Abstract
Background
Tropheryma whipplei (T. whipplei) is a rod-shaped, Gram-positive, acid-fast bacterium. Classical Whipple’s disease, a rare chronic infectious condition affecting multiple systems, is traditionally attributed to T. whipplei infection. The conventional treatment regimen consists of a one-year course of oral doxycycline (100 mg twice daily) and hydroxychloroquine (600 mg daily), followed by lifelong doxycycline maintenance therapy. However, the literature lacks discussion on short-term antimicrobial treatment for acute T. whipplei infections, such as pulmonary abscesses caused by this pathogen.
Presentation
This case report describes a 57-year-old male presenting with a pulmonary abscess. The patient underwent bronchoscopic alveolar lavage and pus cavity irrigation. The collected sample was subjected to pathogen targeted next-generation sequencing (tNGS) analysis. The tNGS results indicated that T. whipplei was the primary etiological agent responsible for the pulmonary abscess. Treatment with 6 weeks amoxicillin clavulanate led to a favorable clinical outcomes.
Conclusion
Existing case reports typically employ treatment protocols for classic Whipple’s disease, such as oral doxycycline combined with hydroxychloroquine or trimethoprim/sulfamethoxazole for a one-year duration. The use of amoxicillin/clavulanic acid for short-term antimicrobial treatment of T. whipplei-induced pulmonary abscesses achieved favorable clinical outcomes. This case study explores the feasibility of short-term antimicrobial therapy for an acute T. whipplei infection.
Keywords: Tropheryma whipplei, pulmonary abscess, tNGS, bronchoscopic lavage
Background
T. whipplei is bacillus belonging to the Actinobacteria phylum, Actinomycetes class, Micrococcales order, and Habitataceae family.1–3 Classical Whipple’s disease, a rare chronic infectious condition affecting multiple systems, is caused by T. whipplei. The disease exhibits a higher incidence rate among Caucasian males, while being uncommon in individuals of Asian or African descent.4 The widespread application of next-generation sequencing technology has led to an increased detection of T. whipplei in respiratory specimens. However, these cases frequently do not present the classic symptoms of Whipple’s disease, such as arthralgia, weight loss, diarrhea, and abdominal pain.5–8 Typically, case reports employ the standard treatment regimen for Whipple’s disease, which involves initial intravenous administration of antibiotics effective against T. whipplei (such as ceftriaxone, amoxicillin, penicillin, and meropenem), followed by a year-long oral therapy with trimethoprim/sulfamethoxazole or doxycycline combined with hydroxychloroquine. However, the literature currently lacks documentation on the use of short-term anti-infective treatment of pulmonary abscesses primarily caused by T. whipplei. This article presents a case where T. whipplei was identified as the primary pathogen in a pulmonary abscess following glucocorticoid pulse therapy. The subsequent literature review discusses the characteristics of this pathogen, its clinical manifestations, and treatment strategies.
Case Report
Medical History
A three-day history of coughing and blood-tinged sputum led to the hospitalization of a 57-year-old male with untreated type 2 diabetes. Two months prior to symptom onset, the patient was diagnosed with optic neuritis, presenting as blurred vision. The patient received short-term glucocorticoid pulse therapy, followed by oral prednisone maintenance therapy. At the onset of symptoms, the patient was taking 20 mg of prednisone daily. The patient had no other underlying medical conditions. He reported a 25-year history of alcohol consumption, consuming 50 milliliters of spirits daily, but had never smoked.
The patient’s cough and hemoptysis began two months after initiating glucocorticoid maintenance therapy. The patient denied experiencing dyspnea, fever, rhinorrhea, or chest pain. The patient reported no history of night sweats. The patient did not experience abdominal pain, joint pain, or diarrhea, and no weight loss was observed. Upon seeking medical attention at our hospital, a chest CT scan revealed a cystic lesion in the left lung with a fluid level and minimal consolidation surrounding the abscess cavity, suggestive of abscess formation. Consequently, a diagnosis of pulmonary abscess was established.
Physical Examination
Upon examination, the patient’s condition was largely unremarkable. Several decayed teeth were observed without accompanying signs of inflammation such as redness, swelling, or purulent discharge. No palpable enlargement of superficial lymph nodes was detected. The skin and mucous membranes exhibited no signs of jaundice. Auscultation revealed decreased breath sounds on the left side, while normal breath sounds were noted on the right side. The cardiac borders were within normal limits, without enlargement. The abdomen was soft and flat, with no palpable liver or spleen below the rib cage. A neurological examination yielded no significant findings.
