Similar to other viral processes, the dengue viral replication process initiates with attachment to the host cell and the formation of an endosome that seals around the virus to allow entry into the cell.164 Viral RNA is then released into the cytoplasm and the virus hijacks host cell mechanisms to further replicate and mature to then infect other cells. DENV is spread through the bite of an Aedes mosquito, which has a life cycle of around 8 to 10 days at room temperature and has both an aquatic and terrestrial phase.163 In addition, humans can serve as reservoirs for dengue in urban settings, while nonhuman primates are reservoirs in a sylvatic cycles.165
Transmission
Dengue virus disease is transmitted to humans from the female Aedes mosquito into skin epithelium during a blood meal, where it infects and replicates in cells of mononuclear lineage.162,163 It can then spread into the lymph node and continue replicating, ultimately leading to a systemic infection. When viremia occurs in humans, Aedes mosquitoes can feed and become a dengue vector and continue to spread the disease. Transmission of dengue infection via a blood transfusion is rare, but has been documented.166
Ocular Manifestations
Ocular symptoms usually include blurred vision, subconjunctival hemorrhages, eye pain, and photophobia167 with many of these symptoms presenting during the acute, self-limiting phase. More severe ophthalmic findings have been described including bilateral maculopathy, vasculitis, optic neuropathy, and endophthalmitis.168 Foveolitis may even persist and cause a chronic central scotoma.167 Notably, uveitis noted in Dengue survivors has responded well to topical corticosteroids.169
One Health Mitigation of Dengue Virus Disease
Dengue virus outbreaks are exacerbated by numerous determinants including urbanization, climate change, insufficient population immunity, and the rapidly evolving nature of DENV.170 Many of these factors have resulted in an increase in DENV vector distribution in addition to inadequate vector control.171 In fact, since the COVID-19 pandemic, there has been a global resurgence in the number of Dengue cases, with cases in America reaching over 9.7 million as of June of 2024, which is more than double that of 2023.172 Climate change is believed to be a major factor in the recent resurgence of DENV cases, as rising global temperatures create warmer environments more conducive to DENV transmission, survival, and increased biting rates.173
Vector control strategies are crucial and provide a comparably practical approach to DENV management, and include interventions such as removing stagnant water, managing waste, manipulating vector habitats, and decreasing the reproducibility of mosquitoes.170 The incorporation of vector geographic distribution prediction models might also prove to be of use in public health planning.174,175 To reduce insecticide resistance, biological controls, like bacterial larvicide with Bacillus thuringiensis israelensis, can serve as alternatives to vector control strategies that rely on insecticides.176 Though the efficacy of the vaccines Dengvaxia (CYD-TDV) and Qdenga (TAK-003) still require more research, they are somewhat limited as Dengvaxia can only be used in someone with previous infection, while Qdenga vaccine protection in the setting of a highly mutable virus could result in more severe infection in the future.177,178
Chikungunya Virus Disease
Chikungunya virus (CHIKV) is an arbovirus belonging to the family Togaviridae. It is an enveloped, single stranded positive sense RNA virus with four different genotypes that are separated by geographic regions: West African, East/Central/South African, Asian, and Indian Ocean.168 It was initially discovered in present day Tanzania in 1952 where the name Chikungunya originated, meaning “disease that bends up joints.”179 There have been cases internationally reported including in the Americas, Africa, Europe, and Asia. Large outbreaks include Comoros in 2005 with over 200,000 infections, Reunion Island with 3500 confirmed cases and more than 200,000 suspected cases,180 and Kenya in 2004.181 Outbreaks in Americas started in 2013 on Saint Martin Island in the Caribbean with nearly 1 million cases reported by the end of 2015.182 In addition, certain areas have been impacted with dual infection of CHIKV and either DENV or ZIKV. While mortality rates are low (0.1%),179 morbidity can be high. Outbreaks tend to correlate with periods of heavy rains, which can make a more opportune habitat for mosquitos.
Life Cycle and Animal Reservoir
CHIKV entry is clathrin-mediated endocytosis that requires an acidic environment for entry and fusion.183 The viral genome codes E1, a fusion protein, and E2, an attachment protein. These 2 proteins then form a complex by conformational change to enhance endocytosis. Fusion releases nucleocapsid into the cytosol where viral replication compartments named spherules form. Nucleocapsids then assemble and develop mature progeny.179 Infections can occur in human epithelial and endothelial cells, fibroblasts, and macrophages.181 This can then spread throughout the body to several organs including liver, muscle, joints and lymphoid tissue.
