Abstract
The signaling pathway that comprises cyclic guanosine monophosphate–adenosine monophosphate (cGAMP or GMP-AMP) synthase (cGAS) and Stimulator of Interferon Genes (STING) is emerging as a druggable target for immunotherapy, with tumor-resident dendritic cells (DC) playing a critical role in mediating its effects. The STING receptor is part of the DNA-sensing cellular machinery, that can trigger the secretion of pro-inflammatory mediators, priming effector T cells and initiating specific antitumor responses. Yet, recent studies have highlighted the dual role of STING activation in the context of cancer: STING can either promote antitumor responses or enhance tumor progression. This dichotomy often depends on the cell type in which cGAS-STING signaling is induced and the activation mode, namely acute versus chronic. Of note, STING activation at the DC level appears to be particularly important for tumor eradication. This review outlines the contribution of the different conventional and plasmacytoid DC subsets and describes the mechanisms underlying STING-mediated activation of DCs in cancer. We further highlight how the STING pathway plays an intricate role in modulating the function of DCs embedded in tumor tissue. Additionally, we discuss the strategies being employed to harness STING activation for cancer treatment, such as the development of synthetic agonists and nano-based delivery systems, spotlighting the current techniques used to prompt STING engagement specifically in DCs.
Keywords: dendritic cell, cGAS-STING pathway, cancer immunotherapy, nanoparticle
Introduction
The paradigm of natural antitumor immunity involves the recognition, processing and presentation of cancer-associated antigens by dendritic cells (DCs) on major histocompatibility (MHC) complexes, triggering effective T cell-mediated antitumor immunity that results in killing of the cancer cells.1–4 Accordingly, DCs are highly specialized tissue-scanning cells equipped with a plethora of receptors to detect damage- or pathogen-associated molecular patterns (DAMPs or PAMPs). Although the immune system’s task of filtering and responding to onco-signals emanating from cancerous tissue is an exceedingly complex one, the wide range of innate receptors expressed by DCs enables them to effectively recognize several by-products associated with tumor growth, such as tumor-derived DNA present in the tumor microenvironment (TME).5–7 The cGAS-STING pathway is an innate DNA-sensing mechanism which was originally described to mediate protective immune responses against infections but was recently found to play a crucial role in driving antitumor immunity. In particular, activation of STING signaling in DCs has the potential to generate spontaneous as well as long-lasting and highly specific antitumor immunity.8–10 This review focuses on the contribution of individual DC subsets to cancer defense and explores emerging developments in STING-mediated activation of DCs for novel cancer therapeutics.
The cGAS-STING Pathway and Its Effects in Cancer Cells
The cGAS-cGAMP-STING signaling pathway, discovered in 2013, is a promising therapeutic target to enhance antitumor immunity. Its activation induces an inflammatory response that converts “cold” tumors into “hot” tumors, ie, cancers that have higher infiltration of immune cells and consequently are more likely to exhibit higher response rates to currently used immunotherapies such as immune checkpoint inhibitors (ICI).8,9,11 STING is a ubiquitous intracellular receptor that is located in the endoplasmic reticulum membrane and activated by binding of the agonist cGAMP. cGAMP is a naturally occurring cyclic dinucleotide (CDN) produced when the enzyme cGAS detects intracellular nucleic acids. Upon binding cGAMP, STING dimerizes and undergoes vesicular trafficking to the Golgi. This triggers downstream signaling which involves activation of TANK binding kinase 1 (TBK1), phosphorylation of interferon (IFN) regulatory factor 3 (IRF3), and induction of nuclear factor kappa B (NF-κB).12,13 As a result, type I IFNs (IFN I) are upregulated, leading to expression and secretion of pro-inflammatory factors that can affect the TME and influence disease progression.14–16 In cancer cells, the cGAS-STING pathway is frequently activated due to genomic instability caused by higher occurrence of mitotic errors compared to healthy cells. Consequently, tumors are rich in micronuclei, and chromatin bridges. Micronuclei consist of residual chromosomes or chromosome fragments that are surrounded by a nuclear envelope that persists after mitosis. These structures are prone to rupture, and by releasing nucleic acids into the cytosol, they have been reported to trigger STING activation.17,18 Interestingly, Flynn et al report that in spite of the ability of these structures to efficiently recruit cGAS, they fail to activate the cGAS-STING pathway.19 Instead, the authors convincingly show that chromatin bridges, single-handedly trigger cGAS activation, and subsequent IFN signaling.19 Similarly to micronuclei, these structures are also byproducts of mitotic processes which persist throughout interphase and can be strongly stretched, until they break due to tension caused by cell migration.17 Self-chromatin can induce STING activation, that in turn, has the potential to impede early tumor progression by upregulating inflammatory genes and by inducing apoptosis as well as cell cycle arrest, in a process mediated by IFN I and NF-κB.20,21 In melanoma, for example, restoration of STING signaling in tumor cells increases their immunogenicity and thus their recognition by T cells.22 Species-specific molecular determinants of self-DNA reactivity of cGAS were also uncovered using a melanoma model, which contributed to a better understanding of innate immunity involved in self-nonself discrimination. By engineering melanoma cells to express several species-specific cGAS transgenes with a doxycycline-inducible system, the authors showed that the key factor that protected mice from tumor-related mortality was the self-DNA reactive cGAS present in melanoma cells.23 In fact, induction of STING in tumor cells can induce cell death, increasing the availability of tumor antigens to be recognized by antigen-presenting cells, such as DCs. Accordingly, besides the self-sustained protection mechanisms supported by STING activation in cancer cells, the continuous engagement of this receptor recruits various immune cells to the TME; therefore, enabling the cGAS-STING pathway in the immune compartment is of critical importance to overcome the generation of tumor-suppressive immune cells by the TME.24,25
STING-Mediated Activation in DCs
Tumor-resident DCs are attractive targets for cancer immunotherapy because potent activation of DCs can initiate effective antitumor immune responses by priming CD4+ and CD8+ T cells.1,26,27 Upon recognition of PAMPs or DAMPs, DCs undergo a severe phenotypic and functional transformation, characterized by the secretion of a wide variety of inflammatory cytokines and the expression of maturation and activation markers such as MHC II and CD86. During this process, DCs become competent in cross-talking with lymphocytes, sustaining the expansion of specific effector cell subsets. As activation of the cGAS-STING pathway is well-known to promote DC activation, efforts have been made to understand the impact of STING activation in DCs within the tumor context. Accordingly, while it is widely accepted that DCs undergo a metabolic shift from oxidative phosphorylation to glycolysis upon activation, Hu et al recently found that increased ATP production via glycolysis facilitates STING-mediated antitumor functions in tumor-resident DCs.28,29 In an attempt to unravel the discrepancies observed with respect to the effects of STING activation in the tumor context in murine models and the human setting, Pang et al discovered that STING stimulation activated plasmacytoid (pDC) and conventional (cDC) DC subsets in both species, resulting in increased production of IFN I.30 The authors also reported differential cell death responses upon STING activation, with rapid ablation occurring particularly for pDCs, highlighting the importance of understanding the impact of STING activation on different DC subsets.