Investigations
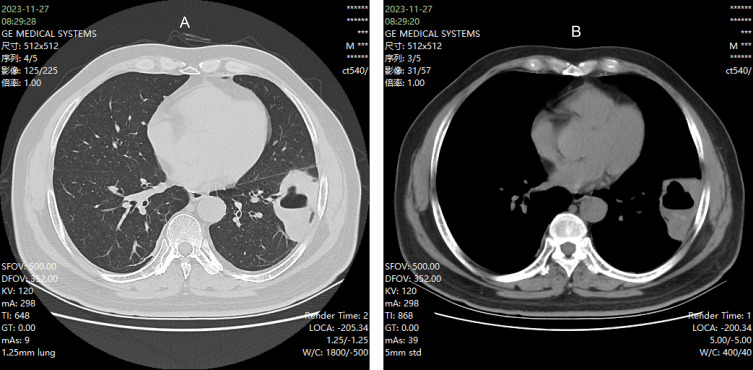
Complete blood count and high-sensitivity C-reactive protein (hs-CRP): The complete blood count revealed a white blood cell count of 14.6× 109/L, with neutrophils at 10.2×10−9/L and lymphocytes at 3.1×10−9/L. hs-CRP was elevated at 79.4 mg/L. Procalcitonin levels were below 0.04 ng/mL. Glycosylated hemoglobin (HbA1c) was measured at 8.1%. Interleukin-6 levels were elevated at 20.95 pg/mL, and ferroprotein levels were significantly increased at 1115.81 ng/mL. Sputum cultures, both aerobic and anaerobic, conducted over a 5-day period, yielded no bacterial growth. A chest computed tomography (CT) scan (Figure 1) revealed a cystic lesion with fluid levels and localized lung involvement in the left lung.
Figure 1.
(A and B) Chest CT before treatment.
Treatment and Prognosis
Prior to the initiation of empiric antibiotic therapy, the patient underwent electronic bronchoscopy. The procedure revealed the presence of blood and purulent sputum throughout the left bronchi. The endoscopic aspiration cleared the intratracheal blood and purulent sputum, revealing a distorted and swollen basal trunk of the left lower lobe bronchus. Alveolar lavage was performed in the lateral basal region of the left lower lobe (Figure 2). The lavage procedure involved rinsing the affected bronchus twice with 120 mL of sterile physiological saline. The recollected turbid alveolar lavage fluid, containing purulent secretions, was submitted for tNGS. Following the diagnostic bronchoscopic alveolar lavage, multiple infusions of sterile normal saline were administered through the bronchoscope. After each infusion, thorough aspiration was performed until the aspirated liquid became clear. A total of 360 mL of normal saline was utilized. Subsequently, empirical antibiotic therapy with amoxicillin/clavulanate (5:1) was initiated (1200 mg intravenous drip at 8-hour intervals). An enhanced chest CT examination was conducted on the second day post-bronchoalveolar lavage treatment, as depicted in Figure 3. The Pus tNGS results (Table 1) predominantly identify T. whipplei (sequence count: 2362; reference content: 53079 copies/mL). Given that pulmonary abscess is typically considered a mixed infection, the pathogens listed in Table 1 are primarily regarded as potential causative agents of pulmonary abscess, excluding viruses such as Human gammaherpesvirus 4, Human betaherpesvirus 5, and Pneumocystis jirovecii, which are known to colonize the lower respiratory tract. Despite the patient’s prior high-dose glucocorticoid treatment (prednisone > 20 mg/d for over 1 month), the clinical presentation and CT images did not support a diagnosis of pneumocystis pneumonia. Consequently, trimethoprim/sulfamethoxazole or echinocandins were not added to the treatment regimen. Following 13 days of intravenous amoxicillin/clavulanic acid treatment, the patient’s infection-related biological markers normalized, and symptoms of cough and hemoptysis resolved. A follow-up chest CT (Figure 4) demonstrated shrinkage of the cavity lesions. Upon discharge, the patient was prescribed oral amoxicillin/clavulanate (914mg 7:1) for 4 weeks. Subsequent outpatient follow-up chest CT (Figure 5) indicated continued improvement of the lesion, leading to the discontinuation of amoxicillin/clavulanate. At approximately 8 weeks post-treatment cessation, a further follow-up chest CT (Figure 6) revealed complete closure of the abscess cavity. The patient’s clinical symptoms, including cough and hemoptysis, have not recurred in the 8 weeks following medication discontinuation.
