ABSTRACT
Life history theory predicts increased parental investment comes with fitness costs, often expressed as negative effects on survival and future reproduction. To better understand the costs of reproduction and life history trade‐offs, we evaluated calcium supplementation at a high‐elevation site in Colorado as a novel approach to experimentally alter reproductive investment in nesting female Tachycineta bicolor (tree swallow). Calcium is a nutrient critical to avian reproduction as the intake of natural calcium is essential for egg production, embryo development, and nestling growth. Altering calcium availability exclusively during the breeding season allowed examination of individual biological responses to experimental modification of reproduction, as well as the reproductive costs associated with egg production and laying an entire clutch. As a functional endpoint and proxy for fitness and longevity, telomere length was measured at the beginning and end of each breeding season. Telomeres—protective “caps” at the ends of chromosomes—have been shown to shorten with aging and a variety of stressors, including higher reproductive output. Results demonstrate that tree swallow mothers supplemented with calcium during the breeding season experience higher reproductive output and produce offspring with longer telomeres, which came at the cost of relatively shorter telomeres during the reproductive season. These findings provide additional support for reproductive trade‐offs, and also challenge previous calcium supplementation studies that suggest excess calcium reduces the cost of reproduction.
Keywords: calcium supplementation, cost of reproduction, life‐history tradeoff, telomeres, tree swallows
We used calcium supplementation in nesting Tachycineta bicolor (tree swallow) and measured telomere length before and after breeding to better understand the costs of reproduction and life history trade‐offs. We found that mothers supplemented with calcium had higher reproductive success and greater telomere shortening, though their offspring had longer telomeres at 12 days old.
1. Introduction
Life history theory is built upon the premise that resources, and the time and energy it takes to acquire them, are limiting, and that allocation of these resources to competing functions can result in trade‐offs (Stearns 1992). Critical to the evolution of life history strategies is the cost of reproduction, where energy and resources allocated to breeding activities are not available for self‐maintenance. Accordingly, an increase in reproductive investment can be linked to a reduction in longevity through a decrease in investment toward self‐maintenance (Williams 1966; Stearns 1992; Roff 2002). Here, using telomere shortening as a proxy for fitness and longevity, we investigated the cost of reproduction in a wild song bird.
Telomeres are highly conserved repetitive DNA sequences that cap the physical ends of eukaryotic chromosomes, protecting them from degradation and loss (Blackburn 1991). Because conventional DNA polymerases cannot completely replicate linear DNA (the end‐replication problem), telomeres shorten with cell division, and thus with aging (Olovnikov 1996). Telomere length varies among individuals of the same age depending on genetic background (Asghar, Hasselquist, and Bensch 2011; Horn et al. 2011; Pepke et al. 2022), as well as with environmental and social stressors that contribute to cellular oxidation (Chatelain, Drobniak, and Szulkin 2020; Gil et al. 2020; Burraco, Lucas, and Salmón 2022). Variation in telomere length has been correlated with survival in numerous wild animal populations, as individuals with relatively short telomeres or a higher rate of telomere shortening tend to have a higher risk of mortality and concomitant reductions in lifespan across many species (Boonekamp et al. 2013; Vedder et al. 2022; Wilbourn et al. 2018; Whittemore et al. 2019). Due to their connection to survival and lifespan, relative telomere length (RTL) and telomere length dynamics (changes over time/longitudinal analyses) have been used as proxies for fitness in a variety of taxa as well, including birds, mammals, and reptiles (e.g., Fairlie et al. 2016; Fitzpatrick et al. 2021; Le Vaillant et al. 2015; Pauliny et al. 2006; Salmón et al. 2017; Angelier et al. 2013; Boonekamp et al. 2013; Wood and Young 2019). However, conclusions from cross‐sectional studies of telomere length can be confounded by measuring telomere length in older individuals, as disappearance of individuals from a population may not be independent of telomere length (Sudyka et al. 2016). Selective disappearance is therefore an important consideration since age‐related telomere shortening shows significant individual variation.
Recent studies demonstrate that the effort invested in reproduction can contribute to telomere shortening (e.g., Bauch, Becker, and Verhulst 2013; Bichet et al. 2020; Sudyka et al. 2019). However, these studies were either cross‐sectional in design or based on annual measurements of telomere length over consecutive breeding seasons, both of which may prove misleading as assessing telomere length over an entire year cannot be linked directly to the reproductive period of interest. Thus, our first objective was to investigate whether biologically relevant differences in reproductive investment influence telomere shortening specifically during the breeding season. Our second objective was to determine if/how telomere length of offspring might be affected by variation in reproductive success of their mothers, as variation in telomere length during early life has been shown to have long‐lasting effects on fitness (e.g., Eastwood et al. 2019; Heidinger et al. 2021, 2012). Lastly, and consistent with the Terminal Investment Hypothesis (Clutton‐Brock 1984), we sought to test whether older individuals invest more into current reproduction compared to younger individuals as reflected by increased telomere shortening, and in contrast to younger birds who invest more in self‐maintenance to enhance their probability of future reproduction and survival (Godfray, Partridge, and Harvey 1991; Klomp 1970).
To investigate whether variation in reproductive investment influences telomere length in tree swallow mothers and offspring, we employed calcium supplementation at a high‐elevation study site to experimentally modify reproductive output. Calcium is essential for egg production, as well as embryo and nestling skeletal development (Balkan, Karakas, and Biricik 2006); however, the diets of insectivorous and granivorous birds tend to contain inadequate amounts of calcium (Graveland and Van Gijzen 1994). Supplementing calcium for breeding birds in calcium‐depleted areas leads to an increase in reproductive success in the form of larger and more eggs produced, as well as higher hatching and fledging success (Dawson and Bidwell 2005; Graveland and Drent 1997; Mänd and Tilgar 2003; Mänd, Tilgar, and Leivits 2000a, 2000b; Tilgar, Mänd, and Leivits 1999; Tilgar, Mänd, and Mägi 2002). In areas where calcium is readily available, avian reproduction may still be limited by calcium due to naturally occurring variation in environmental conditions that make calcium‐specific foraging more costly, such as the harsh weather at the start of the breeding season—a typical occurrence at our high elevation study site in Colorado. We chose calcium supplementation over clutch enlargement methods, as supplementing mothers with calcium facilitates interrogation of differences in individual biological response to the experimental modification of reproduction regardless of the measure (e.g., egg size, number of eggs, etc.). Calcium supplementation also allows accounting for the reproductive costs associated with egg production and laying an entire clutch.
To confirm that calcium supplementation positively influenced reproductive parameters at our study site, we first conducted a 4‐year calcium‐supplementation trial with female tree swallows. In the two subsequent breeding seasons, we used calcium supplementation to modify reproductive output. Blood samples were collected from mothers immediately before egg‐laying and shortly before fledging to determine the direct relationship between reproductive investment and telomere shortening in our study species. The results of our investigation, carried out in a natural ecosystem, represent an important contribution to the knowledge of life‐history and telomere dynamics in vertebrates.
2. Materials and Methods
2.1. Study System
We studied female Tachycineta bicolor (tree swallows), insectivorous passerine birds that nest readily in human‐made nest boxes (Robertson and Rendell 1990). A typical clutch size is four to seven eggs, and incubation lasts 14–15 days (Stocek 1970). Once hatched, both parents provide care for the altricial nestlings until fledging, which takes 17–23 days (Stocek 1970). Numerous telomere studies have used tree swallows as a wild study species (e.g., Belmaker et al. 2018; Haussmann et al. 2003; Haussmann, Winkler, and Vleck 2005; Ouyang et al. 2016), and the short lifespan of tree swallows enables detection of changes in telomere length more quickly than long‐lived organisms (Haussmann et al. 2003). Studying a species that uses nest boxes also comes with certain logistical advantages, such as the ability to trap breeding females and offspring readily and repeatedly. Ninety (90) 5"×5"×8" pine nest boxes were placed at our high‐elevation study area during each of the first 4 years of the study, the number of which increased to 200 nest boxes for the last 2 years. Although 110 nest boxes were added for the 2017–2018 breeding season, an increase in the number of nests used by tree swallows over those years was not observed.
The calcium supplementation study took place over six summers (2013–2018), while telomere length was measured only during the summers of 2017 and 2018. Our study site was located at the Colorado State University Mountain Campus in Larimer County, Colorado, USA (N40.5611, W105.5978). The selected site was within a mountain valley at an elevation of 2750 m, the highest elevation of any avian calcium supplementation study to date. To date, the area has not been affected by anthropogenic acid deposition and natural sources of calcium are available (Binkley et al. 2003; Clow and Sueker 2000; Mast et al. 2011). The nest box trail runs along the edge of a riparian area and consists of nest boxes mounted on t‐posts, ~1.5 m above the ground and at a distance of at least 10 m between boxes.
2.2. Study Design and Sampling
Starting in early May during the years of 2013–2018, tree swallow nest boxes were checked daily for the start of nest construction. Once nest initiation was observed, indicated by a shallow grass ring at the base of the nest box, the nest box was randomly assigned to either the calcium supplemented group or the non‐supplemented control group. When initiation of the next nest was spotted, that nest box was assigned to the other group, and so on. Also, during group assignment, females were captured at the nest by covering the entrance once she was inside, or by using a mesh trap door. Once in hand, females were banded and tarsus length (cm), wing length (cm), and mass (g) measured. Birds were then classified into age categories of 1‐year old or greater than 1 year depending on the presence or absence of gray/dull plumage around the beak and eyes (Pyle 1997). After taking physical measurements, blood samples were taken using brachial venipuncture; between 10 and 30 μL blood was collected using an insulin syringe and a 27‐gauge needle as suggested by Owen (2011). Blood was transferred to a heparinized capillary tube and was stored at −20°C until analysis in the laboratory (Criscuolo et al. 2009).