Nonhuman primates such as chimpanzees and baboons have been implicated as natural reservoirs for disease in more rural areas.184 Other reservoirs include bats and rodents, although rodents do not exhibit clinical signs and may be dead-end hosts.185 In urban areas, mosquito-to-human-to-mosquito is the most common pattern of transmission with no animal reservoirs identified.186
Transmission
CHIKV can be transmitted to humans by mosquitos of the Aedes genus via blood meals. CHIKV can also be transmitted vertically intrapartum or peripartum, with rare reports of intrauterine transmissions.187,188 Two transmission cycles have been noted for chikungunya: rural/sylvatic and urban. The rural cycle involves mosquito-animal reservoir transmission with occasional spillover into humans.182 Nonhuman primates are the major reservoir in this cycle.181 Urban introduction has occurred largely via Aedes aegypti or Aedes albopictus, with a mosquito-to-human transmission cycle.179 In recent times, the virus has become adapted to urban cycles, which may result in large outbreaks even when lower levels of virus circulation are present.182
Ocular Manifestations
Ocular symptoms can vary based on phase of the disease. In the acute phase, symptoms may include photophobia, retro-orbital pain, and redness of the eye.168 Later in the disease course, patients may have anterior uveitis and keratic precipitates, and there have been reports of retinitis with good recovery.168
One Health Mitigation of Chikungunya Virus Disease
The global spread of CHIKV has been heavily influenced by international travel.189–191 Ecological studies reveal an inverse relationship of a city’s spatial distribution of arbovirus incidence and sanitation, sewage-treatment networks, solid waste management, and urban water drainage. In addition, arbovirus incidence increases with an increase in rainfall depth. Aedes vector control systems utilize environmental methods that range from capturing, killing, or sterilizing mosquitos, including novel interfering RNA formulations used to kill mosquito larvae.192–195 In addition to these studies focused on vector spatial distribution surveillance, studies evaluating case surveillance data from domestic and imported cases of CHIKV disease may increase our knowledge in vector-human transmission dynamics, with possible implications in outbreak predictions and the development of outbreak mitigation strategies.196 The most advanced vaccine against chikungunya virus is VLA1553, which has proved to be well-tolerated and efficacious.197,198 However, since vaccine is a live, attenuated virus, the development of candidate vaccines that are more tolerable by immunocompromised and pregnant patients might prove to be beneficial in a vaccine that would need to be widely distributed.199
CONCLUSION
Given the distinct ophthalmic manifestations observed within a range of emerging infectious diseases including viral hemorrhagic fevers and a number of vector-borne illnesses, it is evident that individual health care provider and public health response, as well as a broad understanding of the disease characteristics, pathogenesis, and a One Health perspective is needed. While clinicians require a keen understanding of the specific ophthalmic manifestations of diseases and existing treatments that address disease mechanism, improved understanding of transmission dynamics may improve the ability to detect risk factors and host-animal-environment interactions that also play a role in disease presentation. Ultimately, a One Health approach is required to address the multiplicity of factors that lead to disease that affect humans, animals, and their shared environments.
The One Health approach may involve multisectoral collaboration to bridge animal and human health dynamics by bringing together experts who evaluate outbreak details from varying perspectives in fields including health, livestock, fisheries, wildlife, environment and disaster respose.32 Moreover, the inclusion of ecologists in One Health efforts can improve assessment and management of environmental risks in disease control and prevention.186,187
One Health approaches to research and global health efforts have improved our understanding of the interconnectedness of pathogens, humans, and the environment, allowing us to develop holistic strategies to prevent and mitigate disease outbreaks. Using this approach when considering ocular manifestations of emerging infectious etiologies may improve disease assessment and management with the broader goal of reducing disease burden and improving clinical outcomes.
Footnotes
K.M.B. and J.T. contributed equally and shared the first authorship.
This project was supported by the National Eye Institute of the National Institutes of Health under award number R01 EY029594 (Yeh). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Grant support is also provided by the Macula Society Retina Research Foundation, ARVO Mallinckrodt Young Investigator Grant, and the Stanley M. Truhlsen Family Foundation Inc. CDC was supported by the National Institutes of Health K08 award EY034892, the Knights Templar Eye Foundation, and a Physician Scientist award from the University of Nebraska Medical Center.
The authors declare that they have no conflicts of interest to disclose.
Contributor Information
K’Mani Blyden, Email: kblyden@augusta.edu.
Joanne Thomas, Email: joanne.jess.thomas@emory.edu.
Parisa Emami-Naeini, Email: parisaemami@gmail.com.
Tolulope Fashina, Email: tfashin1@gmail.com.
Christopher D. Conrady, Email: cconrady@unmc.edu.
Thomas A. Albini, Email: TAlbini@med.miami.edu.
Jessica Carag, Email: jessica.carag@emory.edu.
Steven Yeh, Email: syeh@unmc.edu.
REFERENCES
- 1.https://www.cdc.gov/onehealth/basics/index.html One Health Basics. One Health. CDC. May 10, 2023. Accessed July 26, 2023.