cDC1s
cDC1s, identified by CD141+ in humans and CD103+ or CD8α+ markers in murine models, play a vital role in antitumor immunity by efficiently presenting tumor antigens on MHC I and thereby activating cytotoxic CD8+ T cells.1,31 Using mice with deficiencies in different subsets of antigen-presenting cells, Wang et al demonstrated that specific STING licensing of cDC1s using cGAMP-loaded micelle nanoparticles (NPs) is essential for tumor rejection in mice. These NPs not only inhibited tumor growth in vivo but also induced a STING-activated cDC1 signature that correlated with higher survival rates among patients with non-small cell lung cancer (NSCLC).32 Another recent study found that the antitumor effects induced by cGAMP encapsulated in virus-like NPs also required STING activation in resident cDC1s (XCR1+ CD172−) but not in cDC2s. The choice of delivery vehicle, route of administration and STING agonist are all crucial factors, as the same study reported that administration of the synthetic CDN ADU-S100 induced cDC1 ablation.33 In fact, ADU-S100 activates STING in cDC1s and not only fails to engage cDC2s but also induces upregulation of T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), a traditional exhaustion marker.34 Of note, another study reported that inhibiting TIM-3 also supports activation of the cGAS-STING pathway and increases the uptake of extracellular DNA in intratumoral cDC1s, emphasizing the role of TIM-3 as a regulator of DNA-sensing mechanisms.35 Interestingly, cDC1 activation can also be prompted by IFN I secreted from STING-activated CD4+ T cells, thus establishing a mechanism that further supports antitumor immunity.36
cDC2s
cDC2s, defined as CD1c+ and CD11b+ cells in humans and in murine animals, respectively, play a crucial role in halting cancer progression by directly promoting CD4+ T helper responses that support cytotoxic responses induced by CD8+ T cells.37 Ulrich-Lewis et al convincingly demonstrated that upon treatment with a DNA vaccine, STING−/− and cDC STING conditional knockout mice show lower Th1 responses compared to wild-type animals.38 In line with previous findings, blocking of TIM-3 has been reported to enhance antitumor immunity mediated by STING agonist in 4T1 tumor models, by regulating cDC2s and improving the release of CD4+ T cells.34
pDCs
Plasmacytoid DCs (pDCs) have a lower ability to act as antigen-presenting cells compared to cDCs. However, they are major producers of IFN and are key players in innate immunity, particularly in mounting responses against viral infections.39,40 In 2016, work by Bode et al demonstrated that human pDCs detect cytosolic DNA and CDNs via the cGAS-STING pathway, leading to robust activation and identifying pDCs as a potential target for new antitumor strategies.41 However, while stimulation of human pDCs with cGAMP or double-stranded immunostimulatory DNA (dsDNA) did induce nuclear translocation of IRF3, NF-κB was not activated.42 Interestingly, STING signaling in pDCs has been shown to be susceptible to TME cues, as it is impaired in melanomas.43 Furthermore, Brewitz et al reported that pDC-secreted IFN can support maturation and cross presentation of cDCs in close proximity, enhancing CD8+ T cell-mediated immunity, and thus pinpointing pDCs as potential targets of interest for novel cancer therapies.44
Tumor-Associated DCs
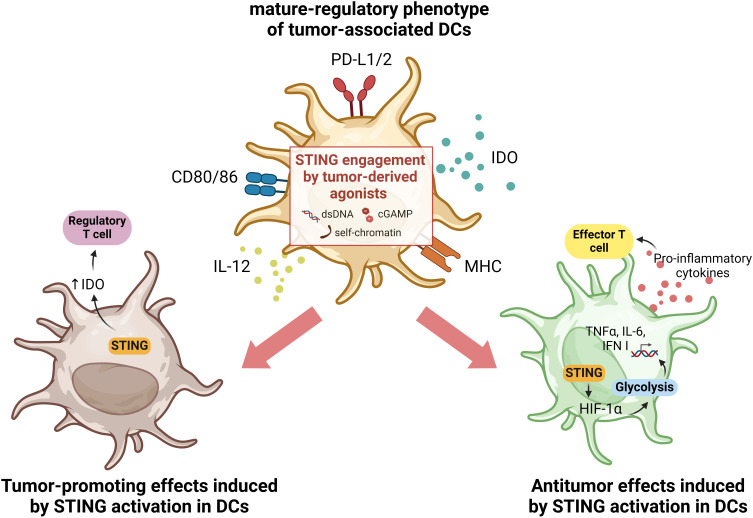
In the context of cancer, all DC subsets may be present within the TME, albeit with different phenotypes and functions compared to DCs present in healthy tissues. Initially identified in NSCLC patients by Maier et al, tumor-associated DCs are characterized by high expression of activation markers such as MHC and CD80/86, as well as markers associated with immune tolerance such as indoleamine 2,3-dioxygenase (IDO) and programmed death-ligand 1 (PD-L1).27,45–47 Thus, tumor-associated DCs are often referred to as mature-regulatory DCs (mregDCs) and both type I and type II conventional DCs, cDC1s and cDC2s respectively, can acquire that phenotype. mregDCs are metabolically very active and play a critical role in modulating the TME by influencing the recruitment and function of other immune cells, exerting significant control over tumor progression.27 By increasing the expression of regulatory genes, mregDCs can interfere with T-cell responses by favoring regulatory T-cell polarization and decreasing the number of antitumor effector T cells.48,49 Accordingly, while several studies describe tumor-suppressing effects of STING signaling, STING activation in the TME has also been reported to induce IDO expression in tumor-associated DCs in a murine model of lung carcinoma. Moreover, the authors showed that only in tumors with low antigenicity did DNA-sensing by STING promote a suppressive TME and thus tumor growth.49 In contrast, activation of STING signaling in tumor-associated DCs in hepatocellular carcinoma models increased the secretion of CXCL9 and IL-12, leading to infiltration and activation of natural killer (NK) cells.50 The STING-mediated antitumor function of DCs can also be induced by oxidative stress in the TME. In fact, Hu and co-authors showed that oxidative stress is required for DC antitumor functions mediated by STING, as it promotes the accumulation of the redox sensor SUMO Specific Peptidase 3 (SENP3) which detects reactive oxygen species (ROS) and enables STING-mediated antitumor responses.51 Oxidative stress, mediated by ROS, is a feature of the TME that can activate immunosuppressive mechanisms and also induce DNA oxidation, which enhances immune recognition by DCs.33,34 Due to the rapid growth of cells during neoplasm formation, tumors are often deprived of oxygen, and the hypoxic TME can intensify the oxidative stress. This leads to a metabolic shift of tumor-resident DCs from oxidative phosphorylation to glycolysis, thus supporting downstream antitumor responses mediated by STING. As a result of STING activation induced by glycolysis, there is upregulation of hypoxia-inducible factor 1 α (HIF-1α) that can sustain a self-perpetuating HIF-1α-mediated glycolysis, prompting DC activation.29 Engagement of STING receptor is essential to start the activation and maturation of antigen-bearing tumor-resident DCs, supporting adaptive immunity by effector T cells (Figure 1).8,29,52
Figure 1.