Following capture and depending on the group assignment, nests were either calcium‐supplemented with oyster shell, (commercially prepared for poultry), or with non‐supplemented local soil (control) in a tray attached to the roof of the nest box. The oyster shell was crushed into fragments of a comparable size to that of grit normally consumed by tree swallows (Mayoh and Zach 1986). Oyster shell has been used in previous calcium supplementation studies (e.g., Johnson and Barclay 1996, Bidwell and Dawson 2005), and is similar in composition to snail shells, which are a natural source of calcium (Dawson and Bidwell 2005). Although we had no way of definitively determining whether mothers or offspring actually consumed the supplemented calcium, evidence of supplemented calcium fragments was found in the nests of supplemented birds.
Once laying began, nests were checked daily to determine clutch completion; once complete, individual egg length and width measurements were taken. Egg volume was calculated using the formula V = 0.51LW 2, where L is the length of the egg, W is the width of the egg, and 0.51 is a species‐specific constant (Hoyt 1979). Once hatched, hatching success was calculated for each nest as the proportion of eggs in the clutch that hatched. Sample sizes differ for each analysis due to missing egg volume measurements before hatching and missing hatching success measurements before fledging. For mean clutch size, 107 calcium supplemented nests were compared to 116 control nests. For mean egg volume per clutch, 88 calcium‐supplemented nests were compared to 93 control nests. For mean hatching success, 105 calcium‐supplemented nests were compared to 112 control nests.
In 2017 and 2018, blood samples were collected from mother birds and offspring when chicks were 12 days old using the same collection and storage methods described above; chicks were also banded at this time. All birds were handled and sampled under a Federal Bird Banding permit from the USGS Bird Banding Laboratory and in accordance with approved guidelines of the Institutional Animal Care and Use Committee of Colorado State University (Protocol # 17‐7304A). For analyzing female tree swallow telomere length over the breeding season, 22 calcium‐supplemented and 26 control birds were sampled before and after breeding. Of the females measured, 29 were 1 year‐old and 19 were older than 1 year. For determining mean offspring telomere length per nest, 18 calcium‐supplemented and 21 control nests were used.
2.3. Telomere Length Measurement
DNA was isolated from whole blood samples using the DNeasy Blood and Tissue kit (Qiagen, Valencia, California) following the manufacturer's protocol. DNA purity and concentration was determined using a NanoDrop 8000 spectrophotometer (Thermo Scientific). The average ratio of absorbance at 260 nm over 280 nm was used to check for protein contamination and the average ratio of absorbance at 260 nm over 230 nm was used to check for salt contamination. If either ratio of absorbance was < 1.8, the extract was excluded from further analysis (Morinha, Magalhães, and Blanco 2020). DNA integrity was visually assessed on an agarose gel as recommended by Seeker et al. (2016). Following the protocol of Criscuolo et al. (2009), quantitative Polymerase Chain Reaction (qPCR) was used to quantify telomere length in blood. Telomere length was determined as the ratio (T/S) of telomere repeat copy number (T) to a control single gene copy number (S), which was then standardized to a reference sample and expressed as RTL. The primers used to amplify the telomere region were as follows: Tel1b (5′‐CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT‐3′) and Tel2b (5′‐GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT‐3′). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was selected as the single‐copy gene; the primers used for amplification of GAPDH were specific GADPH‐F (5′‐GGTAGATGGGAGTTCAGTTGTG‐3′) and GAPDH‐R (5′‐AGAAACAAAGCACTGTCAGGG‐3′). Multiplexed qPCR was used with 3 μL of sample DNA at 3 ng/μl, Tel1b/Tel2b primers at a concentration of 900 nM, and GAPDH‐F/GAPDH‐R primers at a concentration of 400 nM in a final volume of 25 μL containing 10 μL of GoTaq qPCR Master Mix (Promega, Madison, Wisconsin, USA). Cycling conditions for the telomere qPCR were as follows: 10 min at 95°C, followed by 30 cycles of 15 s at 95°C, 30 s at 54.5°C, and 30 s at 72°C. For GAPDH amplification, cycling conditions were as follows: 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 60.5°C, and 30 s at 72°C. DNA samples were run in triplicate and a reference sample was run on every plate to compare measurements between plates. QPCR plates included serial dilutions (0.2, 0.4, 2, 10, 30, and 50 ng) of DNA from the same reference bird to create a reference curve to control for amplifying efficiency of the qPCR. All plates had standard curves with R 2 > 0.98, as recommended by Morinha, Magalhães, and Blanco (2020). A tree swallow blood sample collected at our study site (but not included in our study) was used as the reference sample. Coefficient of determination was > 0.98 and efficiencies within 100 ± 10% (Telomere standard curve: mean = 1.99, standard deviation = 0.01; GAPDH standard curve: mean = 1.94, standard deviation = 0.01). Within sample triplicates, if coefficient of variation (CV; repeatability) was > 0.14 for a sample T/S value, one of the triplicates was dropped. Samples were excluded if one of the remaining sample duplicate CVs was still > 0.14. Average repeatability of T/S values was 0.037 across all samples, and was 0.034 for reference samples. Reproducibility for samples run on multiple plates was measured as the standard deviation of cycle threshold (Cq) values for those samples. Reproducibility for telomeric values was 1.68, while reproducibility for GAPDH was 1.58.
2.4. Statistical Analysis
All statistical analyses were performed in R 3.5.2. The “lm” function was used in baseR and the “lmer” function in the “lmer4” package (Bates et al. 2015) to fit linear models and linear mixed models to test for effects of calcium supplementation on reproductive traits, telomere shortening, and telomere length on breeding tree swallows and their offspring. Normality and heteroscedastity assumptions were checked visually by plotting residuals of models. An information‐theoretic approach for model selection and ranking (Burnham and Anderson 2002) was used for each model set using the package MuMin in R (Barton and Barton 2015). A model of no effect was considered in each model set and we considered the model with the lowest AICc value in each set to be best supported by the data (Burnham and Anderson 2002; Burnham, Anderson, and Huyvaert 2011).
We first analyzed egg volume, hatching success, and brood size in relation to calcium treatment to determine whether these reproductive parameters were influenced by calcium supplementation. As clutch and brood size are highly correlated (r = 0.66), only clutch size was used in our analysis. Linear mixed effects models for each of the three response variables of interest were constructed, which included “box” as a random effect in each model, and each candidate model set included a treatment effect model (calcium or control), a year effect model, an additive model including treatment and year, an interaction model for treatment and year, and an intercept‐only model indicating no effects. Since the study spanned 6 years, during which time weather conditions at our site varied considerably, a year effect model was also included, as was an additive model for treatment and year to account for possible compounding effects of annual variation in weather conditions and calcium supplementation, and an interaction effect to account for the influence of calcium availability varying by year.
An information‐theoretic approach was used for model selection and ranking for each of the three model sets (Burnham and Anderson 2002). The model with the lowest AICc value in each set was considered best supported by the data. The ∆AICc (difference between each model, we, and the top‐ranking model) and Akaike weights (w i ; estimates of the probability that i is the best model given the data and the model set) were also calculated. AICc model selection was also used to analyze samples collected only during the 2017–2018 breeding seasons to ensure differences in reproductive performances between calcium and control nests during the years that telomere measurements were taken.
To measure change in RTL of the mother during the breeding season, we calculated D, a measure of temporal RTL shortening adjusted for the regression to the mean, following Kelly and Price (2005) using the equation:
where
X 1 is the RTL for mothers at time‐point one (pre‐breeding), X 2 is the RTL for mothers at time‐point two (when chicks were 12 days‐old), r is the correlation between X 1 and X 2, s is standard deviation, and s 2 is variance. RTL measurements at both time‐points were transformed to a log‐normal scale to achieve normality. We then fit D as the response variable in linear mixed effects models and included RTL for mothers at time‐point one as a covariate in every candidate model to account for baseline telomere length. We also included qPCR plate as a covariate in every candidate model to account for differences in repeatability between plates.
To analyze telomere length dynamics in mother tree swallows, a global model consisting of variables that could be influencing telomere length was constructed. These predictor variables included treatment, age, clutch size, and year. As all of our predictor variables have potential for additive effects, all combination of predictor variables included in the global model were compared and ranked based on AICc. Two‐way interactions were only included in the global model if such relationships were considered plausible a priori. The same models were also used when replacing clutch size with brood size to examine whether there was a difference between these two variables.
Average chick RTL per nest at Day 12 was also analyzed using linear mixed effect models. A global model consisting of variables that could be influencing chick RTL was constructed. These predictor variables included treatment, mother RTL at time‐point one, mother age, and year, along with “nest ID” as a random effect included in each model. As all of our predictor variables have potential for additive effects, all combination of predictor variables included in the global model were compared and ranked based on AICc. Again, two‐way interactions were only included in the global model if such relationships were considered plausible a priori.