- 2. Mackenzie JS, Jeggo M. The One Health approach—why is it so important? Trop Med Infect Dis. 2019;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uyeki TM, Milton S, Abdul Hamid C, et al. Highly pathogenic avian influenza A(H5N1) virus infection in a dairy farm worker N Engl J Med. 2024;390:2028–2029. [DOI] [PubMed] [Google Scholar]
- 4. CDC. Avian influenza current situation summary. Centers for Disease Control and Prevention. June 6, 2024. Accessed June 7, 2024. https://www.cdc.gov/flu/avianflu/avian-flu-summary.htm
- 5. CDC. CDC confirms three human cases of h5 bird flu among colorado poultry workers. CDC Newsroom. July 30, 2024. Accessed August 18, 2024. https://www.cdc.gov/media/releases/2024/s0725-three-human-cases-of-h5-bird-flu.html
- 6. American Academy of Ophthalmology. Novel H5N1 Bird Flu Outbreak. May 31, 2024. Accessed August 18, 2024. http://www.aao.org/education/bird-flu.
- 7. Hussein HA. Brief review on ebola virus disease and one health approach. Heliyon. 2023;9:e19036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jimenez CEP, Keestra S, Tandon P, et al. Biosecurity and water, sanitation, and hygiene (WASH) interventions in animal agricultural settings for reducing infection burden, antibiotic use, and antibiotic resistance: a One Health systematic review. Lancet Planet Health. 2023;7:e418–e434. [DOI] [PubMed] [Google Scholar]
- 9. Lebov J, Grieger K, Womack D, et al. A framework for One Health research. One Health. 2017;3:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopkins SR, Lafferty KD, Wood CL, et al. Evidence gaps and diversity among potential win-win solutions for conservation and human infectious disease control. Lancet Planet Health. 2022;6:e694–e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacob ST, Crozier I, Fischer WA, et al. Ebola virus disease. Nat Rev Dis Primer. 2020;6:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. PMC. An overview of Ebola virus disease. Accessed October 8, 2023. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5175058/#
- 13. Rugarabamu S, Mboera L, Rweyemamu M, et al. Forty-two years of responding to Ebola virus outbreaks in Sub-Saharan Africa: a review. BMJ Glob Health. 2020;5:e001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. PREVAIL III Study Group. Sneller MC, Reilly C, et al. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380:924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buseh AG, Stevens PE, Bromberg M, et al. The Ebola epidemic in West Africa: challenges, opportunities, and policy priority areas. Nurs Outlook. 2015;63:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu DS, Weng TH, Wu XX, et al. The lifecycle of the Ebola virus in host cells. Oncotarget. 2017;8:55750–55759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salata C, Calistri A, Alvisi G, et al. Ebola Virus entry: from molecular characterization to drug discovery. Viruses. 2019;11:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoenen T, Groseth A, Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17:593–606. [DOI] [PubMed] [Google Scholar]
- 19. Judson SD, Munster VJ. The multiple origins of ebola disease outbreaks. J Infect Dis. 2023;228:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morvan JM, Deubel V, Gounon P, et al. Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic. Microbes Infect. 1999;1:1193–1201. [DOI] [PubMed] [Google Scholar]
- 21. Rouquet P, Froment JM, Bermejo M, et al. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001-2003. Emerg Infect Dis. 2005;11:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kadanali A, Karagoz G. An overview of Ebola virus disease. North Clin Istanb. 2015;2:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. [DOI] [PubMed] [Google Scholar]
- 24. Alexander KA, Sanderson CE, Marathe M, et al. What factors might have led to the emergence of Ebola in West Africa? PLoS Negl Trop Dis. 2015;9:e0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors—final report. N Engl J Med. 2017;377:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet Lond Engl. 2016;388:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mate SE, Kugelman JR, Nyenswah TG, et al. Molecular evidence of sexual transmission of Ebola Virus. N Engl J Med. 2015;373:2448–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clare G. Ebola and the eye. Community Eye Health. 2020;33:81–82. [PMC free article] [PubMed] [Google Scholar]
- 30. Shantha JG, Crozier I, Hayek BR, et al. Ophthalmic manifestations and causes of vision impairment in ebola virus disease survivors in Monrovia, Liberia. Ophthalmology. 2017;124:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sneller MC, Reilly C, Badio M, et al. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380:924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sikakulya FK, Mulisya O, Munyambalu DK, et al. Ebola in the Eastern Democratic Republic of Congo: One Health approach to infectious disease control. One Health. 2020;9:100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Formenty P, Boesch C, Wyers M, et al. Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d’Ivoire. J Infect Dis. 1999;179 Suppl 1(suppl 1):120–126. [DOI] [PubMed] [Google Scholar]
- 34. Olivero J, Fa JE, Real R, et al. Recent loss of closed forests is associated with Ebola virus disease outbreaks. Sci Rep. 2017;7:14291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leendertz SAJ, Wich SA, Ancrenaz M, et al. Ebola in great apes— current knowledge, possibilities for vaccination, and implications for conservation and human health. Mammal Rev. 2017;47:98–111. [Google Scholar]