Illustration of the dual effects of STING activation in tumor-associated DCs. Tumor-associated DCs often display a mature-regulatory phenotype, characterized by the upregulation of both co-stimulatory as well as co-inhibitory molecules. In this context, it has been reported that the activation of STING in tumor-associated DCs may also play a dual role. On the one hand, engagement of the STING receptor can sustain antitumor effects by inducing HIF-1α, which supports glycolysis in DCs, associated with an active and mature phenotype of these cells. This induces an IFN I-mediated response with secretion of pro-inflammatory cytokines that trigger effector T cell responses fighting the tumor (right panel). Conversely, activation of the STING pathway has also been reported to support pro-tumorigenesis, by inducing regulation of IDO expression and consequently enhancing regulatory T cell polarization (left panel). Created with BioRender.com.
STING Activation Strategies and Application in Cancer Therapies
Due to the strong immunostimulatory effect of STING signaling in immune oncology, much effort has focused on identifying the optimal agonist. Recent reports show that not only the induction of STING in immune cells can reduce TME-induced immunosuppression but also highlight that some agonists have the potential to enhance T cell infiltration at the tumor site, addressing a critical limitation of the success of adoptive cell therapy (ACT).53,54 Both Benoit-Lizon and Li have reported that STING agonists can directly activate T cells with no significant associated toxicity, improving effector responses mediated by CD8+ T cells, and Th1 and Th9, respectively.55,56 As STING induction may enhance anti-tumor immunity triggered by ICI and ACT, and as these therapies can be combined, it is possible that a suitable agonist may be the missing link for improved clinical success.56,57 Furthermore, Xu et al have shown that CAR T cells stimulated with STING agonists cGAMP or 5.6-dimethylxanthenone-4-acetic acid (DMXAA) improved the control of tumor development as it enhanced the permanence of CAR T cells at the tumor site. Of note, the authors also show that sustained anti-tumor responses required co-treatment with ICI or anti-GR-1 Ab, which deplete myeloid-derived suppressor cells.54 The relevance of the cGAS-STING pathway as a druggable target in immune-oncology is largely acknowledged for its classical inflammatory response, but also for its ability to induce anti-cancer immunity via IFN I–independent mechanisms (described in10). Hines et al have nicely reviewed candidate agonist molecules currently being evaluated clinically.58 In broad terms, STING-activating agents can be categorized into CDNs and non-CDNs of natural or of synthetic origin.
Natural Agonists in the Tumor Context
cGAMP is a CDN that is expressed in both physiological and pathological conditions.59 It can activate STING directly in the cell of origin or it can act on surrounding cells.60,61 However, due to its hydrophilic nature, it cannot passively diffuse through the cell membrane, requiring protein transporters for intercellular access. Solute Carrier Family 19 Member 1 (SLC19A1) was the first reported cGAMP importer, but several others have been identified subsequently and were thoroughly reviewed by Blest et al.60 Nevertheless, none of them is particularly efficient in mediating cGAMP internalization in primary cells, leading to very low accumulation of this agonist in DCs.60,62,63 Furthermore, cGAMP is rapidly destroyed by nucleases in the plasma, which limits its uptake by antigen-presenting cells under physiological conditions, thereby preventing constitutive STING activation and collateral chronic inflammation.64,65 In cancer, DCs are more exposed to STING agonists secreted by cancer cells in the TME because of chromosomal instability and DNA leakage. This is particularly notable in primary tumors, where cancer cell DNA-sensing mechanisms can autonomously trigger antitumor immunity. Consequently, byproducts of the cGAS-STING pathway such as cGAMP or dsDNA are fed into the TME through necrosis or cell leakage, boosting immunity via DC activation.7,66 In fact, recent studies have shown that the success rate of radiation as an anticancer therapy is dependent on STING signaling. Ionizing radiation may promote the activation of exosomes loaded with tumor-derived dsDNA that mediate the cargo transfer to DCs, initiating pro-inflammatory cascades.67 This hypothesis is supported by another study where cancer cells were treated with another DNA damaging agent, a topoisomerase I inhibitor. This promoted the activation of DCs through delivery of tumor-associated DNA by exosomes.68 Furthermore, ionizing radiation induces genotoxicity and immunogenic cell death not only in irradiated cells but also in neighboring cells, increasing the exposure of DCs to natural tumor-derived STING agonists.69–71
Synthetic Agonists
Synthetic agonists comprise CDNs and small molecules, often developed to improve the pharmacokinetics of STING agonists or to maximize STING signaling. The use of natural or synthetic CDNs as a standalone or combined cancer therapy is limited not only by their hydrophilicity, but also by their small size which causes rapid diffusion out of the tumors even when administered intratumorally.72 CDNs can be isolated from bacteria, such as cyclic di-GMP or cyclic di-AMP, or synthesized and modified to enhance their stability and immunogenicity for systemic administration.73,74 Despite achieving robust CD8+ T cell-mediated specific responses in preclinical models, prominent examples of synthetic CDNs like ADU-S100 did not perform well in clinical trials.75–78 Other CDNs have since been tested in clinical trials but all produced underwhelming results, as reviewed by others.79,80 To overcome the limitations of CDNs, other small molecules that sustain STING-mediated antitumor immune responses have been developed. For example, DMXAA was developed as an angiogenic disrupting agent in 2002, but it gained distinction for its potent immunomodulatory effects on the STING pathway, leading to unprecedented success in inducing tumor regression in pre-clinical models.81–83 However, DMXAA failed to replicate the results obtained in preclinical studies when used to treat NSCLC patients in combination with chemotherapy in Phase III trials. This disappointing result was traced to the drug’s high specificity exclusively for mouse STING.84–86 Several other non-CDN small molecules, most of which share structural similarities to DMXAA, are currently under development.80,87 For instance, dimeric amidobenzimidazole ligand (diABZI) is a synthetic non-CDN small molecule that demonstrated a 400-fold higher potency over cGAMP in activating peripheral blood mononuclear cells.65 Nonetheless, small molecules lack strong cell selectivity across different subsets of immune cells, which leads to their uptake by lymphocytes and subsequent cell death. This highlights the need for drug delivery systems able to translate STING agonists into clinical applications.78,88–90
STING Agonist Delivery Systems
Nano-based drug delivery systems offer promising advantages in modulating cargo pharmacokinetics and enabling targeted delivery, which can reduce drug-related side effects and enhance therapeutic effects.91,92 Liposomes were the first platforms investigated for encapsulating CDNs, due to their cationic lipid structure and aqueous core. In fact, encapsulation of cyclic di-GMP resulted in a STING-dependent enhanced antitumor response in metastatic melanoma mouse models.93 Similarly, Doshi et al demonstrated that intravenous administration of ADU-S100 loaded into liposomes improved the therapeutic efficacy of that STING agonist and the responses to an immune checkpoint inhibitor (ICI) in both immunogenic and non-immunogenic in vivo tumor models.94 CDNs were also successfully delivered using pegylated (PEG)-lipid discoid NPs, promoting efficient penetration into tumors after intravenous injection, and thus exposing most tumor cells to the loaded STING agonist in mice bearing colon adenocarcinomas.95 The recently FDA-approved solid lipid nanoparticles (LNP) have also been exploited to this end, as they are known to successfully encapsulate nucleic acids and have intrinsic adjuvant properties. In line with this, Zhivaki et al have shown in vivo that LNPs loaded with mRNA encoding a self-DNA reactive variant of cGAMP had stronger immunostimulatory activity than other mRNA-encoded antigens also delivered by LNPs.96 Mesoporous silica NPs also have been shown to be suitable for CDN delivery, achieving remarkable loading capacities and sustained release, resulting in a robust immune response in melanoma models.97 Furthermore, several other polymeric-based NPs have successfully improved the delivery of CDNs, boosting the natural potency of cGAMP and inducing a “hot” immune TME in different tumor models.98,99 Accordingly, chitosan-based NPs, for instance, have been explored for their ability to induce cGAS-STING signaling, making them a promising adjuvant for use in novel formulations.100,101 Moreover, different ions, including Cu2+, Zn2+, Mn2+, have been reported to support cGAS-STING signaling, but their exact mechanism has not yet been unveiled.102–105 In line with these findings, manganese has been deemed as essential not only in inducing cGAS-STING as a defense mechanism against dsDNA, but also in initiating innate immune responses to tumors by promoting maturation of DCs and macrophages and also supporting downstream effector T-cell responses.106 The transporters for many of ions mentioned earlier are dysregulated in cancer (reviewed in107), indicating that altered ion transport may be linked to cGAS-STING-mediated tumor immune evasion.