3. Results
3.1. Calcium Supplementation and Reproductive Parameters
Two‐hundred and twenty‐four nests were initiated and monitored over the six‐year timeframe of the study. Sample size varied among years, as well as for each analysis, due to differences in nest survival over the various breeding seasons. Only a few individuals were captured multiple times over the 6‐year period, and only the first capture of these individuals was used in the study in order to maintain independence. In analyzing clutch size, the “treatment” model was ranked highest and carried the highest model weight (w i = 0.80; Table 1A): calcium supplemented nests had statistically significantly larger mean clutch sizes compared to control nests (p = 0.002; Figure 1A and Table 2A). Specifically, clutch size in the control nests was estimated to be smaller by 0.59 eggs (95% CI: −0.97, −0.22) compared to the calcium supplemented nests. In analyzing egg volume, the additive “treatment + year” model ranked highest and carried all the model weight (w i = 1.0; Table 1B); supplemented nests had significantly higher mean egg volumes compared to control nests (p < 0.001; Figure 1B and Table 2B). In particular, egg volume in control nests was estimated to be 44.51mm3 (95% CI: −22.35, −66.66) smaller than those in calcium supplemented nests. Finally, in analyzing hatching success, the additive “treatment + year” model carried the highest model weight (w i = 1.0; Table 1C); a significantly higher proportion of eggs hatched in supplemented nests compared to control nests (p = 0.002; Figure 1C and Table 2C). Specifically, hatching success in control nests was estimated to be 13.91% (95% CI: −22.45, −5.36) lower than that of calcium supplemented nests.
TABLE 1.
Mothers at nests with supplemented calcium produced and hatched more and larger eggs, with some variation by year, as indicated by the model set and rankings exploring the importance of calcium supplementation (“Treatment”) and “year” on mean clutch size per nest, mean hatching success per nest, and mean egg volume per nest in Tree Swallows at a high‐elevation valley in northern Colorado during 2013–2018. The number of parameters (K), −2 log‐likelihood (−2LL), and model weights (w i ) are shown for each model and the models are ranked by their AICc differences relative to the best model in the set (∆AICc i ). The minimum AICc value for mean clutch size per nest was 649.72, for mean egg volume per nest it was 2260.40, and for mean hatching success per nest it was 2296.42. “Box” was included as a random effect in each model.
| Dependent variable | Model | K | ∆AICc i | −2LL | w i |
|---|---|---|---|---|---|
| (A) Mean clutch size per nest | Treatment | 4 | 0.00 | −315.14 | 0.80 |
| Treatment + year | 9 | 3.59 | −311.60 | 0.13 | |
| Intercept | 3 | 5.40 | −318.88 | 0.05 | |
| Treatment × year | 14 | 10.28 | −309.36 | 0.00 | |
| Year | 8 | 11.26 | −316.52 | 0.00 | |
| (B) Mean egg volume per nest | Treatment + year | 9 | 0.00 | −1075.09 | 1.00 |
| Treatment × year | 13 | 31.76 | −1095.54 | 0.00 | |
| Year | 8 | 37.61 | −1099.58 | 0.00 | |
| Treatment | 4 | 75.74 | −1122.95 | 0.00 | |
| Intercept | 3 | 82.01 | −1127.13 | 0.00 | |
| (C) Mean hatching success per nest | Treatment + Year | 9 | 0.00 | −1093.98 | 1.00 |
| Treatment × Year | 13 | 31.71 | −1114.39 | 0.00 | |
| Year | 8 | 37.55 | −1118.42 | 0.00 | |
| Treatment | 4 | 73.95 | −1140.93 | 0.00 | |
| Intercept | 3 | 80.29 | −1145.14 | 0.00 |
FIGURE 1.
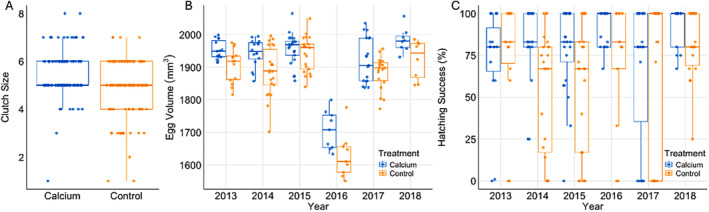
Female tree swallows supplemented with calcium laid and hatched more, larger eggs than those in control groups with some variation over years, as shown by: (A) Tree swallow clutch size per nest of calcium supplemented (n = 107) compared to control nests (n = 116); (B) tree swallow clutch size per nest of calcium supplemented (n = 88) compared to control nests (n = 93); and (C) tree swallow hatching success of calcium supplemented (n = 105) compared to control nests (n = 112) over the course of the 6 years of the study at a high elevation site in northern Colorado.
TABLE 2.
Results of the top linear mixed models explaining variation in reproductive output in tree swallows showed a treatment effect on mean (A) clutch size, (B) egg volume, and (C) hatching success.
| A | Clutch | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 26.81 | −15.39—69.01 | 0.212 |
| Treatment (control) | −0.59 | −0.97—‐0.22 | 0.002 |
| ICC | 1.00 | ||
| NBoxID | 94 | ||
| Observations | 224 | ||
| Marginal R 2 | 0.000 | ||
| B | Volume | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 1625.96 | 1596.36–1655.56 | < 0.001 |
| Treatment (control) | 44.51 | 22.35–66.66 | < 0.001 |
| Year (2014) | −19.10 | −55.81 – 17.61 | 0.306 |
| Year (2015) | 307.39 | 271.50–343.2 | < 0.001 |
| Year (2016) | 13.64 | −30.96 – 58.24 | 0.547 |
| Year (2017) | 273.27 | 236.62–309.91 | < 0.001 |
| Year (2018) | 262.85 | 216.57–309.14 | < 0.001 |
| NBoxID | 82 | ||
| Observations | 181 | ||
| Marginal R 2 | 0.795 | ||
| C | Hatching success | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 79.61 | 67.25–91.97 | < 0.001 |
| Treatment (control) | −13.91 | −22.45—‐5.36 | 0.001 |
| Year (2014) | −5.92 | −21.08—9.23 | 0.442 |
| Year (2015) | −3.90 | −18.60—10.80 | 0.601 |
| Year (2016) | 8.35 | −8.28—24.97 | 0.323 |
| Year (2017) | −7.10 | −22.57—8.36 | 0.366 |
| Year (2018) | 14.08 | −2.15—30.31 | 0.089 |
| NBoxID | 93 | ||
| Observations | 217 | ||
| Marginal R 2 | 0.093 | ||
Note: Bold values indicate significance at the p = 0.05 level.
For determining reproductive performance during only the 2017–2018 years, we used the same methods described above, but on a subset of 54 individuals across the two breeding seasons. In our analysis of clutch size for 2017 and 2018, the “treatment” model again ranked highest and carried the highest model weight (w i = 0.44; Table 3A); calcium‐supplemented nests had statistically significantly larger mean clutch sizes compared to control nests (p < 0.001; Table 4A). Clutch size in the control nests was estimated to be smaller by 0.66 eggs (95% CI: −1.23, −0.09) compared to the calcium‐supplemented nests. In analyzing egg volume for 2017–2018, the “treatment × year” model ranked highest and carried the highest model weight (w i = 0.97; Table 3B); calcium supplemented nests had larger mean clutch sizes compared to control nests (p < 0.001; Table 4B). Specifically, egg volume in the control nests was estimated to be smaller by 36.29 mm3 eggs (95% CI: −73.24, 0.66) compared to the calcium supplemented nests. In analyzing hatching success for 2017–2018, the “treatment × year” model ranked highest and carried the highest model weight (w i = 0.36; Table 3C); calcium supplemented nests in 2018 had significantly higher hatching success compared to control nests (p = 0.001; Table 4C).
TABLE 3.
Mothers at nests with supplemented calcium produced and hatched more and larger eggs, with some variation by year, as indicated by the model set and rankings exploring the importance of calcium supplementation (“Treatment”) and “year” on mean clutch size per nest, mean hatching success per nest, and mean egg volume per nest in Tree Swallows at a high‐elevation valley in northern Colorado during 2017–2018. The number of parameters (K), −2 log‐likelihood (−2LL), and model weights (w i ) are shown for each model and the models are ranked by their AICc differences relative to the best model in the set (∆AICc i ). The minimum AICc value for mean clutch size per nest was 482.53, for mean egg volume per nest it was 601.69, and for mean hatching success per nest it was 482.53. “Box” was included as a random effect in each model.
| Dependent Variable | Model | K | ∆AICc i | −2LL | w i |
|---|---|---|---|---|---|
| (A) Mean Clutch Size per Nest | Treatment | 4 | 0 | −109.39 | 0.44 |
| Treatment × year | 6 | 11.96 | −107.65 | 0.24 | |
| Treatment + year | 5 | 14.12 | −109.33 | 0.15 | |
| Intercept | 3 | 18.41 | −111.66 | 0.14 | |
| Year | 4 | 21.13 | −111.75 | 0.04 | |
| (B) Mean egg volume per nest | Treatment × year | 6 | 0 | −277.84 | 0.97 |
| Treatment + year | 5 | 6.92 | −282.57 | 0.03 | |
| Year | 4 | 19.64 | −290.15 | 0.00 | |
| Treatment | 4 | 20.41 | −290.53 | 0.00 | |
| Intercept | 3 | 32.22 | −297.61 | 0.00 | |
| (C) Mean hatching success per nest | Treatment × year | 5 | 0 | −236.01 | 0.36 |
| Year | 4 | 0.74 | −238.74 | 0.25 | |
| Intercept | 2 | 1.34 | −240.15 | 0.18 | |
| Treatment + year | 4 | 2.36 | −238.39 | 0.11 | |
| Treatment | 3 | 2.62 | −239.68 | 0.10 |
TABLE 4.