- 36. Olivero J, Fa JE, Real R, et al. Mammalian biogeography and the Ebola virus in Africa. Mammal Rev. 2017;47:24–37. [Google Scholar]
- 37. Meseko CA, Egbetade AO, Fagbo S. Ebola virus disease control (in West Africa): an ecological, one health approach. Pan Afr Med J. 2015;21:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Egbetade AO, Sonibare AO, Meseko CA, et al. Implications of Ebola virus disease on wildlife conservation in Nigeria. Pan Afr Med J. 2015;22 (suppl 1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plourde AR, Bloch EM. A literature review of Zika Virus. Emerg Infect Dis. 2016;22:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. [DOI] [PubMed] [Google Scholar]
- 41. Krauer F, Riesen M, Reveiz L, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré syndrome: systematic review. PLoS Med. 2017;14:e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Labib BA, Chigbu DI. Pathogenesis and manifestations of Zika virus-associated ocular diseases. Trop Med Infect Dis. 2022;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo J, Ma X, Xu X, et al. Zika virus infection and development of drug therapeutics. Appl Microbiol. 2022;2:782–799. [Google Scholar]
- 45. Vorou R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: what we know and what we should investigate urgently. Int J Infect Dis. 2016;48:85–90. [DOI] [PubMed] [Google Scholar]
- 46. Oliveira Melo AS, Malinger G, Ximenes R, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. [DOI] [PubMed] [Google Scholar]
- 47. Martines RB. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–160. [DOI] [PubMed] [Google Scholar]
- 48. Centeno-Tablante E, Medina-Rivera M, Finkelstein JL, et al. Update on the transmission of Zika virus through breast milk and breastfeeding: a systematic review of the evidence. Viruses. 2021;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gregory CJ, Oduyebo T, Brault AC, et al. Modes of transmission of Zika virus. J Infect Dis. 2017;216(suppl_10):S875–S883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barjas-Castro ML, Angerami RN, Cunha MS, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion (Paris). 2016;56:1684–1688. [DOI] [PubMed] [Google Scholar]
- 51. Ventura CV, Maia M, Travassos SB, et al. Risk factors associated with the ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol. 2016;134:912–918. [DOI] [PubMed] [Google Scholar]
- 52. de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016;134:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Troumani Y, Touhami S, Jackson TL, et al. Association of anterior uveitis with acute Zika virus infection in adults. JAMA Ophthalmol. 2021;139:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fontes BM. Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol. 2016;79:63–63. [DOI] [PubMed] [Google Scholar]
- 55. Oliver GF, Carr JM, Smith JR. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: a review. Clin Experiment Ophthalmol. 2019;47:372–380. [DOI] [PubMed] [Google Scholar]
- 56. Miner JJ, Sene A, Richner JM, et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 2016;16:3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun J, Wu D, Zhong H, et al. Presence of Zika virus in conjunctival fluid. JAMA Ophthalmol. 2016;134:1330–1332. [DOI] [PubMed] [Google Scholar]
- 58. Tan JJL, Balne PK, Leo YS, et al. Persistence of Zika virus in conjunctival fluid of convalescence patients. Sci Rep. 2017;7:11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Brien E, Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health. 2019;7:100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organization. Handbook for Integrated Vector Management. World Health Organization; 2012: 67. [Google Scholar]
- 61. Eddy C, Sase E. Part 1: the Zika virus threat and prevention challenges: an all-hazards and one health approach to pandemic and global epidemic prevention and mitigation. J Environ Health. 2021;84:8–19. [Google Scholar]
- 62. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmiege D, Perez Arredondo AM, Ntajal J, et al. One Health in the context of coronavirus outbreaks: a systematic literature review. One Health. 2020;10:100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen B, Tian EK, He B, et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther. 2020;5:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peiris JSM. Coronaviruses. Med Microbiol. 2012:587–593. doi: 10.1016/B978-0-7020-4089-4.00072-X. [DOI] [Google Scholar]
- 67. Mulabbi EN, Tweyongyere R, Byarugaba DK. The history of the emergence and transmission of human coronaviruses. Onderstepoort J Vet Res. 2021;88:1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Woo PCY, Lau SKP, Huang Y, et al. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. 2009;234:1117–1127. [DOI] [PubMed] [Google Scholar]
- 69. Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nziza J, Goldstein T, Cranfield M, et al. Coronaviruses detected in bats in close contact with humans in Rwanda. EcoHealth. 2020;17:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.http://www.frontiersin.org/articles/10.3389/fpubh.2020.00155/full. http://www.frontiersin.org/articles/10.3389/fpubh.2020.00155/full Frontiers. Role of the eye in transmitting human coronavirus: what we know and what we do not know. Accessed September 5, 2023.
