Abstract
Purpose
Cervical cancer remains a significant health concern, particularly in developing countries, where it is a leading cause of cancer-related deaths among women. Innovative technologies have emerged to improve the efficiency, cost-effectiveness, and sensitivity of cervical cancer screening and treatment methods. This study aims to explore the various approaches for the detection and treatment of human papillomavirus (HPV), cervical dysplasia (CD), and cervical cancer, highlighting new technologies and updated screening strategies in developing areas.
Patients and Methods
A comprehensive literature search was conducted using PubMed to identify relevant publications on the subject of cervical cancer screening and HPV detection.
Results
HPV infection and cervical cancer continue to pose significant global health challenges. Emerging technologies such as rapid, low-cost HPV testing combined with high-resolution digital colposcopy and artificial intelligence interpretation hold promise for efficient and sensitive screening. Advancements in HPV vaccine distribution, high-risk HPV screening, DNA methylation assays, dual-stain cytology, lab-on-chip assays, and deep learning technologies offer new avenues for improved detection and risk stratification.Research and innovations in detection and treatment methods are crucial for reducing the burden of these diseases worldwide.
Conclusion
Screening for HPV and CD plays a pivotal role in reducing the risk of cervical cancer-related mortality. The development of novel technologies, along with efforts to enhance global health equity and integrate cervical cancer prevention with HIV screening and treatment programs, represent critical steps toward achieving comprehensive cervical cancer screening on a global scale.
Keywords: human papillomavirus, cervical automated visual evaluation, digital colposcope, HPV self-swab
Introduction
Cervical cancer is the fourth most common cancer globally and a leading cause of cancer deaths among women with an estimated 660,000 new cases and 350,000 deaths in 2022.1 Low and middle-income countries have disproportionately high burdens of cancer, with around 90% of cervical cancer deaths in low-resource communities.2 Over 95% of cervical cancer cases are attributed to infection by the human papillomavirus (HPV).1
In developed countries, effective primary prevention (vaccination) and secondary prevention (regular screening programs) prevent most cervical cancer cases. A single cervical cancer screening in a woman’s lifetime significantly reduces the risk of developing cervical cancer (a risk ratio of 0.65 when comparing single lifetime screening to no screening cohorts)3 However, in low and middle-income areas, access to preventative measures is limited, delaying diagnosis of dysplastic lesions and cervical cancer until advanced stages.4,5 Access to treatment and limited treatment modalities also result in higher mortality rates.6 Further, in resource-limited countries increasing HIV prevalence and decreased funding for antiretroviral therapy, potentiates the pathogenesis of HPV.7
Increased access to vaccines, genomic testing, and artificial intelligence technologies have the potential to vastly improve both primary and secondary prevention of cervical cancer. This article will describe these technologies which have the potential to dramatically lower cervical cancer mortality rates around the world.
Background
HPV Infection and Oncogenesis
HPV is the most common viral infection of the reproductive tract, with most unvaccinated men and women contracting the virus at some point in their lives.8 While most HPV infections do not cause symptoms and clear spontaneously within 1–2 years of infection, persistent infection with high-risk types may cause cervical cancer, among other diseases.2 Around 5–10% of all women who contract HPV develop persistent infection. In the cervix, abnormal cell growth progresses over the course of months and years to premalignant glandular or squamous intraepithelial lesions, classified histopathologically as cervical intraepithelial neoplasia (CIN). The majority of low-grade CIN lesions regress spontaneously, whereas higher-grade lesions pose higher risk for malignancy.9