Results of the top linear mixed models explaining the variation in reproductive output in tree swallows showed an effect of experimental calcium treatment on mean (A) clutch size, (B) egg volume, and (C) hatching success for the 2017–2018 years.
| A | Clutch | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 5.15 | 4.73–5.58 | < 0.001 |
| Treatment (control) | −0.66 | −1.23 to −0.09 | 0.024 |
| ICC | 0.17 | ||
| NBoxID | 55 | ||
| Observations | 69 | ||
| Marginal R 2/conditional R 2 | 0.074/0.231 | ||
| B | Volume | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 1920.44 | 1894.33–1946.54 | < 0.001 |
| Treatment (control) | −36.29 | −73.24–0.66 | 0.054 |
| Year (2018) | 62.52 | 15.75–109.29 | 0.01 |
| Treatment (control) × year (2018) | −26.59 | −94.28–41.10 | 0.434 |
| ICC | 0.19 | ||
| NBoxID | 48 | ||
| Observations | 54 | ||
| Marginal R 2/conditional R 2 | 0.246/0.387 | ||
| C | Hatching success | ||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 87.37 | 80.52–94.21 | < 0.001 |
| Treatment (control) | 12.05 | 2.90–21.20 | 0.011 |
| Year (2018) | 1.12 | −6.58–8.81 | 0.772 |
| Treatment (control) × year (2018) | −20.13 | (31.98 to −8.27) | 0.001 |
| ICC | 0.79 | ||
| NBoxID | 46 | ||
| Observations | 58 | ||
| Marginal R 2/conditional R 2 | 0.172/0.828 | ||
Note: Bold values indicate significance at the p = 0.05 level.
3.2. Calcium Supplementation and Telomere Length
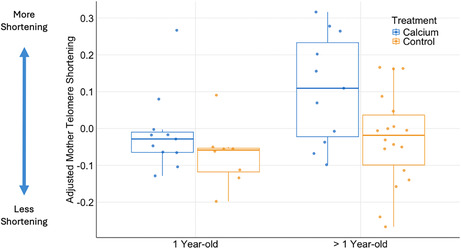
In analyzing telomere length in mother tree swallows, the “Treatment + Age” model carried the highest model weight (w i = 0.42; Table 5); supplemented nests had higher adjusted telomere shortening compared to controls (p = 0.006; Figure 2A and Table 6). Specifically, control mothers were estimated to have 0.11 lower adjusted telomere shortening (95% CI: −0.18, 0.03) compared to calcium‐supplemented nests. In looking at maternal age, one‐year‐old mothers had significantly lower adjusted telomere shortening compared to older mothers (p = 0.004; Figure 2B and Table 6). Specifically, one‐year‐old mothers were estimated to have −0.08 less adjusted telomere shortening (95% CI: −0.15, 0.00) compared to that of calcium supplemented mothers. We reran model selection with brood size in place of clutch size and the “Treatment + Age” model was again the top model (Table 7).
TABLE 5.
Mothers at nests with supplemented calcium showed the most telomere shortening over the course of the breeding season as indicated by their high model weights and rankings among the set of models exploring the importance of calcium supplementation (“Treatment”), “Age,” “Year,” and clutch size (“Clutch”) on telomere shortening in breeding female Tree Swallows. Telomere length at time point one was included as a covariate in each model to account for baseline telomere length. qPCR plate was included as a covariate in each model. The number of parameters (K), −2 log‐likelihood (−2LL), and model weights (wi) are shown for each model and the models are ranked by their AICc differences relative to the best model in the set (∆AICc).
| Model | K | ∆AICc i | −2LL | w i |
|---|---|---|---|---|
| Treatment + age | 6 | 0.00 | 35.81 | 0.42 |
| Treatment × age | 7 | 1.24 | 36.57 | 0.23 |
| Treatment | 5 | 2.09 | 33.46 | 0.15 |
| Treatment + clutch | 6 | 4.27 | 33.70 | 0.04 |
| Treatment + year | 6 | 4.71 | 33.46 | 0.03 |
| Null model | 4 | 5.55 | 30.48 | 0.03 |
| Age | 5 | 6.01 | 31.50 | 0.02 |
| Clutch | 5 | 6.02 | 31.51 | 0.01 |
| Treatment × clutch | 7 | 6.71 | 33.87 | 0.01 |
| Age × clutch | 7 | 6.93 | 33.76 | 0.01 |
| Age + clutch | 6 | 7.07 | 32.30 | 0.01 |
| Age × year | 7 | 7.12 | 33.63 | 0.01 |
| Calcium × year | 7 | 7.13 | 33.63 | 0.01 |
| Year × clutch | 7 | 7.63 | 33.41 | 0.01 |
| Year | 5 | 8.04 | 30.48 | 0.01 |
| Age + year | 6 | 8.57 | 31.53 | 0.00 |
| Year + clutch | 6 | 8.65 | 31.51 | 0.00 |
FIGURE 2.
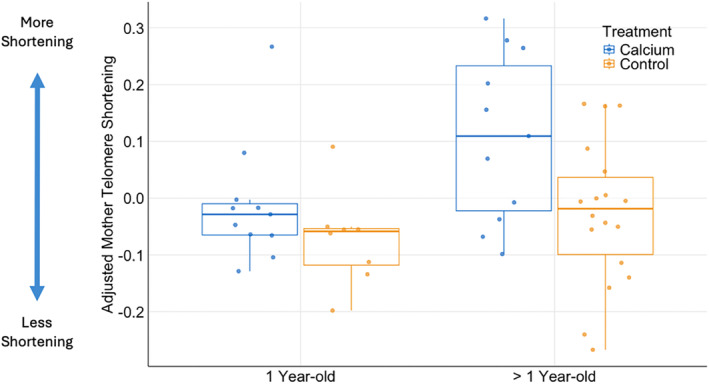
Female tree swallows supplemented with calcium showed increased telomere shortening as indicated by female telomere shortening adjusted for regression to the mean for calcium (blue; n = 22) versus control nests (yellow; n = 26). Older female tree swallows also showed increased telomere shortening as indicated by female telomere shortening adjusted to regression to the mean for 1‐year‐old (n =29) and older than 1‐year‐old birds (n =19). Adjustments for regression to the mean scales the mean of the data to zero.
TABLE 6.
Results of the top linear model explaining the variation in mother telomere shortening over the course of the breeding season showed an effect of experimental calcium treatment and age.
| Mother telomere shortening | |||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 0.16 | 0.02 to 0.30 | 0.029 |
| Treatment (control) | −0.11 | −0.18 to −0.03 | 0.006 |
| Age (1 year old) | −0.08 | −0.15 to −0.00 | 0.041 |
| Mother TL | −0.01 | −0.05 to 0.03 | 0.748 |
| Plate | −0.01 | −0.02 to 0.00 | 0.156 |
| Observations | 48 | ||
| Marginal R 2/conditional R 2 | 0.245/0.175 | ||
Note: Bold values indicate significance at the p = 0.05 level.
TABLE 7.
Mothers at nests with supplemented calcium show the most telomere shortening during the breeding season as indicated by their high model weights and rankings among the set of models exploring the importance of calcium supplementation (“Treatment),” “Age,” “Year,” and brood size (“Brood”) on telomere shortening in breeding female Tree Swallows. Telomere length at time point one was included as a covariate in each model to account for baseline telomere length. qPCR plate was included as a covariate in each model. The number of parameters (K), −2 log‐likelihood (−2LL), and model weights (w i ) are shown for each model and the models are ranked by their AICc differences relative to the best model in the set (∆AICci).
| Model | K | ∆AICc i | −2LL | w i |
|---|---|---|---|---|
| Treatment + age | 6 | 0.00 | 35.81 | 0.42 |
| Treatment × age | 7 | 1.24 | 36.57 | 0.23 |
| Treatment | 5 | 2.09 | 33.46 | 0.15 |
| Treatment + year | 6 | 4.71 | 33.46 | 0.04 |
| Treatment + brood | 6 | 5.36 | 33.16 | 0.03 |
| Null model | 4 | 5.55 | 30.48 | 0.03 |
| Age | 5 | 6.01 | 31.50 | 0.02 |
| Calcium × brood | 7 | 6.76 | 33.84 | 0.01 |
| Brood | 5 | 6.79 | 31.12 | 0.01 |
| Age × year | 7 | 7.12 | 33.63 | 0.01 |
| Treatment × year | 7 | 7.13 | 33.63 | 0.01 |
| Age + brood | 6 | 7.56 | 32.06 | 0.01 |
| Age + brood | 7 | 8.00 | 33.22 | 0.01 |
| Year | 5 | 8.04 | 30.48 | 0.01 |
| Age + year | 6 | 8.57 | 31.53 | 0.01 |
| Year + brood | 6 | 9.41 | 31.14 | 0.00 |
| Year × brood | 7 | 9.90 | 32.28 | 0.00 |
The “Treatment + Mother Pre‐breeding Relative Telomere Length” model carried the most model weight (w i = 0.44; Table 8) for offspring RTL; calcium supplemented offspring had significantly longer average RTL per nest at 12 days compared to control chicks (p = 0.011; Figure 3A), with control nests having offspring with an estimated 0.63 shorter RTL (95% CI: −1.10, −0.15) compared to calcium supplemented nests (Table 9). In addition, offspring RTL was significantly positively correlated with mother pre‐breeding RTL (p = 0.011; Figure 3B), with an estimated correlation of 0.33 (95% CI: 0.08, 0.59).
TABLE 8.