- 73. Shantha JG, Fashina T, Stittleburg V, et al. COVID-19 and the eye: Systemic and laboratory risk factors for retinopathy and detection of tear film SARS-CoV-2 RNA with a triplex RT-PCR assay. PLoS One. 2022;17:e0277301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) affect the eyes? A Review Of Coronaviruses And Ocular Implications In Humans And Animals. Ocul Immunol Inflamm. 2020;28:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wood JLN, Leach M, Waldman L, et al. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos Trans R Soc B Biol Sci. 2012;367:2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Plowright RK, Ahmed AN, Coulson T, et al. Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nat Commun. 2024;15:2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kosik I, Yewdell JW. Influenza hemagglutinin and neuraminidase: yin–yang proteins coevolving to thwart immunity. Viruses. 2019;11:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Webster RG, Bean WJ, Gorman OT. Evolution and ecology of influenza A viruses. Microbiol Rev 1992. Accessed June 16, 2024. https://journals.asm.org/doi/epdf/10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Perroncito E. Epizoozia tifoide nei gallinacei. Ann Accad Agri Torino. 1878:87–126. [Google Scholar]
- 81. Alexander DJ, Brown IH. History of highly pathogenic avian influenza. Rev Sci Tech Int Off Epizoot. 2009;28:19–38. [DOI] [PubMed] [Google Scholar]
- 82. Lupiani B, Reddy SM. The history of avian influenza. Comp Immunol Microbiol Infect Dis. 2009;32:311–323. [DOI] [PubMed] [Google Scholar]
- 83. Krauss S, Stucker KM, Schobel SA, et al. Long-term surveillance of H7 influenza viruses in American wild aquatic birds: are the H7N3 influenza viruses in wild birds the precursors of highly pathogenic strains in domestic poultry? Emerg Microbes Infect. 2015;4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wille M, Barr IG. Resurgence of avian influenza virus. Science. 2022;376:459–460. [DOI] [PubMed] [Google Scholar]
- 85. Samji T. Influenza A: understanding the viral life cycle. Yale J Biol Med. 2009;82:153–159. [PMC free article] [PubMed] [Google Scholar]
- 86. Boulo S, Akarsu H, Ruigrok RWH, et al. Nuclear traffic of influenza virus proteins and ribonucleoprotein complexes. Virus Res. 2007;124:12–21. [DOI] [PubMed] [Google Scholar]
- 87. Nguyen KT, Holloway MP, Altura RA. The CRM1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol. 2012;3:137–151. [PMC free article] [PubMed] [Google Scholar]
- 88.https://www.cdc.gov/bird-flu/virus-transmission/index.html CDC. Transmission of avian influenza A viruses between animals and people. Avian Influenza (Bird Flu). June 5, 2024. Accessed June 16, 2024.
- 89. Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet Lond Engl. 1998;351:472–477. [DOI] [PubMed] [Google Scholar]
- 90. Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. [DOI] [PubMed] [Google Scholar]
- 91. Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. [DOI] [PubMed] [Google Scholar]
- 92. Claas EC, Kawaoka Y, de Jong JC, et al. Infection of children with avian-human reassortant influenza virus from pigs in Europe. Virology. 1994;204:453–457. [DOI] [PubMed] [Google Scholar]
- 93. Castrucci MR, Donatelli I, Sidoli L, et al. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193:503–506. [DOI] [PubMed] [Google Scholar]
- 94. Hinshaw VS, Webster RG, Easterday BC, et al. Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Burrough ER, Magstadt DR, Petersen B, et al. Highly pathogenic avian influenza A (H5N1) Clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis. 2024;30:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock Highly pathogenic avian influenza (HPAI) detections in livestock. Animal and Plant Health Inspection Service. Accessed June 16, 2024.
- 97.https://www.cdc.gov/bird-flu/spotlights/h5n1-response-07192024.html CDC. CDC A(H5N1) bird flu response update, July 19, 2024. Avian Influenza (Bird Flu). July 22, 2024. Accessed August 18, 2024.