Figure 2.

Anterior medial basal segment of left lower lobe.
Figure 3.
(A and B) Enhanced Chest CT after Electronic Bronchoscopy Treatment.
Table 1.
The Result of tNGS
| Genus Name | Name of Pathogen | Reads Accum | Reference content (Copies/mL) |
|---|---|---|---|
| Tropheryma | Tropheryma whipplei | 2362 | 53079 |
| Streptococcus | Streptococcus cristatus | 975 | 21910 |
| Streptococcus | Streptococcus mitis | 710 | 15955 |
| Neisseria | Neisseria subflava | 614 | 13798 |
| Eikenella | Eikenella corrodens | 107 | 2404 |
| Haemophilus | Haemophilus parainfluenzae | 101 | 2270 |
| Actinomyces | Actinomyces graevenitzii | 94 | 2112 |
| Lymphocryptovirus | Human gammaherpesvirus 4 | 91 | 2045 |
| Pneumocystis | Pneumocystis jirovecii | 13 | 292 |
| Cytomegalovirus | Human betaherpesvirus 5 | 9 | 202 |
Figure 4.
(A and B) Chest CT on discharge.
Figure 5.
(A and B) Chest CT Scanning at discontinuation of medication.
Figure 6.
(A and B) Chest CT after discontinuing medication for 8 weeks.
Discussion
Tropheryma whipplei is characterized as a rod-shaped, Gram-positive, acid-fast bacterium.1–3 This microorganism is ubiquitously present in the environment9,10 and can colonize human oral cavities and intestines, often being detected in saliva and feces.11–13 Classical Whipple’s disease, a rare chronic infectious condition, is traditionally associated with T. whipplei, despite only a small percentage of carriers becoming symptomatic.14 The disease exhibits a higher incidence rate among Caucasian males, while being uncommon in individuals of African or Asian descent.4 T. whipplei infection can manifest systemically, potentially leading to central nervous system involvement, endocarditis, and pneumonia.15,16 The bacterium presents significant challenges for in vitro cultivation, requiring the presence of living eukaryotic host cells.17 The cultivation process is characterized by prolonged duration, susceptibility to contamination, and low sensitivity.18,19 Consequently, diagnosis primarily relies on nucleic acid testing methodologies.12,13,20 The advent of next-generation sequencing (NGS) technology has facilitated increased detection of T. whipplei in respiratory specimens. A notable study revealed that 70 out of 1725 bronchoalveolar lavage fluid (BALF) samples (4.0%) tested positive for T. whipplei, indicating a significant pulmonary prevalence. This finding underscores the importance of considering T. whipplei as a potential contributing factor in certain lung diseases, even in immunocompetent patients.21,22 Interestingly, these cases often do not present with the classic symptoms of Whipple’s disease, such as arthralgia, weight loss, diarrhea, and abdominal pain.5–8 T. whipplei infection has a variety of clinical manifestations, but studies tend to focus on specific clinical phenotypes, potentially limiting the full understanding of the infection’s clinical spectrum. The absence of recognized diagnostic criteria has led to the adoption of treatment plans typically used for classical Whipple’s disease in related case reports. Antimicrobial treatment regimens vary across studies, reflecting a lack of standardized treatment guidelines. A comprehensive review indicates that commonly employed antibiotics include penicillin, streptomycin, tetracycline, ceftriaxone, meropenem, trimethoprim-sulfamethoxazole, doxycycline, and hydroxychloroquine. The currently recommended treatment protocol involves initiating therapy with intravenous ceftriaxone or meropenem for 14 days, followed by oral trimethoprim-sulfamethoxazole for 12 months. For patients intolerant of ceftriaxone, meropenem serves as an alternative; similarly, doxycycline is suggested for those unable to tolerate trimethoprim-sulfamethoxazole. In some cases, lifelong treatment with doxycycline may be necessary. Patients presenting with late-stage symptoms involving the eyes, heart, or central nervous system pose significant treatment challenges and are associated with high relapse and mortality rates.23 This case report presents a patient who developed a pulmonary abscess following oral prednisone maintenance therapy after short-term glucocorticoid pulse treatment for optic neuritis. The abscess was acutely infected by T. whipplei. Bronchoscopic lavage of the purulent cavity was performed, and subsequent high-throughput gene testing of the lavage fluid identified T. whipplei as the predominant pathogen. The use of bronchoscopy for thorough purulent irrigation was assessed to significantly reduce the pathogen load in the infected lesion. Considering the low virulence characteristics of the pathogen, the