Relative telomere length (TL) of nestling Tree Swallows at 12 days old was dependent on whether their nest was supplemented with calcium (“Treatment”) and the relative telomere length of their mothers pre‐breeding as indicated by model weights and rankings for models exploring the importance of calcium supplementation on telomere length in nestling Tree Swallows. qPCR plate was included as a fixed effect in each model. “Box” was included as a random effect in each model. The number of parameters (K), −2 log‐likelihood (−2LL), and model weights (w i ) are shown for each model and the models are ranked by their AICc differences relative to the best model in the set (∆AICc i ).
| Model | K | ∆i | ‐2LL | w i |
|---|---|---|---|---|
| Treatment + mother TL | 6 | 0.00 | −46.88 | 0.44 |
| Treatment × mother TL | 7 | 2.36 | −46.66 | 0.13 |
| Mother TL + year | 7 | 2.63 | −48.20 | 0.12 |
| Treatment | 6 | 3.95 | −50.19 | 0.06 |
| Mother TL | 5 | 3.97 | −50.20 | 0.06 |
| Treatment + mother age | 5 | 4.04 | −48.90 | 0.06 |
| Mother TL *year | 6 | 5.10 | −48.02 | 0.03 |
| Treatment + year | 7 | 5.55 | −49.66 | 0.03 |
| Treatment + mother age | 6 | 5.86 | −49.81 | 0.02 |
| Treatment × mother age | 7 | 6.16 | −48.56 | 0.02 |
| Mother TL × mother age | 7 | 6.42 | −48.68 | 0.02 |
| Treatment × year | 7 | 8.36 | −49.65 | 0.01 |
| Null model | 4 | 13.35 | −56.17 | 0.00 |
| Year | 5 | 14.04 | −55.24 | 0.00 |
| Mother age | 5 | 14.26 | −55.35 | 0.00 |
| Mother age + year | 6 | 15.74 | −54.75 | 0.00 |
| Mother age × year | 7 | 18.46 | −54.70 | 0.00 |
FIGURE 3.
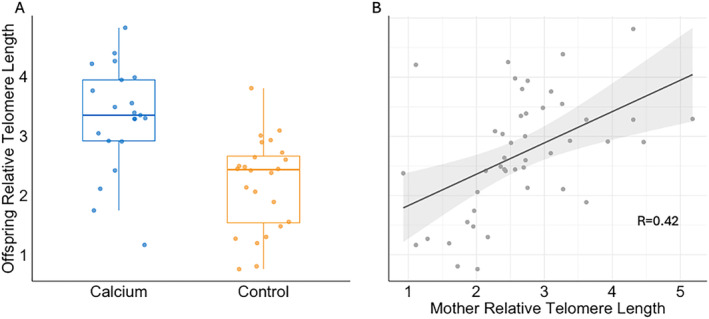
In 12‐day‐old nestling tree swallows, relative telomere length differs between calcium supplemented nests and control nests (A), and is highly correlated with mother telomere length pre‐breeding (B).
TABLE 9.
Results of the top linear model explaining the variation in juvenile telomere length (TL) over the course of the breeding season showed an effect of experimental calcium treatment and mother telomere length.
| Juvenile telomere length | |||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 1.45 | 0.48–2.42 | 0.004 |
| Treatment (control) | −0.63 | −1.10 to −0.15 | 0.011 |
| Mother TL | 0.33 | 0.08–0.59 | 0.011 |
| Plate | 0.10 | 0.04–0.16 | 0.002 |
| ICC | 0.03 | ||
| NBoxID | 32 | ||
| Observations | 45 | ||
| Marginal R 2/conditional R 2 | 0.515/0.527 | ||
Note: Bold values indicate significance at the p = 0.05 level.
4. Discussion
During avian reproduction, parental investment contributes to higher levels of oxidative stress (Alonso‐Alvarez et al. 2004; Metcalfe and Alonso‐Alvarez 2010). When reproductive investment exceeds what is sustainable for parents, the costs to longevity are evidenced by accelerated telomere shortening (Sudyka et al. 2014). Indeed, studies in a range of organisms with experimentally increased brood size have shown that costly reproductive events have a negative impact on adult lifespan (Reichert et al. 2014) and the early life of offspring (Boonekamp et al. 2013; Herborn et al. 2014; Nettle et al. 2013). Traditionally, brood‐manipulation studies have reflected the relationship between investment in reproduction and telomere attrition; however, these studies did not account for investment in egg production, and telomere shortening was not specific to the breeding period. Measuring telomere length pre‐breeding facilitated assessment of telomere shortening during egg production, incubation, and nestling care. Here, we investigated the role that calcium supplementation plays in the relationship between reproductive investment and telomere length dynamics.
4.1. Calcium and Reproductive Parameters
Our results provide evidence that calcium is a limiting factor in tree swallow reproduction. Experimentally supplemented nests had higher reproductive productivity in the form of larger average egg volume, improved hatching success, and larger clutch size than control nests. While a variety of other studies have examined the effects of calcium supplementation on reproductive success in birds (e.g., Espín et al. 2016; Graveland and Berends 1997; Mänd and Tilgar 2003; Poulin and Brigham 2001; Tilgar et al. 2004; Wilkin et al. 2009), few have focused on the effect in areas where calcium is naturally available, and none have examined these effects in systems at elevations over 2000 m. Birds breeding at high elevation face a host of challenges that make living and breeding more metabolically costly than at lower elevations, resulting in relatively lower reproductive success with increasing elevation (Altshuler and Dudley 2006; Johnson et al. 2018; Nagy and Grabherr 2009). Although calcium supplementation appears to be advantageous in terms of reproductive output at our high‐elevation study site, the stress associated with increased reproductive effort comes at a direct cost to the mother, as evidenced by increased telomere shortening.
4.2. Calcium Supplementation and Telomere Shortening in Mother Tree Swallows
Mother tree swallows supplemented with calcium experienced increased telomere shortening compared to non‐supplemented control mothers. On average, supplemented females in our study also produced more, larger eggs, and hatched more eggs compared to control mothers. 0.86 additional chicks Supplemented females also and suffered more telomere shortening relative to controls., suggesting that producing and raising more offspring is costly in terms of stress and predicted lifespan.
The observed increase in telomere shortening may reflect a higher cost of breeding for supplemented mothers, likely due to the increased energy and resource expenditure that accompanies producing and raising more offspring. However, brood size was not included in the top model for telomere shortening, indicating that it was not necessarily the number of offspring produced, but rather, the calcium supplementation itself that resulted in brood sizes above what is optimal for each tree swallow mother.
Parents should ideally produce the number of offspring that maximizes their overall fitness, meaning that an individual should not produce the maximum number of offspring possible per year, but rather, the maximum number that simultaneously optimizes longevity (Krebs and Charnov 1974; Lack 1947). The optimum number of offspring has been studied widely and varies with parental condition, as well as with the environmental conditions present during reproduction (e.g., De Heij, Van den Hout, and Tinbergen 2006; Murphy et al. 2000; Pettifor, Perrins, and McCleery 2001). Many studies have concluded that reproductive costs are “investment‐dependent,” as they are only apparent once reproductive efforts have surpassed what parents were prepared to sustain (Reichert et al. 2014; Santos and Nakagawa 2012). This was true of our study system, as increased telomere shortening was the cost of reproduction that mother tree swallows experienced after exceeding their optimum clutch size.
Telomere shortening has previously been linked to reproductive investment via exposure to oxidative stress (Epel et al. 2004; Haussmann and Marchetto 2010; Wiersma et al. 2004; Von Zglinicki 2002). Because reproduction entails elevated levels of cellular replication, higher investments in reproduction are expected to increase an individual's exposure to oxidative damage through increased reactive oxygen species (ROS), or decreased investment into antioxidant defenses, both of which contribute to telomere shortening (Blount et al. 2016; Selman et al. 2012).
In addition to calcium supplementation, and as expected, age class was also an important factor in predicting telomere shortening during the breeding season, with older birds experiencing more telomere shortening compared to younger, one‐year‐old birds. Ouyang et al. (2016) also found that tree swallow reproductive output increased with age, and that this higher reproductive output coincided with shorter telomeres. Our results support the Terminal Investment Hypothesis, as older birds produced more and larger eggs compared to younger birds, perhaps due to the fewer chances they have to reproduce in the future (Clutton‐Brock 1984; Stearns 1992). In the context of life history theory, younger birds may lay smaller clutches and exert less energy to keep their probability of future survival and fecundity high (Godfray, Partridge, and Harvey 1991; Klomp 1970). Contrary to many studies that show telomere length shortens faster for larger individuals (e.g., Ringsby et al. 2015; Pepke et al. 2022; Sheldon et al. 2021), tarsus length was not included in the top model of our study, a finding likely due to calcium treatment and age having larger effects than body size in this system.
4.3. Calcium Supplementation and Telomere Length in Nestling Tree Swallows
The cost of reproduction in birds can manifest in terms of parental survival, future reproduction, and/or offspring survival. The cost of reproduction may be observed as an effect on offspring (i.e., early‐life telomere length), especially when parental effort is increased (Knowles, Wood, and Sheldon 2010; Linden and Møller 1989; Martin 2004; Pettifor, Perrins, and McCleery 2001; Sudyka et al. 2014; Voirin et al. 2023). Here, calcium supplementation and pre‐breeding RTL of the mother were the most important factors in determining offspring telomere length. Even though mothers in experimentally supplemented nests showed increased reproductive output at the cost of faster rates of telomere shortening, their offspring had longer average RTL compared to control chicks. RTL of chicks in supplemented nests at 12 days old (mean = 3.23 ± 0.15 SE) was longer compared to that of control nests (mean = 2.25 ± 0.17 SE).
It may be that calcium‐supplemented mothers bear the extra cost associated with increased reproductive investment, leaving their offspring to reap the developmental benefits of increased calcium. In other calcium supplementation studies that did not measure telomere length, offspring benefited from excess calcium, even when their parents experienced increased reproductive success. Dawson and Bidwell (2005) found that supplementing calcium to breeding tree swallows not only led to larger clutch sizes, but also had a positive effect on offspring growth rate. Similarly, Tilgar, Mänd, and Mägi (2002) found that supplementing nesting Great Tits with calcium also led to larger clutch and brood sizes, while simultaneously leading to higher mean tarsus lengths of offspring. Previous supplementation studies have credited increases in the number of offspring and offspring growth to an overall reduction in cost of reproduction. Here, however, we provide evidence to the contrary; calcium supplementation resulted in more telomere shortening in mothers breeding at our high‐elevation study site.