Nano-Based Platforms for Efficient Activation of STING in DCs
Several preclinical studies have explored different strategies to effectively deliver STING agonists to the TME and support STING-mediated tumor cell clearance. The interplay between cGAS-STING signaling and ions in the cancer context motivated the development of new strategies to tackle DCs with manganese nano-based systems.108–110 Using hybrid exosomes formulated with mannose ligands to target DCs, and protein nano-based structures without specific DC targeting, the effect of Mn2+ ions on STING activation of cDCs and bone marrow-derived DCs, respectively, was assessed in breast cancer tumor models. In both approaches, the authors included a DNA-damaging agent in their formulation that induces immunogenic cell death (ICD), thereby increasing the prevalence of dsDNA and conferring synergistic effects on STING engagement and further supporting antitumor responses mediated by the engagement of DCs with natural killer (NK) cells or with T-cells.111,112 Inducing tumor cell ICD to engage the cGAS-STING pathway has also been explored by Gu et al, in a study where colorectal cancer mouse models were co-treated with oxaliplatin-loaded liposomes and with ADU-S100.113 The authors observed that liposome-based immunotherapy not only induced cDC maturation, but also enhanced infiltration by CD4+ and CD8+ T cells. The effect of ADU-S100-loaded liposomes in DCs has also been assessed in colorectal and melanoma murine models. In this case, DCs were directly targeted by incorporating WH (a peptide with high affinity to Clec9a) into liposomes, and this resulted in enhanced potency of the synthetic STING agonist.94 The same DC-targeting strategy was used in another study, where poly(lactide-co-glycolide) (PLGA)-NPs encapsulating 2’3’-cGAMP were coated with another ligand of Clec9a, peptide CBP-12, and administered to animals with melanoma or breast cancer.114 The natural STING agonist 2’3’-cGAMP has also been encapsulated in virus-like particles and in liposomes and used to treat melanoma models. While there was no DC-targeting strategy for liposomes, in the virus-like particles a fusion glycoprotein was used for this purpose.33,115 To treat MC38-OVA tumor-bearing mice, 2’3’-cGAMP was loaded into polymeric STING-activating NPs (PolySTING) and the antitumor effects observed were attributed specifically to cDC1 activation.32 A formulation with the same name, consisting of a prodrug platform that was used to deliver diABZI to DCs via mannose ligands, resulted in selective targeting of DCs in the TME and boosted not only cDC1/CD8+ T-cell crosstalk and lymphocyte infiltration, but also supported maturation of other DC subsets in tumor-draining lymph nodes.116 The recent observations made by Dosta et al, in which CDN-NP-mediated antitumor effects were assessed in melanoma models, further support the observations reported above. CDN-NPs behaved as a potent immune agonist, sustaining cDC activation either through direct uptake or through posterior transfer of NPs internalized by cancer cells.117 By exploiting natural intercellular mechanisms, Jang et al showed that DC targeting can be achieved to some extent by using CDN-loaded HEK293-derived extracellular vesicles (ExoSTING) and that this formulation can induce maturation of all DC subsets.118 To provide clarity on the efforts made to directly activate DCs in order to drive antitumor immunity, a summary of the effects of nano-based systems for activating the STING signaling pathway in DCs is provided in Table 1.
Table 1.
Strategies for Activation of the STING Pathway in DCs
| Delivery System | Cargo | Size (nm) | DC-targeting Approach | DC Subset Analyzed | Model | Route of Administration | Ref |
|---|---|---|---|---|---|---|---|
| Hybrid exosomes | SN38 and MnO2 | 149 | Unspecific (ICD of cancer cells and stimulation of STING) | cDC (CD11c+) | 4T1 tumor model | i.v. | [112] |
| Protein-based nanostructure (Man@Mn2+-Ft@Lap) | Mn2+ and β-lapachone | 100 | Mannose ligands | BMDC | 4T1 tumor model | i.v. | [111] |
| Liposomes | Oxaliplatin | 122 | Unspecific (ICD of cancer cells and stimulation of STING through co-administration of ADU-S100) | cDC1 and cDC2 (CD11c+) | CT26 murine model | Liposomes: i.v. ADU-S100: i.t. |
[113] |
| Liposomes | ADU-S100 | 121.3 | WH peptide (Clec9a targeting) | cDC1 (CD103+) | MC38 and B16F10 murine tumor | i.v. | [94] |
| PLGA NPs | 2’3’-cGAMP | 157.5 | CBP-12 EPBM NP coating (Clec9a targeting) | cDC1 (CD8α+) | B16-OVA melanoma and 4T1 breast cancer | s.c. | [114] |
| Liposomes | 2’3’-cGAMP | 160 | Unspecific | BMDC | B16-F10 melanoma model | in vitro stimulation | [115] |
| Virus-like particles | 2’3’-cGAMP | 158 | Viral fusion glycoprotein | cDC1 and cDC2 (CD11c+) | B16-F10 melanoma model | i.t. | [33] |
| PolySTING | 2’3’-cGAMP | 25 | Not specified | cDC1 (CD8α+ and CD103+) | MC38-OVA tumor-bearing | i.t. | [32] |
| PolySTING | diABZI | - | Mannose ligands | cDC1 (CD8α+ and CD103+) | B16F10 and 4T1 tumor models | i.v. | [116] |
| CDN-NPs | Synthetic CDN | 41 | Unspecific | cDC (CD11c+) | B16-F10 melanoma model | i.v. | [117] |
| ExoSTING | MR SS-2 CDA or cAIM(PS)2 Difluor (Rp/Sp) | 50–200 | EV import | pDC (CD123+), cDC (CD11c+) | B16F10, EG7.OVA and CT26 models | i.t. | [118] |
Abbreviations: BMDC, bone marrow-derived dendritic cell; i.v., intravenously; i.t., intratumorally; s.c., subcutaneous.