mixed infection status, and the absence of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus, a treatment regimen of amoxicillin/clavulanic acid for 6 weeks was implemented, aligning with the standard anti-infection course for pulmonary abscesses. Mid-term follow-up chest CT scans demonstrated continuous improvement of the lesion. At 8 weeks post-treatment cessation, complete closure and absorption of the lung abscess cavity were observed. Existing research suggests that when T. whipplei manifests as classical Whipple’s disease through chronic infection, long-term treatment with established regimens is necessary. However, this case report indicates that an acute infection leading to a pulmonary abscess may not require prolonged treatment. The patient’s infection episode occurred against an immunosuppressive background of glucocorticoid use. Effective anti-infection therapy and reduced glucocorticoid dosages facilitated immune reconstitution and pathogen clearance. Nonetheless, further research is warranted to corroborate these observations.
Previous case reports primarily relied on metagenomic next-generation sequencing (mNGS) for pathogen diagnosis.5,6,8,21,22 However, this report also employed tNGS for diagnosis, as demonstrated in a case report of T. whipplei pneumonia.24 Unlike mNGS, which directly extracts DNA/RNA from samples for high-throughput sequencing, tNGS uses pre-designed specific primers that exclusively target and amplify the pathogen’s gene. Essentially, tNGS is a form of ultra-multiplex polymerase chain reaction (PCR), capable of detecting low concentrations of pathogenic nucleic acids more rapidly and sensitively, at a lower cost, and with reduced interference from human-derived nucleic acids.25 A large-scale study on the diagnostic performance of tNGS in infectious diseases concluded that tNGS outperformed traditional culture methods, particularly in cases involving Mycobacterium tuberculosis, non-tuberculosis Mycobacteria, and anaerobic bacteria. The study demonstrated that tNGS can cover approximately 96.7% of the pathogenic targets associated with community-acquired pneumonia.26 As a relatively cost-effective and efficient means of pathogen diagnosis, tNGS is an emerging molecular diagnostic technology with promising applications in multiple fields. This technology is anticipated to become a crucial tool for clinical microbiology diagnosis in the future.27
Whipple may exhibit an increased infection rate in immunocompromised individuals, including those using glucocorticoids, immunosuppressants, those with AIDS, or long COVID-19 infection.5,8,16,28,29 In this case, the patient had type 2 diabetes and received pulse therapy with glucocorticoids, followed by low-dose maintenance treatment two months prior to the onset of a pulmonary abscess. This pathophysiological status is consistent with the disease’s predisposing population. Patients with pneumonia who have compromised immune function are prone to deteriorate into severe pneumonia, often accompanied by mixed infections of multiple pathogens. A recent study have shown that the most commonly detected pathogens in pneumonia patients with compromised immune function include Klebsiella pneumoniae, Pneumocystis carinii, Pseudomonas aeruginosa, Staphylococcus, Aspergillus, Candida, as well as cytomegalovirus, Epstein-Barr virus, respiratory syncytial virus.30 This study indicates that in pneumonia patients with compromised immune function, the use of bronchoalveolar lavage can significantly enhance the pathogen diagnosis rate, thereby improving treatment outcomes. In other aspects, another study has shown that bronchoscopy lavage to have a high yield in immunocompromised patients, even when performed only to rule-out tuberculosis.31 In the treatment of pyogenic infections, draining pus is a commonly used therapeutic method. We discovered that the pulmonary abscess cavity in this patient was connected to the bronchus. Therefore, we used sterile saline to thoroughly flush the abscess cavity. One day later, enhanced chest CT scan showed that the pus in the lesion cavity had been cleared, achieved the desired drainage effect and avoid the bronchopleural fistula issue that can easily occur with percutaneous puncture drainage. It is crucial to fully suppress the patient’s cough reflex, minimize bronchial mucosal damage and bleeding when performing bronchoalveolar lavage, so as ensure the recovery volume of bronchoalveolar lavage fluid, and use 37°C sterile saline to reduce the tracheal spasm and asthma attacks. Most intraoperative decreases in partial pressure of oxygen are