Costanzo et al. (2016) directly manipulated brood size and found that nestlings and parents in enlarged nests had shorter telomeres and displayed more telomere shortening compared to control and reduced nests, pointing to calcium as the reason for increased offspring growth and survival, even with increased nestling competition. In our study, supplemented mothers suffered the adverse effects of increased productivity as shortened telomeres. Concurrently, 12 day old offspring in calcium‐supplemented nests had longer telomeres compared to 12 day old offspring in control nests.
The RTL of chicks was also dependent on the pre‐breeding telomere length of the mothers. In birds, studies of telomere length inheritance have found stronger correlations between mothers and offspring than between fathers and offspring (Asghar et al. 2015; Horn et al. 2011; Reichert et al. 2015), such that maternal effects might provide better explanations for offspring telomere length. Interestingly, germline cells may be more vulnerable to telomere shortening than somatic tissues as they are especially susceptible to oxidative stress (Metcalfe and Alonso‐Alvarez 2010).
5. Conclusion
Many brood and clutch manipulation experiments have concluded that birds raising enlarged broods suffer the consequences and costs of reproduction via increased telomere shortening, and thus potential life shortening (Bauch, Becker, and Verhulst 2013; Reichert et al. 2014). However, many of these studies were cross‐sectional or assessed as yearly shortening of telomere length, while many longitudinal studies focused either on long‐lived species or used captive populations of short‐lived species.
In the current study, telomere length was measured in female tree swallows before and after breeding, allowing evaluation of telomere shortening exclusively during the breeding period, therefore our inferences are specific to the effects of calcium supplementation on breeding. Studying a wild, short‐lived avian species also facilitated better understanding of telomere length dynamics in birds with a fast pace of life under natural conditions. Calcium supplementation was employed as a novel means of testing the link between reproductive investment and telomere shortening. While previous calcium supplementation studies concluded that excess calcium increases reproductive output and simultaneously reduced the cost of reproduction, we found that supplemental calcium increased reproductive output at the expense of increased telomere shortening—and by extension shortened lifespan—in the supplemented mothers. While calcium‐supplemented tree swallow mothers bear the burden associated with increased reproductive investment, their offspring appear to reap the developmental benefits of increased calcium in that telomeres were longer in chicks from supplemented nests.
Our results provide additional support for using telomere length dynamics to elucidate constraints on life history trade‐offs. Future work should include telomere length assessment to better understand classic life history trade‐offs in various species and under differing environmental conditions.
Author Contributions
Marina D. Rodriguez: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (lead), investigation (lead), methodology (lead), project administration (lead), visualization (lead), writing – original draft (lead). Susan M. Bailey: investigation (supporting), methodology (supporting), resources (equal), supervision (equal), writing – review and editing (equal). Paul F. Doherty Jr.: conceptualization (supporting), formal analysis (supporting), methodology (supporting), resources (supporting), supervision (equal), writing – review and editing (equal). Kathryn P. Huyvaert: conceptualization (equal), investigation (supporting), methodology (supporting), supervision (equal), writing – review and editing (equal).
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank Lynn E. Taylor, Colorado State University Telomeres & Telomerase Lab, and the Colorado State University Mountain Campus for their support. This project was funded by the National Science Foundation Graduate Research Fellowship (award number 006784).
Funding: This work was supported by National Science Foundation Graduate Research Fellowship Program (006784).
Contributor Information
Marina D. Rodriguez, Email: mdrod@colostate.edu.
Kathryn P. Huyvaert, Email: kate.huyvaert@wsu.edu.
Data Availability Statement
The data that support the findings of this study are openly available via Dryad at https://datadryad.org/stash/share/1OdeICdgS3M2zsfeCXJhS9cRq5nLhWjzxfD1WQLBgKQ.
References
- Alonso‐Alvarez, C. , Bertrand S., Devevey G., Prost J., Faivre B., and Sorci G.. 2004. “Increased Susceptibility to Oxidative Stress as a Proximate Cost of Reproduction.” Ecology Letters 7, no. 5: 363–368. [Google Scholar]
- Altshuler, D. L. , and Dudley R.. 2006. “The Physiology and Biomechanics of Avian Flight at High Altitude.” Integrative and Comparative Biology 46, no. 1: 62–71. [DOI] [PubMed] [Google Scholar]
- Angelier, F. , Vleck C. M., Holberton R. L., and Marra P. P.. 2013. “Telomere Length, Non‐breeding Habitat and Return Rate in Male A Merican Redstarts.” Functional Ecology 27, no. 2: 342–350. [Google Scholar]
- Asghar, M. , Bensch S., Tarka M., Hansson B., and Hasselquist D.. 2015. “Maternal and Genetic Factors Determine Early Life Telomere Length.” Proceedings of the Royal Society B: Biological Sciences 282, no. 1799: 20142263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar, M. , Hasselquist D., and Bensch S.. 2011. “Are Chronic Avian Haemosporidian Infections Costly in Wild Birds?” Journal of Avian Biology 42, no. 6: 530–537. [Google Scholar]
- Balkan, M. , Karakas R., and Biricik M.. 2006. “Changes in Eggshell Thickness, Shell Conductance and Pore Density During Incubation in the Peking Duck (Anas platyrhynchos f. Dom).” Ornis Fennica 83, no. 3: 117–123. [Google Scholar]
- Barton, K. , and Barton M. K.. 2015. “Package ‘Mumin’ Version.” 1(18), 439.
- Bates, D. , Mächler M., Bolker B., and Walker S., 2015. “Fitting Linear Mixed‐Effects Models Using lme4.” Journal of Statistical Software 67, no. 1: 1–48. [Google Scholar]
- Bauch, C. , Becker P. H., and Verhulst S.. 2013. “Telomere Length Reflects Phenotypic Quality and Costs of Reproduction in a Long‐Lived Seabird.” Proceedings of the Royal Society B: Biological Sciences 280, no. 1752: 20122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker, A. , Hallinger K. K., Glynn R. A., Haussmann M. F., and Winkler D. W.. 2018. “Is There a Context‐Dependent Advantage of Extra‐Pair Mating in Tree Swallows?” Ornithological Advances 135, no. 4: 998–1008. [Google Scholar]
- Bichet, C. , Bouwhuis S., Bauch C., Verhulst S., Becker P. H., and Vedder O.. 2020. “Telomere Length Is Repeatable, Shortens With Age and Reproductive Success, and Predicts Remaining Lifespan in a Long‐Lived Seabird.” Molecular Ecology 29, no. 2: 429–441. [DOI] [PubMed] [Google Scholar]
- Bidwell, M. T. , and Dawson R. D.. 2005. “Calcium Availability Limits Reproductive Output of Tree Swallows (Tachycineta Bicolor) in a Nonacidified Landscape.” Auk 122, no. 1: 246–254. [Google Scholar]
- Binkley, D. , Singer F., Kaye M., and Rochelle R.. 2003. “Influence of Elk Grazing on Soil Properties in Rocky Mountain National Park.” Forest Ecology and Management 185, no. 3: 239–247. [Google Scholar]
- Blackburn, E. H. 1991. “Structure and Function of Telomeres.” Nature 350, no. 6319: 569–573. [DOI] [PubMed] [Google Scholar]
- Blount, J. D. , Vitikainen E. I., Stott I., and Cant M. A.. 2016. “Oxidative Shielding and the Cost of Reproduction.” Biological Reviews 91, no. 2: 483–497. [DOI] [PubMed] [Google Scholar]
- Boonekamp, J. J. , Simons M. J., Hemerik L., and Verhulst S.. 2013. “Telomere Length Behaves as Biomarker of Somatic Redundancy Rather Than Biological Age.” Aging Cell 12, no. 2: 330–332. [DOI] [PubMed] [Google Scholar]
- Burnham, K. , and Anderson D.. 2002. Model Selection and Multi‐Model Inference. 2nd ed. New York: Springer. [Google Scholar]
- Burnham, K. P. , Anderson D. R., and Huyvaert K. P.. 2011. “AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons.” Behavioral Ecology and Sociobiology 65: 23–35. [Google Scholar]
- Burraco, P. , Lucas P. M., and Salmón P.. 2022. “Telomeres in a Spatial Context: A Tool for Understanding Ageing Pattern Variation in Wild Populations.” Ecography 2022, no. 6: e05565. [Google Scholar]
- Chatelain, M. , Drobniak S. M., and Szulkin M.. 2020. “The Association Between Stressors and Telomeres in Non‐human Vertebrates: A Meta‐Analysis.” Ecology Letters 23, no. 2: 381–398. [DOI] [PubMed] [Google Scholar]