STING Controversy – the Tumor-Promoting Effect
Recent studies have highlighted the dual role of STING-induced responses in immune oncology. While activation of the STING pathway is crucial for natural antitumor immune responses in low immunogenic primary tumors, overactivation can lead to unproductive nonimmunogenic cell ablation, rendering patients more vulnerable to relapse or metastasis.33,78 In metastatic tumors with high chromosomal instability, targeting STING may further support tumor immune evasion.119,120 In fact, chronic activation of the cGAS-STING pathway, even at low levels, can sustain immunoregulatory gene expression such as IDO in DCs, thereby supporting tumor outgrowth and metastasis.49,121,122 Moreover, non-canonical activation of the STING pathway can also induce tumor-promoting responses, as such activation has been reported to support breast cancer cell survival in patient-derived xenograft models.123 Persistent inflammation following STING engagement in phagocytic cells engulfing dead cells may be tumor-promoting, which is consistent with the theory that prolonged, unresolved inflammation contributes to oncogenesis. Thus, targeting the cGAS-STING pathway in DCs is particularly challenging, as DCs appear to waver between pro- and antitumor T-cell responses.
Concluding Remarks
The net effect of STING activation on cancer is heavily influenced by several factors, including the timing and magnitude of STING stimulation and the STING-responsive target cells. As this receptor is ubiquitously expressed in different cells and independent of pathological states, identification of an optimal STING agonist candidate has proven to be difficult, based on the results of clinical trials.80,90,124 Nevertheless, it is uniformly recognized that DCs play a crucial role in stimulating antitumor immune responses, with STING engagement being essential to prompt this process through IFN I secretion.10 However, because the development of DC-targeted vaccines for cancer therapy remains challenging, no standalone STING-based therapies have been successful to date.77,125 Instead, current approaches combine the administration of STING agonists with ICIs such as PD-L1 or CTLA-4, because at optimal doses, STING induction prompts antitumor immunity mediated by IFN I signaling and it facilitates tumor infiltration by T cells, thereby converting “cold” tumors into “hot” tumors that are more prone to respond to ICI therapies.8,9,126–129 However, the underwhelming results of this approach underline the challenge of applying STING agonists in the clinic. The major hurdles hindering the clinical success of STING engagement in cancer immunotherapy are not only the poor pharmacokinetic and physicochemical properties of CDNs that limit their systemic administration, but also the frequent impairment of STING signaling in multiple cancers, although its restoration may be achieved by epigenetic reprogramming.80,130–132 To overcome these difficulties, small molecule STING agonists have been tested in combination with immune checkpoint blockade, but these attempts were reported to induce toxicity in lymphocytes, highlighting that antitumor immunity requires STING licensing specifically in myeloid cells.88,133
In summary, the cGAS-STING pathway is an undisputed target for novel developments in cancer immunotherapy, mediating the orchestration of critical antitumor responses by tumor-resident DCs. However, although STING signaling is essential to combat cancer progression, recent evidence has revealed its dual role, where chronic activation can paradoxically support tumor growth. Thus, the future of immuno-oncology relies on a deeper understanding of the effects of STING activation on different DC subsets in the tumor context, as well as on the development of efficient, tailored STING agonist delivery systems that will allow for optimal antitumor immune responses while mitigating potential side effects.
Acknowledgment
The image was created with Biorender. This work was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Action – Innovative Training Network DIRNANO (grant agreement No. 956544), the County of Salzburg, Cancer Cluster Salzburg [grant number 20102-P1601064-FPR01-2017], and by the Priority program ACBN, University of Salzburg.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–652. doi: 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M, Mujal AM, Pollack JL, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell. 2019;177(3):556–571.e16. doi: 10.1016/j.cell.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang MC, Tullett KM, Lee YS, et al. Differential uptake and cross‐presentation of soluble and necrotic cell antigen by human DC subsets. Eur J Immunol. 2016;46(2):329–339. doi: 10.1002/eji.201546023 [DOI] [PubMed] [Google Scholar]
- 4.Fu C, Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol. 2018;9:423775. doi: 10.3389/fimmu.2018.03059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson N, Ablasser A. The cGAS–STING pathway and cancer. Nat Cancer. 2022;3(12):1452–1463. doi: 10.1038/s43018-022-00468-w [DOI] [PubMed] [Google Scholar]
- 6.Kang TH, Mao C-P, Kim YS, et al. TLR9 acts as a sensor for tumor-released DNA to modulate anti-tumor immunity after chemotherapy. J Immunol Cancer. 2019;7(1):1–8. doi: 10.1186/s40425-019-0738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo S-R, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Hu S, Chen X, et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci. 2017;114(7):1637–1642. doi: 10.1073/pnas.1621363114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Zhao X, Zheng Z, et al. cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors. Cell Mol Life Sci 2024;81(1):149. doi: 10.1007/s00018-024-05191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.X-D L, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–1394. doi: 10.1126/science.1244040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeltema D, Abbott K, Yan N. STING trafficking as a new dimension of immune signaling. J Exp Med. 2023;220(3):e20220990. doi: 10.1084/jem.20220990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonugunta VK, Sakai T, Pokatayev V, et al. Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 2017;21(11):3234–3242. doi: 10.1016/j.celrep.2017.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548–569. doi: 10.1038/s41577-021-00524-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria O, De Gassart A, Coso S, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci. 2015;112(50):15408–15413. doi: 10.1073/pnas.1512832112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arwert EN, Milford EL, Rullan A, et al. STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat Cell Biol. 2020;22(7):758–766. doi: 10.1038/s41556-020-0527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi: 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie KJ, Carroll P, Martin C-A, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi: 10.1038/nature23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn PJ, Koch PD, Mitchison TJ. Chromatin bridges, not micronuclei, activate cGAS after drug-induced mitotic errors in human cells. Proc Natl Acad Sci. 2021;118(48):e2103585118. doi: 10.1073/pnas.2103585118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranoa DRE, Widau RC, Mallon S, et al. STING promotes homeostasis via regulation of cell proliferation and chromosomal stability. Cancer Res. 2019;79(7):1465–1479. doi: 10.1158/0008-5472.CAN-18-1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Q, Chen X, Chen J, et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis 2021;12(1):13. doi: 10.1038/s41419-020-03341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falahat R, Berglund A, Putney RM, et al. Epigenetic reprogramming of tumor cell–intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc Natl Acad Sci. 2021;118(15):e2013598118. doi: 10.1073/pnas.2013598118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosallanejad K, Kennedy SN, Bahleda KM, et al. Species-specific self-DNA detection mechanisms by mammalian cyclic GMP-AMP synthases. Sci Immunol. 