temporary, which can be resolved by oxygen inhalation, encouraging coughing, and prone positioning ventilation. Postoperative fever is also common, it is an absorption heat in most cases that does not require special intervention. However, we must also be vigilant for potential dissemination of infection foci. Additionally, based on personal experience, it is strongly recommended to perform preoperative enhanced CT evaluation for patients highly suspected of having tuberculous cavities or aspergillus cavities before bronchoalveolar lavage. This is because these patients’ abscess lesions may undergo massive hemorrhage during operation, the bronchoscopy intervention team and vascular intervention team still need to be backup. Following the successful control of the patient’s optic neuritis, glucocorticoid treatment was gradually discontinued, and insulin was administered to maintain the patient’s blood sugar within the ideal range. Consequently, the patient’s immunosuppression status improved. Subsequent follow-up chest CT scans revealed continuous improvement of pulmonary lesions after treatment discontinuation. This case suggests that in acute T. whipplei infections where the host’s immune status can be reconstructed, timely discontinuation of immunosuppressive drugs and glucocorticoids, along with improvement of cellular and humoral immune levels, may obviate the need for long-term treatment with drugs such as doxycycline or trimethoprim/sulfamethoxazole. However, this report has limitations. Due to the absence of gastrointestinal symptoms, gastroscopy was not performed, precluding the acquisition of duodenal tissue pathology. Moreover, the patient’s reluctance to undergo repeat bronchoscopy, given the continuous improvement in chest CT imaging and absence of recurrent respiratory symptoms, prevents the determination of complete T. whipplei clearance from the patient’s body. Consequently, an extended follow-up period is necessary.
Pulmonary abscess is a severe respiratory infection associated with substantial morbidity and mortality. The paucity of clinical evidence and international guidelines supporting pulmonary abscess management underscores the urgent need for larger prospective studies to enhance the detection and evaluation of this neglected disease.32 To the best of our knowledge, most pulmonary abscesses involve co-infection with multiple pathogens and correlate with the patient’s underlying condition. Common pathogens include periodontal pathogens, Klebsiella pneumoniae, and Staphylococcus aureus, while common triggering factors encompass poor oral hygiene and long-term alcohol abuse.33 This case demonstrates that T. whipplei can serve as the primary pathogen in pulmonary abscesses among immunocompromised populations. Given the diversity of infectious pathogens, the use of next-generation sequencing is recommended for pathogen identification. This approach has the potential to guide treatment, improve success rates, and reduce misdiagnosis and treatment failure.
Conclusion
Acute T. whipplei infection in pulmonary tissue can manifest as a lung abscess. Because polymicrobial infections are frequently present in pulmonary abscesses, the use of second-generation pathogen sequencing can facilitate diagnosis and provide valuable guidance for antibiotic selection. By reducing the pathogen burden at the infection site and promoting immune status recovery, bronchoscopy lavage enables short-term antibiotic monotherapy to achieve favorable therapeutic outcomes. This approach holds significant potential for minimizing antibiotic exposure duration. For T. whipplei-infected patients with reversible immunosuppression, discontinuation of immunosuppressants and glucocorticoids is recommended when feasible. Upon successful immune reconstitution, an acute T. whipplei infection may not necessitate prolonged antibiotic treatment. This case establishes a precedent for short-term antibiotic therapy in acute T. whipplei infection. Although the patient currently exhibits clinical cure, the risk of relapse persists even after symptom improvement. Relapses typically occur several years later, necessitating long-term monitoring. Further research on short-term antimicrobial treatment for acute T. whipplei infection is warranted to validate this approach.
Acknowledgments
The authors express their sincere gratitude to the patients who participated in this study for their invaluable support.
Funding Statement
This research received no external funding.
Ethics Approval and Informed Consent
This study received approval from the Ethics Committee of Yuyao People’s Hospital.