- Charnov, E. L. , and Krebs J. R.. 1974. “On Clutch Size and Fitness.” IBIS 116, no. 2: 217–219. [Google Scholar]
- Clow, D. W. , and Sueker J. K.. 2000. “Relations Between Basin Characteristics and Stream Water Chemistry in Alpine/Subalpine Basins in Rocky Mountain National Park, Colorado.” Water Resources Research 36, no. 1: 49–61. [Google Scholar]
- Clutton‐Brock, T. H. 1984. “Reproductive Effort and Terminal Investment in Iteroparous Animals.” American Naturalist 123, no. 2: 212–229. [Google Scholar]
- Costanzo, A. , Parolini M., Bazzi G., et al. 2016. “Brood Size, Telomere Length, and Parent‐Offspring Color Signaling in Barn Swallows.” Behavioral Ecology 28, no. 1: 204–211. [Google Scholar]
- Criscuolo, F. , Bize P., Nasir L., et al. 2009. “Real‐Time Quantitative PCR Assay for Measurement of Avian Telomeres.” Journal of Avian Biology 40, no. 3: 342–347. [Google Scholar]
- Dawson, R. D. , and Bidwell M. T.. 2005. “Dietary Calcium Limits Size and Growth of Nestling Tree Swallows Tachycineta Bicolor in a Non‐acidified Landscape.” Journal of Avian Biology 36, no. 2: 127–134. [Google Scholar]
- De Heij, M. E. , Van den Hout P. J., and Tinbergen J. M.. 2006. “Fitness Cost of Incubation in Great Tits (Parus Major) is Related to Clutch Size.” Proceedings of the Royal Society B: Biological Sciences 273, no. 1599: 2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood, J. R. , Hall M. L., Teunissen N., et al. 2019. “Early‐Life Telomere Length Predicts Lifespan and Lifetime Reproductive Success in a Wild Bird.” Molecular Ecology 28, no. 5: 1127–1137. [DOI] [PubMed] [Google Scholar]
- Epel, E. S. , Blackburn E. H., Lin J., et al. 2004. “Accelerated Telomere Shortening in Response to Life Stress.” Proceedings of the National Academy of Sciences 101, no. 49: 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín, S. , Ruiz S., Sánchez‐Virosta P., and Eeva T.. 2016. “Effects of Calcium Supplementation on Growth and Biochemistry in Two Passerine Species Breeding in a ca‐Poor and Metal‐Polluted Area.” Environmental Science and Pollution Research 23: 9809–9821. [DOI] [PubMed] [Google Scholar]
- Fairlie, J. , Holland R., Pilkington J. G., Pemberton J. M., Harrington L., and Nussey D. H.. 2016. “Lifelong Leukocyte Telomere Dynamics and Survival in a Free‐Living Mammal.” Aging Cell 15, no. 1: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, L. J. , Olsson M., Pauliny A., While G. M., and Wapstra E.. 2021. “Individual Telomere Dynamics and Their Links to Life History in a Viviparous Lizard.” Proceedings of the Royal Society B 288, no. 1951: 20210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil, D. , Alfonso‐Iñiguez S., Pérez‐Rodríguez L., Muriel J., and Monclús R.. 2020. “Harsh Conditions During Early Development Influence Telomere Length in an Altricial Passerine: Links With Oxidative Stress and Corticosteroids.” Journal of Evolutionary Biology 32, no. 1: 111–125. [DOI] [PubMed] [Google Scholar]
- Godfray, H. C. J. , Partridge L., and Harvey P. H.. 1991. “Clutch size.” Annual Review of Ecology and Systematics 22: 409–429. [Google Scholar]
- Graveland, J. , and Berends A. E.. 1997. “Timing of the Calcium Intake and Effect of Calcium Deficiency on Behaviour and Egg Laying in Captive Great Tits, Parus Major.” Physiological Zoology 70, no. 1: 74–84. [DOI] [PubMed] [Google Scholar]
- Graveland, J. , and Drent R. H.. 1997. “Calcium Availability Limits Breeding Success of Passerines on Poor Soils.” Journal of Animal Ecology 66: 279–288. [Google Scholar]
- Graveland, J. , and Van Gijzen T.. 1994. “Arthropods and Seeds Are Not Sufficient as Calcium Sources for Shell Formation and Skeletal Growth in Passerines.” Ardea 55, no. 1–2: 299–314. [Google Scholar]
- Haussmann, M. F. , and Marchetto N. M.. 2010. “Telomeres: Linking Stress and Survival, Ecology and Evolution.” Current Zoology 56, no. 6: 714–727. [Google Scholar]
- Haussmann, M. F. , Winkler D. W., O'Reilly K. M., Huntington C. E., Nisbet I. C., and Vleck C. M.. 2003. “Telomeres Shorten More Slowly in Long‐Lived Birds and Mammals Than in Short–Lived Ones.” Proceedings of the Royal Society of London. Series B: Biological Sciences 270, no. 1522: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann, M. F. , Winkler D. W., and Vleck C. M.. 2005. “Longer Telomeres Associated With Higher Survival in Birds.” Biology Letters 1, no. 2: 212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger, B. J. , Blount J. D., Boner W., Griffiths K., Metcalfe N. B., and Monaghan P.. 2012. “Telomere Length in Early Life Predicts Lifespan.” Proceedings of the National Academy of Sciences 109, no. 5: 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger, B. J. , Kucera A. C., Kittilson J. D., and Westneat D. F.. 2021. “Longer Telomeres During Early Life Predict Higher Lifetime Reproductive Success in Females but Not Males.” Proceedings of the Royal Society B 288, no. 1951: 20210560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborn, K. A. , Heidinger B. J., Boner W., et al. 2014. “Stress Exposure in Early Post‐Natal Life Reduces Telomere Length: An Experimental Demonstration in a Long‐Lived Seabird.” Proceedings of the Royal Society B: Biological Sciences 281, no. 1782: 20133151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, T. , Robertson B. C., Will M., Eason D. K., Elliott G. P., and Gemmell N. J.. 2011. “Inheritance of Telomere Length in a Bird.” PLoS One 6, no. 2: e17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, D. F. 1979. “Practical Methods of Estimating Volume and Fresh Weight of Bird Eggs.” Auk 96, no. 1: 73–77. [Google Scholar]
- Johnson, L. S. , and Barclay R. M.. 1996. “Effects of Supplemental Calcium on the Reproductive Output of a Small Passerine Bird, the House Wren (Troglodytes aedon).” Canadian Journal of Zoology 74, no. 2: 278–282. [Google Scholar]
- Johnson, L. S. , Iser K. M., Molnar H. A., Nguyen A. V., and Connor C. L.. 2018. “Clutch and Egg Size of Tree Swallows Along an Elevational Gradient.” Journal of Field Ornithology 89, no. 3: 234–241. [Google Scholar]
- Kelly, C. , and Price T. D.. 2005. “Correcting for Regression to the Mean in Behavior and Ecology.” American Naturalist 166, no. 6: 700–707. [DOI] [PubMed] [Google Scholar]
- Klomp, H. 1970. “The Determination of Clutch‐Size in Birds a Review.” Ardea 55, no. 1–2: 1–124. [Google Scholar]
- Knowles, S. C. , Wood M. J., and Sheldon B. C.. 2010. “Context‐Dependent Effects of Parental Effort on Malaria Infection in a Wild Bird Population, and Their Role in Reproductive Trade‐Offs.” Oecologia 164: 87–97. [DOI] [PubMed] [Google Scholar]
- Lack, D. 1947. “The Significance of Clutch‐Size.” IBIS 89: 302–352. [Google Scholar]
- Le Vaillant, M. , Viblanc V. A., Saraux C., et al. 2015. “Telomere Length Reflects Individual Quality in Free‐Living Adult King Penguins.” Polar Biology 38: 2059–2067. [Google Scholar]
- Linden, M. , and Møller A. P.. 1989. “Cost of Reproduction and Covariation of Life History Traits in Birds.” Trends in Ecology & Evolution 4, no. 12: 367–371. [DOI] [PubMed] [Google Scholar]
- Mänd, R. , and Tilgar V.. 2003. “Does Supplementary Calcium Reduce the Cost of Reproduction in the Pied Flycatcher Ficedula Hypoleuca?” IBIS 145, no. 1: 67–77. [Google Scholar]
- Mänd, R. , Tilgar V., and Leivits A.. 2000a. “Calcium, Snails, and Birds: A Case Study.” Web Ecology 1, no. 1: 63–69. [Google Scholar]
- Mänd, R. , Tilgar V., and Leivits A.. 2000b. “Reproductive Response of Great Tits, Parus Major, in a Naturally Base‐Poor Forest Habitat to Calcium Supplementation.” Canadian Journal of Zoology 78, no. 5: 689–695. [Google Scholar]
- Martin, T. E. 2004. “Avian Life‐History Evolution Has an Eminent Past: Does It Have a Bright Future?” Auk 121, no. 2: 289–301. [Google Scholar]
- Mast, M. A. , Turk J. T., Clow D. W., and Campbell D. H.. 2011. “Response of Lake Chemistry to Changes in Atmospheric Deposition and Climate in Three High‐Elevation Wilderness Areas of Colorado.” Biogeochemistry 103: 27–43. [Google Scholar]
- Mayoh, K. R. , and Zach R.. 1986. “Grit Ingestion by Nestling Tree Swallows and House Wrens.” Canadian Journal of Zoology 64, no. 10: 2090–2093. [Google Scholar]
- Metcalfe, N. B. , and Alonso‐Alvarez C.. 2010. “Oxidative Stress as a Life‐History Constraint: The Role of Reactive Oxygen Species in Shaping Phenotypes From Conception to Death.” Functional Ecology 24, no. 5: 984–996. [Google Scholar]
- Morinha, F. , Magalhães P., and Blanco G.. 2020. “Standard Guidelines for the Publication of Telomere qPCR Results in Evolutionary Ecology.” Molecular Ecology Resources 20, no. 3: 635–648. [DOI] [PubMed] [Google Scholar]