2023;8(79):eabp9765. doi: 10.1126/sciimmunol.abp9765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JC, Liu X, Fitzgerald K, et al. Brief report: STING expressed in tumor and non-tumor compartments has distinct roles in regulating anti-tumor immunity. Cancer Immunol Immunother. 2023;72(5):1327–1335. doi: 10.1007/s00262-022-03327-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Mirlekar B, Johnson BM, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610(7931):373–380. doi: 10.1038/s41586-022-05254-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 27.Li J, Zhou J, Huang H, Jiang J, Zhang T, Ni C. Mature dendritic cells enriched in immunoregulatory molecules (mregDCs): a novel population in the tumour microenvironment and immunotherapy target. Clin J Transl Med. 2023;13(2):e1199. doi: 10.1002/ctm2.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Møller SH, Wang L, P-C H. Metabolic programming in dendritic cells tailors immune responses and homeostasis. Cell Mol Immunol 2022;19(3):370–383. doi: 10.1038/s41423-021-00753-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Z, Yu X, Ding R, et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023;133(7). doi: 10.1172/JCI166031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang ES, Daraj G, Balka KR, et al. Discordance in STING-induced activation and cell death between mouse and human dendritic cell populations. Front Immunol. 2022;13:794776. doi: 10.3389/fimmu.2022.794776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böttcher JP, e Sousa CR. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4(11):784–792. doi: 10.1016/j.trecan.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Li S, Wang M, et al. STING licensing of type I dendritic cells potentiates antitumor immunity. Sci Immunol. 2024;9(92):eadj3945. doi: 10.1126/sciimmunol.adj3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jneid B, Bochnakian A, Hoffmann C, et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci Immunol. 2023;8(79):eabn6612. doi: 10.1126/sciimmunol.abn6612 [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Pang S, Hui Z, et al. Blocking Tim-3 enhances the anti-tumor immunity of STING agonist ADU-S100 by unleashing CD4+ T cells through regulating type 2 conventional dendritic cells. Theranostics. 2023;13(14):4836. doi: 10.7150/thno.86792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De mingo Pulido Á, Hänggi K, Celias DP, et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity. 2021;54(6):1154–1167.e7. doi: 10.1016/j.immuni.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei X, de Groot DC, Welters MJ, et al. CD4+ T cells produce IFN-I to license cDC1s for induction of cytotoxic T-cell activity in human tumors. Cell Mol Immunol 2024;21:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei X, Khatri I, de Wit T, et al. CD4+ helper T cells endow cDC1 with cancer-impeding functions in the human tumor micro-environment. Nat Commun. 2023;14(1):217. doi: 10.1038/s41467-022-35615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich-Lewis JT, Draves KE, Roe K, O’Connor MA, Clark EA, Fuller DH. STING is required in conventional dendritic cells for DNA vaccine induction of type IT helper cell-dependent antibody responses. Front Immunol. 2022;13:861710. doi: 10.3389/fimmu.2022.861710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 40.Ziegler-Heitbrock L, Ohteki T, Ginhoux F, Shortman K, Spits H. Reclassifying plasmacytoid dendritic cells as innate lymphocytes. Nat Rev Immunol. 2023;23(1):1–2. doi: 10.1038/s41577-022-00806-0 [DOI] [PubMed] [Google Scholar]
- 41.Bode C, Fox M, Tewary P, et al. Human plasmacytoid dendritic cells elicit a type I interferon response by sensing DNA via the cGAS‐STING signaling pathway. Eur J Immunol. 2016;46(7):1615–1621. doi: 10.1002/eji.201546113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deb P, Dai J, Singh S, Kalyoussef E, Fitzgerald-Bocarsly P. Triggering of the cGAS–STING pathway in human plasmacytoid dendritic cells inhibits TLR9-mediated IFN production. J Immunol. 2020;205(1):223–236. doi: 10.4049/jimmunol.1800933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monti M, Ferrari G, Grosso V, et al. Impaired activation of plasmacytoid dendritic cells via toll-like receptor 7/9 and STING is mediated by melanoma-derived immunosuppressive cytokines and metabolic drift. Front Immunol. 2024;14:1227648. doi: 10.3389/fimmu.2023.1227648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brewitz A, Eickhoff S, Dähling S, et al. CD8+ T cells orchestrate pDC-XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity. 2017;46(2):205–219. doi: 10.1016/j.immuni.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier B, Leader AM, Chen ST, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257–262. doi: 10.1038/s41586-020-2134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Chen H, Mo H, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39(12):1578–1593.e8. doi: 10.1016/j.ccell.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 47.Smalley I, Chen Z, Phadke M, et al. Single-cell characterization of the immune microenvironment of melanoma brain and leptomeningeal metastases. Clin Cancer Res. 2021;27(14):4109–4125. doi: 10.1158/1078-0432.CCR-21-1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemos H, Mohamed E, Huang L, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76(8):2076–2081. doi: 10.1158/0008-5472.CAN-15-1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Wu Q, Chen T, et al. Blocking CD47 promotes antitumour immunity through CD103+ dendritic cell–NK cell axis in murine hepatocellular carcinoma model. J Hepatol. 2022;77(2):467–478. doi: 10.1016/j.jhep.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 51.Hu Z, Teng X-L, Zhang T, et al. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Molecular Cell. 2021;81(5):940–952.e5. doi: 10.1016/j.molcel.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 52.Andzinski L, Spanier J, Kasnitz N, et al. Growing tumors induce a local STING dependent type I IFN response in dendritic cells. Int J Cancer. 2016;139(6):1350–1357. doi: 10.1002/ijc.30159 [DOI] [PubMed] [Google Scholar]
- 53.Conde E, Vercher E, Soria-Castellano M, et al. Epitope spreading driven by the joint action of CART cells and pharmacological STING stimulation counteracts tumor escape via antigen-loss variants. J Immun Cancer. 2021;9(11):e003351. doi: 10.1136/jitc-2021-003351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu N, Palmer DC, Robeson AC, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218(2). doi: 10.1084/jem.20200844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Lu L, Lu J, et al. cGAS-STING–mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci, trans med. 2020;12(549):eaay9013. doi: 10.1126/scitranslmed.aay9013 [DOI] [PubMed] [Google Scholar]