- 98. Hayden F, Croisier A. Transmission of Avian influenza viruses to and between humans. J Infect Dis. 2005;192:1311–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Olofsson S, Kumlin U, Dimock K, et al. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005;5:184–188. [DOI] [PubMed] [Google Scholar]
- 100. Matrosovich MN, Matrosovich TY, Gray T, et al. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci USA. 2004;101:4620–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Webster RG. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Arch Virol Suppl. 1997;13:105–113. [DOI] [PubMed] [Google Scholar]
- 102. Lewis D. Avian flu to human influenza. Annu Rev Med. 2006;57:139–154. [DOI] [PubMed] [Google Scholar]
- 103. Belser JA, Wadford DA, Xu J, et al. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J Virol. 2009;83:7075–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Belser JA, Lash RR, Garg S, et al. The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect Dis. 2018;18:e220–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bui VN, Ogawa H, Ngo LH, et al. H5N1 highly pathogenic avian influenza virus isolated from conjunctiva of a whooper swan with neurological signs. Arch Virol. 2013;158:451–455. [DOI] [PubMed] [Google Scholar]
- 106. Yamamoto Y, Nakamura K, Yamada M, et al. Corneal opacity in domestic ducks experimentally infected with H5N1 highly pathogenic avian influenza virus. Vet Pathol. 2016;53:65–76. [DOI] [PubMed] [Google Scholar]
- 107. Belser JA, Maines TR, Creager HM, et al. Oseltamivir inhibits influenza virus replication and transmission following ocular-only aerosol inoculation of ferrets. Virology. 2015;484:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Belser JA, Sleeman K, Pearce MB, et al. Oseltamivir inhibits H7 influenza virus replication in mice inoculated by the ocular route. Antimicrob Agents Chemother. 2012;56:1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ryu S, Kim BI, Lim JS, et al. One Health perspectives on emerging public health threats. J Prev Med Pub Health. 2017;50:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gilbert M, Slingenbergh J, Xiao X. Climate change and avian influenza. Rev Sci Tech Int Off Epizoot. 2008;27:459. [PMC free article] [PubMed] [Google Scholar]
- 111. Caserta LC, Frye EA, Butt SL, et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature. 2024. Published online July 25. doi: 10.1038/s41586-024-07849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Eisfeld AJ, Biswas A, Guan L, et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature. 2024;633:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lang AS, Kelly A, Runstadler JA. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J Gen Virol. 2008;89:509–519. [DOI] [PubMed] [Google Scholar]
- 114. Wedari NLPH, Sukrama IDM, Budayanti NNS, et al. One Health concept and role of animal reservoir in avian influenza: a literature review. Bali Med J. 2021;10:515–520. [Google Scholar]
- 115. Lehmann T, Marcet PL, Graham DH, et al. Globalization and the population structure of Toxoplasma gondii . Proc Natl Acad Sci USA. 2006;103:11423–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Smith NC, Goulart C, Hayward JA, et al. Control of human toxoplasmosis. Int J Parasitol. 2021;51:95–121. [DOI] [PubMed] [Google Scholar]
- 117. Kalogeropoulos D, Sakkas H, Mohammed B, et al. Ocular toxoplasmosis: a review of the current diagnostic and therapeutic approaches. Int Ophthalmol. 2022;42:295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Khurana S, Batra N. Toxoplasmosis in organ transplant recipients: Evaluation, implication, and prevention. Trop Parasitol. 2016;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Aguirre AA, Longcore T, Barbieri M, et al. The One Health approach to toxoplasmosis: epidemiology, control, and prevention strategies. Ecohealth. 2019;16:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. McAuley J, Boyer KM, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. [DOI] [PubMed] [Google Scholar]
- 121. Dubey JP, Weigel RM, Siegel AM, et al. Sources and reservoirs of Toxoplasma gondii infection on 47 swine farms in Illinois. J Parasitol. 1995;81:723–729. [PubMed] [Google Scholar]
- 122. Frenkel JK. Toxoplasma in and around us. BioScience. 1973;23:343–352. [Google Scholar]
- 123. Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii . Proc R Soc B Biol Sci. 2000;267:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. [DOI] [PubMed] [Google Scholar]
- 125. Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136:973–988. [DOI] [PubMed] [Google Scholar]
- 126. Lachenmaier SM, Deli MA, Meissner M, et al. Intracellular transport of Toxoplasma gondii through the blood–brain barrier. J Neuroimmunol. 2011;232:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Coppens I, Romano JD. Hostile intruder: toxoplasma holds host organelles captive. PLOS Pathog. 2018;14:e1006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Koethe M, Schade C, Fehlhaber K, et al. Survival of Toxoplasma gondii tachyzoites in simulated gastric fluid and cow’s milk. Vet Parasitol. 2017;233:111–114. [DOI] [PubMed] [Google Scholar]
- 129. Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii . Nature. 1985;315:416–419. [DOI] [PubMed] [Google Scholar]
- 130. Conrady CD, Pradeep T, Yu Y, et al. Association of proton pump inhibitor/histamine2 blocker use and ocular toxoplasmosis: findings from a large US National Database. Ophthalmol Retina. 2023;7:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Greigert V, Bittich-Fahmi F, Pfaff AW. Pathophysiology of ocular toxoplasmosis: facts and open questions. PLoS Negl Trop Dis. 2020;14:e0008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep. 2010;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. de Barros RAM, Torrecilhas AC, Marciano MAM, et al. Toxoplasmosis in human and animals around the world. Diagnosis and perspectives in the One Health approach. Acta Trop. 2022;231:106432. [DOI] [PubMed] [Google Scholar]
- 134. Flegr J, Prandota J, Sovičková M, et al. Toxoplasmosis—a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9:e90203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nogareda F, Strat YL, Villena I, et al. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol Infect. 2014;142:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Du F, Zhang Q, Yu Q, et al. Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet Parasitol. 2012;187:53–56. [DOI] [PubMed] [Google Scholar]
- 137. VanWormer E, Conrad PA, Miller MA, et al. Toxoplasma gondii, source to sea: higher contribution of domestic felids to terrestrial parasite loading despite lower infection prevalence. EcoHealth. 2013;10:277–289. [DOI] [PubMed] [Google Scholar]
- 138. Gao YM, Ding H, Lamberton PHL, et al. Prevalence of Toxoplasma gondii in pet dogs in mainland China: a meta-analysis. Vet Parasitol. 2016;229:126–130. [DOI] [PubMed] [Google Scholar]
- 139. Chu CS, Stolbrink M, Stolady D, et al. Severe Falciparum and Vivax malaria on the Thailand-Myanmar border: a review of 1503 cases. Clin Infect Dis. 2023;77. Published online doi: 10.1093/cid/ciad262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Milner DA. Malaria pathogenesis. Cold Spring Harb Perspect Med. 2018;8:a025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Meibalan E, Marti M. Biology of malaria transmission. Cold Spring Harb Perspect Med. 2017;7:a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lewallen S, Taylor TE, Molyneux ME, et al. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–861. [DOI] [PubMed] [Google Scholar]
- 143.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3804549/ PMC. Using malarial retinopathy to improve the classification of children with cerebral malaria. Accessed September 6, 2023.