Consent for Publication
Informed consent for publication of the medical histories and photographs was obtained from the patient. Additionally, institutional consent for case publication was granted by Yuyao People’s Hospital.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- 1.Wilson KH, Blitchington R, Frothingham R, Wilson JA. Phylogeny of the Whipple’s-disease-associated bacterium. Lancet. 1991;338(8765):474–475. doi: 10.1016/0140-6736(91)90545-z [DOI] [PubMed] [Google Scholar]
- 2.Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. New Engl J Med. 1992;327(5):293–301. doi: 10.1056/nejm199207303270501 [DOI] [PubMed] [Google Scholar]
- 3.La Scola B, Fenollar F, Fournier PE, Altwegg M, Mallet MN, Raoult D. Description of Tropheryma whipplei gen. nov. sp. nov. the Whipple’s disease bacillus. Int J System Evolutionary Microbiol. 2001;51(Pt 4):1471–1479. doi: 10.1099/00207713-51-4-1471 [DOI] [PubMed] [Google Scholar]
- 4.Elchert JA, Mansoor E, Abou-Saleh M, Cooper GS. Epidemiology of Whipple’s disease in the USA between 2012 and 2017: a population-based national study. Dig Dis Sci. 2019;64(5):1305–1311. doi: 10.1007/s10620-018-5393-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Zhang B, Zhang Z, et al. Case report: tropheryma whipplei hide in an AIDS patient with pneumocystis pneumonia. Front Public Health. 2021;9:663093. doi: 10.3389/fpubh.2021.663093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du ZM, Chen P. Co-infection of Chlamydia psittaci and Tropheryma whipplei: a case report. World J Clin Cases. 2023;11(29):7144–7149. doi: 10.12998/wjcc.v11.i29.7144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladna M, George J, Forsmark CE. Whipple’s disease presenting with a chief complaint of dyspnea and cough from pulmonary invasion without evidence of gastrointestinal involvement. Cureus. 2024;16(2):e54554. doi: 10.7759/cureus.54554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan W, Xu J, Yang F, Wu X, Ying K. Tropheryma whipplei infection in the lung of a patient with long COVID: a case report. BMC Infect Dis. 2024;24(1):292. doi: 10.1186/s12879-024-09183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiwald M, Schuhmacher F, Ditton HJ, von Herbay A. Environmental occurrence of the Whipple’s disease bacterium (Tropheryma whippelii). Appl Environ Microbiol. 1998;64(2):760–762. doi: 10.1128/aem.64.2.760-762.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schöniger-Hekele M, Petermann D, Weber B, Müller C. Tropheryma whipplei in the environment: survey of sewage plant influxes and sewage plant workers. Appl Environm Microbiol. 2007;73(6):2033–2035. doi: 10.1128/aem.02335-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutly F, Hinrikson HP, Seidel T, Morgenegg S, Altwegg M, Bauerfeind P. Tropheryma whippelii DNA in saliva of patients without Whipple’s disease. Infection. 2000;28(4):219–222. doi: 10.1007/s150100070039 [DOI] [PubMed] [Google Scholar]
- 12.Maibach RC, Dutly F, Altwegg M. Detection of tropheryma whipplei DNA in feces by PCR using a target capture method. J Clin Microbiol. 2002;40(7):2466–2471. doi: 10.1128/jcm.40.7.2466-2471.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenollar F, Laouira S, Lepidi H, Rolain JM, Raoult D. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: usefulness of saliva and stool specimens for first-line screening. Clin Infectious Dis. 2008;47(5):659–667. doi: 10.1086/590559 [DOI] [PubMed] [Google Scholar]
- 14.Fenollar F, Trani M, Davoust B, et al. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J Infect Dis. 2008;197(6):880–887. doi: 10.1086/528693 [DOI] [PubMed] [Google Scholar]
- 15.Bousbia S, Papazian L, Auffray JP, et al. Tropheryma whipplei in patients with pneumonia. Emerg Infectious Dis. 2010;16(2):258–263. doi: 10.3201/eid1602.090610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagier JC, Fenollar F, Raoult D. Acute infections caused by Tropheryma whipplei. Future Microbiol. 2017;12:247–254. doi: 10.2217/fmb-2017-0178 [DOI] [PubMed] [Google Scholar]
- 17.Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. New Engl J Med. 2000;342(9):620–625. doi: 10.1056/nejm200003023420903 [DOI] [PubMed] [Google Scholar]