- 56.Benoit-Lizon I, Jacquin E, Vargas TR, et al. CD4 T cell-intrinsic STING signaling controls the differentiation and effector functions of T H 1 and T H 9 cells. J Immuno Cancer. 2022;10(1):e003459. doi: 10.1136/jitc-2021-003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullinax JE, Hall M, Prabhakaran S, et al. Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Front Oncol. 2018;8:44. doi: 10.3389/fonc.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hines JB, Kacew AJ, Sweis RF. The development of STING agonists and emerging results as a cancer immunotherapy. Curr Oncol Rep. 2023;25(3):189–199. doi: 10.1007/s11912-023-01361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carozza JA, Böhnert V, Nguyen KC, et al. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nat Cancer. 2020;1(2):184–196. doi: 10.1038/s43018-020-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blest HT, Chauveau L. cGAMP the travelling messenger. Front Immunol. 2023;14:1150705. doi: 10.3389/fimmu.2023.1150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ablasser A, Schmid-Burgk JL, Hemmerling I, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503(7477):530–534. doi: 10.1038/nature12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. SLC19A1 is an importer of the immunotransmitter cGAMP. Molecular Cell. 2019;75(2):372–381.e5. doi: 10.1016/j.molcel.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luteijn RD, Zaver SA, Gowen BG, et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature. 2019;573(7774):434–438. doi: 10.1038/s41586-019-1553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carozza JA, Cordova AF, Brown JA, et al. ENPP1’s regulation of extracellular cGAMP is a ubiquitous mechanism of attenuating STING signaling. Proc Natl Acad Sci. 2022;119(21):e2119189119. doi: 10.1073/pnas.2119189119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garland KM, Sheehy TL, Wilson JT. Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chem Rev 2022;122(6):5977–6039. doi: 10.1021/acs.chemrev.1c00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuertes MB, Woo S-R, Burnett B, Fu Y-X, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34(2):67–73. doi: 10.1016/j.it.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diamond JM, Vanpouille-Box C, Spada S, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. 2018;6(8):910–920. doi: 10.1158/2326-6066.CIR-17-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitai Y, Kawasaki T, Sueyoshi T, et al. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198(4):1649–1659. doi: 10.4049/jimmunol.1601694 [DOI] [PubMed] [Google Scholar]
- 69.Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from α-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62(19):5436–5442. [PubMed] [Google Scholar]
- 70.Demaria S, Formenti SC. Radiotherapy effects on anti-tumor immunity: implications for cancer treatment. Frontiers Media S A. 2013;128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci. 2000;97(5):2099–2104. doi: 10.1073/pnas.030420797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Meng F, Yeo Y. Delivery of STING agonists for cancer immunotherapy. Curr Opin Biotechnol 2024;87:103105. doi: 10.1016/j.copbio.2024.103105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328(5986):1703–1705. doi: 10.1126/science.1189801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–518. doi: 10.1038/nature10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knelson EH, Ivanova EV, Tarannum M, et al. Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol Res. 2022;10(8):947–961. doi: 10.1158/2326-6066.CIR-22-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaidi AH, Kelly RJ, Gorbunova A, et al. Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8+ T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget. 2021;12(4):292. doi: 10.18632/oncotarget.27886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meric-Bernstam F, Sweis RF, Hodi FS, et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28(4):677–688. doi: 10.1158/1078-0432.CCR-21-1963 [DOI] [PubMed] [Google Scholar]
- 78.Sivick KE, Desbien AL, Glickman LH, et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 2018;25(11):3074–3085.e5. doi: 10.1016/j.celrep.2018.11.047 [DOI] [PubMed] [Google Scholar]
- 79.Amouzegar A, Chelvanambi M, Filderman JN, Storkus WJ, Luke JJ. STING agonists as cancer therapeutics. Cancers. 2021;13(11):2695. doi: 10.3390/cancers13112695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motedayen Aval L, Pease JE, Sharma R, Pinato DJ. Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J Clin Med. 2020;9(10):3323. doi: 10.3390/jcm9103323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prantner D, Perkins DJ, Lai W, et al. 5, 6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem. 2012;287(47):39776–39788. doi: 10.1074/jbc.M112.382986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. doi: 10.1016/j.celrep.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ching L, Cao Z, Kieda C, Zwain S, Jameson M, Baguley B. Induction of endothelial cell apoptosis by the antivascular agent 5, 6-dimethylxanthenone-4-acetic acid. Br. J. Cancer. 2002;86(12):1937–1942. doi: 10.1038/sj.bjc.6600368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lara PN Jr, Douillard J-Y, Nakagawa K, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non–small-cell lung cancer. J Clin Oncol. 2011;29(22):2965–2971. doi: 10.1200/JCO.2011.35.0660 [DOI] [PubMed] [Google Scholar]
- 85.Conlon J, Burdette DL, Sharma S, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5, 6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190(10):5216–5225. doi: 10.4049/jimmunol.1300097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao P, Ascano M, Zillinger T, et al. Structure-function analysis of STING activation by c [G (2′, 5′) pA (3′, 5′) p] and targeting by antiviral DMXAA. Cell. 2013;154(4):748–762. doi: 10.1016/j.cell.2013.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hao X, Chen Z, Li H, Wei M, Zuo Z, Su Q. Small-molecule drugs in immunotherapy. Mini Reviews in Med Chem. 2023;23(13):1341–1359. doi: 10.2174/1389557522666220930154527 [DOI] [PubMed] [Google Scholar]
- 88.Gulen MF, Koch U, Haag SM, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8(1):427. doi: 10.1038/s41467-017-00573-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53(1):115–126.e5. doi: 10.1016/j.immuni.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou Y, Zhang M, Zhou J. Recent trends in STING modulators: structures, mechanisms, and therapeutic potential. Drug Discovery Today. 2023;28(9):103694. doi: 10.1016/j.drudis.2023.103694 [DOI] [PubMed] [Google Scholar]
- 91.Huang P, Wang X, Liang X, et al. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019;85:1–26. doi: 10.1016/j.actbio.2018.12.028 [DOI] [PubMed] [Google Scholar]
- 92.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS nano. 2015;9(1):16–30. doi: 10.1021/nn5062029 [DOI] [PubMed] [Google Scholar]
- 93.Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J Control Release. 2015;216:149–157. doi: 10.1016/j.jconrel.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 94.Doshi AS, Cantin S, Prickett LB, Mele DA, Amiji M. Systemic nano-delivery of low-dose STING agonist targeted to CD103+ dendritic cells for cancer immunotherapy. J Control Release. 2022;345:721–733. [DOI] [PubMed] [Google Scholar]