- 144. Seydel KB, Kampondeni SD, Valim C, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Looareesuwan S, Warrell DA, White NJ, et al. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am J Trop Med Hyg. 1983;32:911–915. [DOI] [PubMed] [Google Scholar]
- 146. Beare NA, Taylor TE, Harding SP, et al. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 147. Paquet-Durand F, Beck SC, Das S, et al. A retinal model of cerebral malaria. Sci Rep. 2019;9:3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wilson AL, Courtenay O, Kelly-Hope LA, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14:e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.https://iris.who.int/handle/10665/310862 World Health Organization. Guidelines for Malaria Vector Control. World Health Organization; 2019. Accessed July 23, 2024.
- 151. Ranson H, Lissenden N. Insecticide resistance in African anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. [DOI] [PubMed] [Google Scholar]
- 152. Sutanto H. Combating malaria with vaccines: insights from the One Health framework. Acta Microbiol Hell. 2024;69:153–166. [Google Scholar]
- 153. Soman Pillai V, Krishna G, Valiya Veettil M. Nipah Virus: Past Outbreaks And Future Containment. Viruses. 2020;12:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Singh RK, Dhama K, Chakraborty S, et al. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—a comprehensive review. Vet Q. 2019;39:26–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rawlinson SM, Zhao T, Rozario AM, et al. Viral regulation of host cell biology by hijacking of the nucleolar DNA-damage response. Nat Commun. 2018;9:3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sejvar JJ, Hossain J, Saha SK, et al. Long-term neurological and functional outcome in Nipah virus infection. Ann Neurol. 2007;62:235–242. [DOI] [PubMed] [Google Scholar]
- 157. Lim CCT, Lee WL, Leo YS, et al. Late clinical and magnetic resonance imaging follow up of Nipah virus infection. J Neurol Neurosurg Psychiatry. 2003;74:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chattu VK, Kumar R, Kumary S, et al. Nipah virus epidemic in southern India and emphasizing “One Health” approach to ensure global health security. J Fam Med Prim Care. 2018;7:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah Virus. Clin Infect Dis. 2009;49:1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mishra G, Prajapat V, Nayak D. Advancements in Nipah virus treatment: analysis of current progress in vaccines, antivirals, and therapeutics. Immunology. 2024;171:155–169. [DOI] [PubMed] [Google Scholar]
- 161. Safdar M, Rehman S ur, Younus M, et al. One Health approach to Nipah virus prevention. Vacunas Engl Ed. 2024;25:264–273. [Google Scholar]
- 162. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.https://www.nature.com/scitable/topicpage/dengue-viruses-22400925/# Dengue Viruses. Learn Science at Scitable. Accessed October 5, 2023.
- 165. Gwee SXW, St John AL, Gray GC, et al. Animals as potential reservoirs for dengue transmission: a systematic review. One Health Amst Neth. 2021;12:100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Levi JE. Dengue virus and blood transfusion. J Infect Dis. 2016;213:689–690. [DOI] [PubMed] [Google Scholar]
- 167. Teoh BT, Sam SS, Abd-Jamil J, et al. Isolation of Ancestral Sylvatic Dengue Virus Type 1, Malaysia. Emerg Infect Dis. 2010;16:1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Rishi E, Thomas J, Fashina T, et al. Emerging pathogenic viral infections of the eye. Annu Rev Vis Sci. 2023;9:71–89. [DOI] [PubMed] [Google Scholar]
- 169. Gupta A, Srinivasan R, Setia S, et al. Uveitis following dengue fever. Eye. 2009;23:873–876. [DOI] [PubMed] [Google Scholar]
- 170. Procopio AC, Colletta S, Laratta E, et al. Integrated One Health strategies in dengue. One Health. 2024;18:100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Descloux E, Mangeas M, Menkes CE, et al. Climate-based models for understanding and forecasting dengue epidemics. PLoS Negl Trop Dis. 2012;6:e1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.https://emergency.cdc.gov/han/2024/han00511.asp Health Alert Network (HAN) - 00511. Increased risk of dengue virus infections in the United States. June 25, 2024. Accessed August 19, 2024.