- 18.Renesto P, Crapoulet N, Ogata H, et al. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet. 2003;362(9382):447–449. doi: 10.1016/s0140-6736(03)14071-8 [DOI] [PubMed] [Google Scholar]
- 19.Raoult D, Fenollar F, Birg ML. Culture of T. whipplei from the stool of a patient with Whipple’s disease. New Engl J Med. 2006;355(14):1503–1505. doi: 10.1056/NEJMc061049 [DOI] [PubMed] [Google Scholar]
- 20.Moter A, Janneck M, Wolters M, et al. Potential role for urine polymerase chain reaction in the diagnosis of whipple’s disease. Clin Infectious Dis. 2019;68(7):1089–1097. doi: 10.1093/cid/ciy664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai LM, Zhu XY, Zhao R, et al. Tropheryma whipplei detected by metagenomic next-generation sequencing in bronchoalveolar lavage fluid. Diagnostic Microbiol Infectious Dis. 2024;109(4):116374. doi: 10.1016/j.diagmicrobio.2024.116374 [DOI] [PubMed] [Google Scholar]
- 22.Lin M, Wang K, Qiu L, et al. Tropheryma whipplei detection by metagenomic next-generation sequencing in bronchoalveolar lavage fluid: a cross-sectional study. Front Cell Infect Microbiol. 2022;12:961297. doi: 10.3389/fcimb.2022.961297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolmans RA, Boel CH, Lacle MM, Kusters JG. Clinical manifestations, treatment, and diagnosis of tropheryma whipplei Infections. Clin Microbiol Rev. 2017;30(2):529–555. doi: 10.1128/cmr.00033-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Yang X, He Z, OuYang C, Yang X, Yang C. Using targeted next-generation sequencing to diagnose severe pneumonia due to tropheryma whipplei and human metapneumovirus: a case report and literature review. Infect Drug Resist. 2024;17:1863–1868. doi: 10.2147/idr.S451477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao CL, Liu K, Zhou YZ. The clinical application of targeted next-generation sequencing and metagenomics next-generation sequencing in pathogenic microorganism detection. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine]. 2024;58(1):114–121. doi: 10.3760/cma.j.cn112150-20230201-00072 [DOI] [PubMed] [Google Scholar]
- 26.Deng Z, Li C, Wang Y, et al. Targeted next-generation sequencing for pulmonary infection diagnosis in patients unsuitable for bronchoalveolar lavage. Front Med Lausanne. 2023;10:1321515. doi: 10.3389/fmed.2023.1321515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong HL, Flurin L, Thoendel MJ, et al. Targeted versus shotgun metagenomic sequencing-based detection of microorganisms in sonicate fluid for periprosthetic joint infection diagnosis. Clin Infectious Dis. 2023;76(3):e1456–e1462. doi: 10.1093/cid/ciac646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shams S, Niloofar R, Beltrame A, et al. Tropheryma whipplei intestinal colonization in immunocompromised children in Iran: a preliminary study. Future Microbiol. 2021;16(15):1161–1166. doi: 10.2217/fmb-2021-0091 [DOI] [PubMed] [Google Scholar]
- 29.Marth T, Raoult D. Whipple’s disease. Lancet. 2003;361(9353):239–246. doi: 10.1016/s0140-6736(03)12274-x [DOI] [PubMed] [Google Scholar]
- 30.Li J, Zhou CE, Wei SC, et al. Diagnostic value of metagenomic next-generation sequencing for pneumonia in immunocompromised patients. Canadian J Infectious Dis Med Microbiol. 2022;2022:5884568. doi: 10.1155/2022/5884568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freund O, Hadad Y, Lagziel T, et al. The added value of bronchoalveolar lavage for pulmonary tuberculosis diagnosis in high-risk hospitalized patients with negative sputum samples. Adv Respir Med. 2023;92(1):15–24. doi: 10.3390/arm92010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperling S, Dahl VN, Fløe A. Lung abscess: an update on the current knowledge and call for future investigations. Curr Opin Pulm Med. 2024;30(3):229–234. doi: 10.1097/mcp.0000000000001058 [DOI] [PubMed] [Google Scholar]
- 33.Mohapatra MM, Rajaram M, Mallick A. Clinical, radiological and bacteriological profile of lung abscess - an observational hospital based study. Open Access Maced J Med Sci. 2018;6(9):1642–1646. doi: 10.3889/oamjms.2018.374 [DOI] [PMC free article] [PubMed] [Google Scholar]