- 95.Dane EL, Belessiotis-Richards A, Backlund C, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nature Mater. 2022;21(6):710–720. doi: 10.1038/s41563-022-01251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhivaki D, Gosselin EA, Sengupta D, et al. mRNAs encoding self-DNA reactive cGAS enhance the immunogenicity of lipid nanoparticle vaccines. Mbio. 2023;14(6):e02506–23. doi: 10.1128/mbio.02506-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park KS, Xu C, Sun X, Louttit C, Moon JJ. Improving STING agonist delivery for cancer immunotherapy using biodegradable mesoporous silica nanoparticles. Adv Ther. 2020;3(10):2000130. doi: 10.1002/adtp.202000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shae D, Becker KW, Christov P, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nature Nanotechnol. 2019;14(3):269–278. doi: 10.1038/s41565-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang-Bishop L, Kimmel BR, Ngwa VM, et al. STING-activating nanoparticles normalize the vascular-immune interface to potentiate cancer immunotherapy. Sci Immunol. 2023;8(83):eadd1153. doi: 10.1126/sciimmunol.add1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S, Zeng Y, Wang K, et al. Chitosan-based nano-micelles for potential anti-tumor immunotherapy: synergistic effect of cGAS-STING signaling pathway activation and tumor antigen absorption. Carbohydr Polym. 2023;321:121346. doi: 10.1016/j.carbpol.2023.121346 [DOI] [PubMed] [Google Scholar]
- 102.Wang P, Wang Y, Li H, et al. A homologous-targeting cGAS-STING agonist multimodally activates dendritic cells for enhanced cancer immunotherapy. Acta Biomater. 2024;177:400–413—. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Gao Y, Li Q, He A, Xu Q, Mou Y. Enhancing dendritic cell activation through manganese-coated nanovaccine targeting the cGAS-STING pathway. Int j Nanomed. 2024;Volume 19:263–280. doi: 10.2147/IJN.S438359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molinaro C, Martoriati A, Pelinski L, Cailliau K. Copper complexes as anticancer agents targeting topoisomerases I and II. Cancers. 2020;12(10):2863. doi: 10.3390/cancers12102863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361(6403):704–709. doi: 10.1126/science.aat1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lv M, Chen M, Zhang R, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30(11):966–979. doi: 10.1038/s41422-020-00395-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu L, Liu P. Cytosolic DNA sensing by cGAS: regulation, function, and human diseases. Signal Transduct Target Ther. 2021;6(1):170. doi: 10.1038/s41392-021-00554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao M, Xie YQ, Lei K, et al. A manganese phosphate nanocluster activates the cGAS‐STING pathway for enhanced cancer immunotherapy. Adv Ther. 2021;4(8):2100065. doi: 10.1002/adtp.202100065 [DOI] [Google Scholar]
- 109.Sun X, Zhang Y, Li J, et al. Amplifying STING activation by cyclic dinucleotide–manganese particles for local and systemic cancer metalloimmunotherapy. Nature Nanotechnol. 2021;16(11):1260–1270. doi: 10.1038/s41565-021-00962-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng Z, Xi M, Zhang C, et al. Biomineralized MnO2 nanoplatforms mediated delivery of immune checkpoint inhibitors with STING pathway activation to potentiate cancer radio-immunotherapy. ACS nano. 2023;17(5):4495–4506. doi: 10.1021/acsnano.2c10352 [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Liu Y, Xue C, et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat Commun. 2022;13(1):5685. doi: 10.1038/s41467-022-33301-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheng L, Zhang P, Liu Y, et al. Multifunctional hybrid exosomes enhanced cancer chemo-immunotherapy by activating the STING pathway. Biomaterials. 2023;301:122259. doi: 10.1016/j.biomaterials.2023.122259 [DOI] [PubMed] [Google Scholar]
- 113.Gu Z, Hao Y, Schomann T, Ossendorp F, Ten Dijke P, Cruz LJ. Enhancing anti-tumor immunity through liposomal oxaliplatin and localized immunotherapy via STING activation. J Control Release. 2023;357:531–544. doi: 10.1016/j.jconrel.2023.04.011 [DOI] [PubMed] [Google Scholar]
- 114.Gou S, Liu W, Wang S, et al. Engineered nanovaccine targeting Clec9a+ dendritic cells remarkably enhances the cancer immunotherapy effects of STING agonist. Nano Lett. 2021;21(23):9939–9950. doi: 10.1021/acs.nanolett.1c03243 [DOI] [PubMed] [Google Scholar]
- 115.Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1(1–2):1600013. doi: 10.1002/adbi.201600013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen DC, Song K, Jokonya S, et al. Mannosylated STING agonist drugamers for dendritic cell-mediated cancer immunotherapy. ACS Cent Sci 2024;10:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dosta P, Cryer AM, Dion MZ, et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nature Nanotechnol. 2023;18(11):1351–1363. doi: 10.1038/s41565-023-01447-7 [DOI] [PubMed] [Google Scholar]
- 118.Jang SC, Economides KD, Moniz RJ, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol. 2021;4(1):497. doi: 10.1038/s42003-021-02004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS–STING pathway in health and disease. Nat Rev Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1 [DOI] [PubMed] [Google Scholar]
- 120.Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–472. doi: 10.1038/nature25432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharma S, Campbell AM, Chan J, et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci. 2015;112(7):E710–E717. doi: 10.1073/pnas.1420217112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemos H, Huang L, Chandler PR, et al. Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J Immunol. 2014;192(12):5571–5578. doi: 10.4049/jimmunol.1303258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheradame L, Guerrera IC, Gaston J, et al. STING protects breast cancer cells from intrinsic and genotoxic-induced DNA instability via a non-canonical, cell-autonomous pathway. Oncogene. 2021;40(49):6627–6640. doi: 10.1038/s41388-021-02037-4 [DOI] [PubMed] [Google Scholar]
- 124.Kong X, Zuo H, Huang H-D, et al. STING as an emerging therapeutic target for drug discovery: perspectives from the global patent landscape. J Adv Res. 2023;44:119–133. doi: 10.1016/j.jare.2022.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harrington K, Brody J, Ingham M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. doi: 10.1093/annonc/mdy424.015 [DOI] [Google Scholar]
- 126.Marloye M, Lawler SE, Berger G. Current patent and clinical status of stimulator of interferon genes (STING) agonists for cancer immunotherapy. Future Sci. 2019;8:87–90. [DOI] [PubMed] [Google Scholar]
- 127.Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12(1):1–12. doi: 10.1186/s13045-019-0721-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nature Med. 2014;20(11):1301–1309. doi: 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 129.Ghaffari A, Peterson N, Khalaj K, et al. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer. 2018;119(4):440–449. doi: 10.1038/s41416-018-0188-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song S, Peng P, Tang Z, et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7(1):39858. doi: 10.1038/srep39858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Falahat R, Berglund A, Perez-Villarroel P, et al. Epigenetic state determines the in vivo efficacy of STING agonist therapy. Nat Commun. 2023;14(1):1573. doi: 10.1038/s41467-023-37217-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin Z, Liu Y, Lin P, Li J, Gan J. Clinical significance of STING expression and methylation in lung adenocarcinoma based on bioinformatics analysis. Sci Rep. 2022;12(1):13951. doi: 10.1038/s41598-022-18278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larkin B, Ilyukha V, Sorokin M, Buzdin A, Vannier E, Poltorak A. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J Immunol. 2017;199(2):397–402. doi: 10.4049/jimmunol.1601999 [DOI] [PMC free article] [PubMed] [Google Scholar]