- 173. Brady OJ, Johansson MA, Guerra CA, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus . Elife. 2015;4:e08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Oliveira S, Rocha J, Sousa CA, et al. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci Rep. 2021;11:9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bohari R, Hin CJ, Matusop A, et al. Wide area spray of bacterial larvicide, Bacillus thuringiensis israelensis strain AM65-52, integrated in the national vector control program impacts dengue transmission in an urban township in Sibu district, Sarawak, Malaysia. PLoS One. 2020;15:e0230910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis. 2016;213:1562–1572. [DOI] [PubMed] [Google Scholar]
- 178. Rivera L, Biswal S, Sáez-Llorens X, et al. Three-year efficacy and safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin Infect Dis. 2022;75:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest, 127:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bessaud M, Peyrefitte CN, Pastorino BAM, et al. Chikungunya virus strains, reunion island outbreak. Emerg Infect Dis. 2006;12:1604–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Vairo F, Haider N, Kock R, et al. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33:1003–1025. [DOI] [PubMed] [Google Scholar]
- 182. de Lima Cavalcanti TYV, Pereira MR, de Paula SO, et al. A review on Chikungunya virus epidemiology, pathogenesis and current vaccine development. Viruses. 2022;14:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Reis EVS, Damas BM, Mendonça DC, et al. In-depth characterization of the Chikungunya virus replication cycle. J Virol. 96:e01732–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Matusali G, Colavita F, Bordi L, et al. Tropism of the Chikungunya virus. Viruses. 2019;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Vourc’h G, Halos L, Desvars A, et al. Chikungunya antibodies detected in non-human primates and rats in three Indian Ocean islands after the 2006 ChikV outbreak. Vet Res. 2014;45:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.https://www.who.int/news-room/fact-sheets/detail/chikungunya Chikungunya fact sheet. Accessed October 5, 2023.
- 187. Torres JR, Falleiros-Arlant LH, Dueñas L, et al. Congenital and perinatal complications of Chikungunya fever: a Latin American experience. Int J Infect Dis. 2016;51:85–88. [DOI] [PubMed] [Google Scholar]
- 188. Faria BS, da Silva LB, Avelar CFR, et al. Vertical transmission of chikungunya virus: a worldwide concern. Braz J Infect Dis. 2024;28:103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Mourad O, Makhani L, Chen LH. Chikungunya: an emerging public health concern. Curr Infect Dis Rep. 2022;24:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hamer DH, Chen LH. Chikungunya: establishing a New Home in the Western Hemisphere. Ann Intern Med. 2014;161:827–828. [DOI] [PubMed] [Google Scholar]
- 191.https://www.cdc.gov/chikungunya/data-maps/chikungunya-us.html CDC. Chikungunya in the United States. Chikungunya Virus. May 20, 2024. Accessed July 28, 2024.
- 192. Barrera R. New tools for Aedes control: mass trapping. Curr Opin Insect Sci. 2022;52:100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tambwe MM, Saddler A, Kibondo UA, et al. Semi-field evaluation of the exposure-free mosquito electrocuting trap and BG-Sentinel trap as an alternative to the human landing catch for measuring the efficacy of transfluthrin emanators against Aedes aegypti . Parasit Vectors. 2021;14:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sippy R, Rivera GE, Sanchez V, et al. Ingested insecticide to control Aedes aegypti: developing a novel dried attractive toxic sugar bait device for intra-domiciliary control. Parasit Vectors. 2020;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wang GH, Gamez S, Raban RR, et al. Combating mosquito-borne diseases using genetic control technologies. Nat Commun. 2021;12:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chou YC, Hsieh CJ, Cheng CA, et al. Epidemiologic characteristics of imported and domestic Chikungunya cases in Taiwan: a 13-year retrospective study. Int J Environ Res Public Health. 2020;17:3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wressnigg N, Hochreiter R, Zoihsl O, et al. Single-shot live-attenuated Chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect Dis. 2020;20:1193–1203. [DOI] [PubMed] [Google Scholar]
- 198. Roques P, Fritzer A, Dereuddre-Bosquet N, et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight. 2022;7:e160173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Bartholomeeusen K, Daniel M, LaBeaud DA, et al. Chikungunya fever. Nat Rev Dis Primer. 2023;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]