- Murphy, M. T. , Armbrecth B., Vlamis E., and Pierce A.. 2000. “Is Reproduction by Tree Swallows Cost Free?” Auk 117, no. 4: 902–912. [Google Scholar]
- Nagy, L. , and Grabherr G.. 2009. The Biology of Alpine Habitats. USA: Oxford University Press. [Google Scholar]
- Nettle, D. , Monaghan P., Boner W., Gillespie R., and Bateson M.. 2013. “Bottom of the Heap: Having Heavier Competitors Accelerates Early‐Life Telomere Loss in the European Starling Sturnus vulgaris .” PLoS One 8, no. 12: e83617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olovnikov, A. M. 1996. “Telomeres, Telomerase, and Aging: Origin of the Theory.” Experimental Gerontology 31, no. 4: 443–448. [DOI] [PubMed] [Google Scholar]
- Ouyang, J. Q. , Lendvai Á. Z., Moore I. T., Bonier F., and Haussmann M. F.. 2016. “Do Hormones, Telomere Lengths, and Oxidative Stress Form an Integrated Phenotype? A Case Study in Free‐Living Tree Swallows.” Integrative and Comparative Biology 56, no. 2: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, J. C . 2011. “Collecting, Processing, and Storing Avian Blood: A Review.” Journal of Field Ornithology 82, no. 4: 339–354. [Google Scholar]
- Pauliny, A. , Wagner R. H., Augustin J., Szép T., and Blomqvist D.. 2006. “Age‐Independent Telomere Length Predicts Fitness in Two Bird Species.” Molecular Ecology 15, no. 6: 1681–1687. [DOI] [PubMed] [Google Scholar]
- Pepke, M. L. , Kvalnes T., Rønning B., et al. 2022. “Artificial Size Selection Experiment Reveals Telomere Length Dynamics and Fitness Consequences in a Wild Passerine.” Molecular Ecology 31, no. 23: 6224–6238. [DOI] [PubMed] [Google Scholar]
- Pettifor, R. A. , Perrins C. M., and McCleery R. H.. 2001. “The Individual Optimization of Fitness: Variation in Reproductive Output, Including Clutch Size, Mean Nestling Mass and Offspring Recruitment, in Manipulated Broods of Great Tits Parus Major.” Journal of Animal Ecology 70, no. 1: 62–79. [Google Scholar]
- Poulin, R. , and Brigham R. M.. 2001. “Effects of Supplemental Calcium on the Growth Rate of an Insectivorous Bird, the Purple Martin (Progne Subis).” Ecoscience 8, no. 2: 151–156. [Google Scholar]
- Pyle, P. 1997. Identification Guide to North American Birds: A Compendium of Information on Identifying, Ageing, and Sexing “Near‐Passerines” and Passerines in the Hand. Bolinas, CA: Slate Creek Press. [Google Scholar]
- Reichert, S. , Rojas E. R., Zahn S., Robin J. P., Criscuolo F., and Massemin S.. 2015. “Maternal Telomere Length Inheritance in the King Penguin.” Heredity 114, no. 1: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert, S. , Stier A., Zahn S., et al. 2014. “Increased Brood Size Leads to Persistent Eroded Telomeres.” Frontiers in Ecology and Evolution 2: 9. [Google Scholar]
- Ringsby, T. H. , Jensen H., Pärn H., et al. 2015. “On Being the Right Size: Increased Body Size Is Associated With Reduced Telomere Length Under Natural Conditions.” Proceedings of the Royal Society B: Biological Sciences 282, no. 1820: 20152331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, R. J. , and Rendell W. B.. 1990. “A Comparison of the Breeding Ecology of a Secondary Cavity Nesting Bird, the Tree Swallow (Tachycineta Bicolor), in Nest Boxes and Natural Cavities.” Canadian Journal of Zoology 68, no. 5: 1046–1052. [Google Scholar]
- Roff, D. A. 2002. “Life History Evolution.” Sunderland, Sinauer Associates, Boston, MA.
- Salmón, P. , Nilsson J. F., Watson H., Bensch S., and Isaksson C.. 2017. “Selective Disappearance of Great Tits With Short Telomeres in Urban Areas.” Proceedings of the Royal Society B: Biological Sciences 284, no. 1862: 20171349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, E. S. A. , and Nakagawa S.. 2012. “The Costs of Parental Care: A Meta‐Analysis of the Trade‐Off Between Parental Effort and Survival in Birds.” Journal of Evolutionary Biology 25, no. 9: 1911–1917. [DOI] [PubMed] [Google Scholar]
- Seeker, L. A. , Holland R., Underwood S., et al. 2016. “Method Specific Calibration Corrects for DNA Extraction Method Effects on Relative Telomere Length Measurements by Quantitative PCR.” PLoS One 11, no. 10: e0164046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman, C. , Blount J. D., Nussey D. H., and Speakman J. R.. 2012. “Oxidative Damage, Ageing, and Life‐History Evolution: Where Now?” Trends in Ecology & Evolution 27, no. 10: 570–577. [DOI] [PubMed] [Google Scholar]
- Sheldon, E. L. , Ton R., Boner W., et al. 2021. “Associations Between DNA Methylation and Telomere Length During Early Life: Insight From Wild Zebra Finches (Taeniopygia guttata).” Molecular Ecology 31, no. 23: 6261–6272. [DOI] [PubMed] [Google Scholar]
- Stearns, S. C. 1992. The Evolution of Life Histories. London, UK: Oxford University Press. [Google Scholar]
- Stocek, R. F. 1970. “Observations on the Breeding Biology of the Tree Swallow.” Cassinia 52: 3–20. [Google Scholar]
- Sudyka, J. , Arct A., Drobniak S., Dubiec A., Gustafsson L., and Cichoń M.. 2014. “Experimentally Increased Reproductive Effort Alters Telomere Length in the Blue Tit (Cyanistes Caeruleus).” Journal of Evolutionary Biology 27, no. 10: 2258–2264. [DOI] [PubMed] [Google Scholar]
- Sudyka, J. , Arct A., Drobniak S., Gustafsson L., and Cichoń M.. 2016. “Longitudinal Studies Confirm Faster Telomere Erosion in Short‐Lived Bird Species.” Journal of Ornithology 157: 373–375. [Google Scholar]
- Sudyka, J. , Arct A., Drobniak S. M., Gustafsson L., and Cichoń M.. 2019. “Birds With High Lifetime Reproductive Success Experience Increased Telomere Loss.” Biology Letters 15, no. 1: 20180637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgar, V. , Mänd R., and Leivits A.. 1999. “Effect of Calcium Availability and Habitat Quality on Reproduction in Pied Flycatcher Ficedula Hypoleuca and Great Tit Parus Major.” Journal of Avian Biology 30: 383–391. [Google Scholar]
- Tilgar, V. , Mänd R., and Mägi M.. 2002. “Calcium Shortage as a Constraint on Reproduction in Great Tits Parus Major: A Field Experiment.” Journal of Avian Biology 33, no. 4: 407–413. [Google Scholar]
- Tilgar, V. , Mänd R., Ots I., Mägi M., Kilgas P., and Reynolds S. J.. 2004. “Calcium Availability Affects Bone Growth in Nestlings of Free‐Living Great Tits (Parus Major), as Detected by Plasma Alkaline Phosphatase.” Journal of Zoology 263, no. 3: 269–274. [Google Scholar]
- Vedder, O. , Moiron M., Bichet C., et al. 2022. “Telomere Length Is Heritable and Genetically Correlated With Lifespan in a Wild Bird.” Molecular Ecology 31, no. 23: 6297–6307. [DOI] [PubMed] [Google Scholar]
- Voirin, C. J. , Tsunekage T., Liu Y., Alexy K. F., and Levin I. I.. 2023. “Brood Size Is Associated With Apparent Telomere Lengthening in Nestling Barn Swallows.” Oecologia 202, no. 1: 29–40. [DOI] [PubMed] [Google Scholar]
- Von Zglinicki, T. 2002. “Oxidative Stress Shortens Telomeres.” Trends in Biochemical Sciences 27, no. 7: 339–344. [DOI] [PubMed] [Google Scholar]
- Whittemore, K. , Vera E., Martínez‐Nevado E., Sanpera C., and Blasco M. A.. 2019. “Telomere Shortening Rate Predicts Species Life Span.” Proceedings of the National Academy of Sciences 116, no. 30: 15122–15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma, P. , Selman C., Speakman J. R., and Verhulst S.. 2004. “Birds Sacrifice Oxidative Protection for Reproduction.” Proceedings of the Royal Society of London. Series B: Biological Sciences 271, no. suppl_5: S360–S363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbourn, R. V. , Moatt J. P., Froy H., Walling C. A., Nussey D. H., and Boonekamp J. J.. 2018. “The Relationship Between Telomere Length and Mortality Risk in Non‐model Vertebrate Systems: A Meta‐Analysis.” Philosophical Transactions of the Royal Society, B: Biological Sciences 373, no. 1741: 20160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin, T. A. , Gosler A. G., Garant D., Reynolds S. J., and Sheldon B. C.. 2009. “Calcium Effects on Life‐History Traits in a Wild Population of the Great Tit (Parus Major): Analysis of Long‐Term Data at Several Spatial Scales.” Oecologia 159: 463–472. [DOI] [PubMed] [Google Scholar]
- Williams, G. C. 1966. “Natural Selection, the Costs of Reproduction, and a Refinement of Lack's Principle.” American Naturalist 100, no. 916: 687–690. [Google Scholar]
- Wood, E. M. , and Young A. J.. 2019. “Telomere Attrition Predicts Reduced Survival in a Wild Social Bird, but Short Telomeres Do Not.” Molecular Ecology 28, no. 16: 3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available via Dryad at https://datadryad.org/stash/share/1OdeICdgS3M2zsfeCXJhS9cRq5nLhWjzxfD1WQLBgKQ.