Over 200 hPV types have been identified in humans, of which 15 types are proven or assumed oncogenic for cervical, anal, oropharyngeal, penile, vulvar, and vaginal cancers.10 High-risk types HPV-16 and HPV-18 are responsible for 71% of global cervical cancer cases.11 Approximately 90% of HPV-associated squamous-cell carcinomas are caused by HPV-16,18,31,33,45,58.12
Co-infection with multiple types of HPV is an increasingly recognized phenomenon with significant clinical implications, particularly in the context of oncogenesis. Studies have reported that, among HPV-positive women, the prevalence rate of multiple HPV infections is between 18.5 and 46%.13–15 The presence of co-infecting HPV types may alter the persistence of HPV infection, modulate immune responses, and influence the progression to high-grade lesions or malignancy.16 However, studies have demonstrated mixed clinical findings regarding the clinical impact of co-infection, with some research suggesting a synergistic effect that enhances oncogenic potential, while others indicate no significant deviation from the risks posed by single-type infections.17 More recent studies have postulated that co-infection with lower-grade HPV types has little impact on the CIN2 + risk associated with a single hrHPV infection.18,19
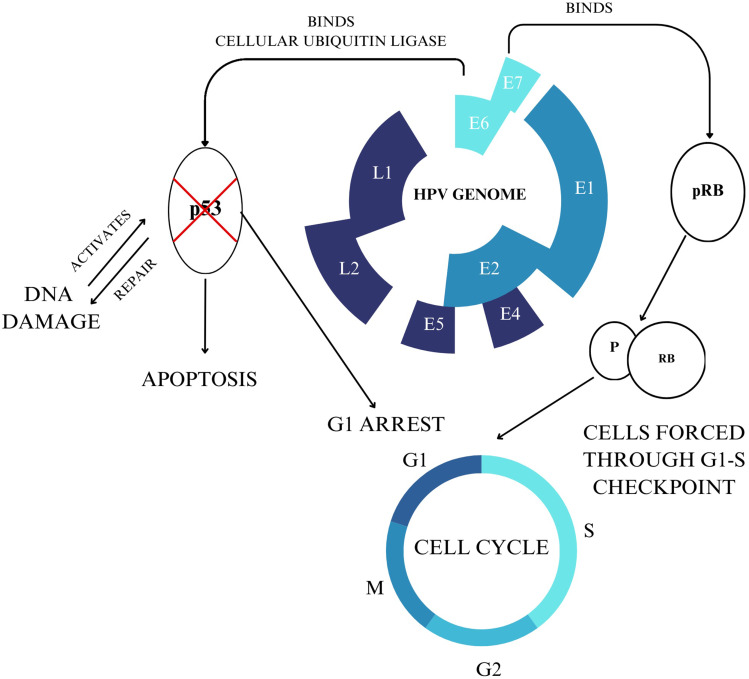
Human papillomaviruses are a type of small non-enveloped circular double-stranded DNA viruses comprising three genomic sections: the early gene-coding region (E), the late gene-coding region (L), and the long control region (LCR). The E region comprises six open reading frames, which regulate viral genome replication and early protein transcription. (Figure 1)20
Figure 1.
HPV Genomic Map and Cervical Cancer Oncogenesis.
The HPV life cycle utilizes the host cell differentiation program of keratinocytes located within the cutaneous and mucosal epithelia. In normal cervical tissue, the only cells that proliferate are the lower basal cells of the epithelium. These differentiated daughter cells migrate upwards through to the epithelial surface as a part of healthy tissue regeneration. Papillomaviruses often integrate viral genetic information into these lower proliferative cells, trafficking viral information through the epithelium and eventually releasing virions at the surface.21,22
HPV infection begins with the attachment of viral particles to the basal epithelial cells in the single-layered squamous cellular junction between the endo- and ectocervix.23 The primary receptor for HPV initial binding is heparan sulfate proteoglycans, which induces a conformational change in the viral capsid upon binding, triggering endocytosis.24 The viral episomal genome is transported to the nucleus, entering through nuclear pores. Upon entry into the dividing cells, the viral replication factors E1 and E2 recruit cellular DNA replication machinery, generating approximately 50–100 episomal copies per nucleus upon initial replication.25 In this first phase of viral DNA replication, HPV genomes are replicated in concert with cellular DNA replication, and viral genetic information is divided equally into daughter cells.25,26 High levels of Proteins E1 and E2 expression signal the early stages of HPV infection; clinically, this is correlated to low-grade dysplastic lesions.20
Viral oncoproteins E6 and E7 play an integral role in oncogenesis. The E6 protein binds to the p53 tumor suppressor protein, leading to its degradation through ubiquitination. Apoptosis is inhibited, contributing to cell proliferation. The E7 protein binds to the retinoblastoma tumor suppressor protein (pRb), which plays a role in the G1-S phase transition. In HPV-infected cells, the E7 protein degrades pRb, forcing cells through premature S-phase entry.27 The degradation of these suppressor proteins results in the classical signs of cancerous cells: unregulated cell proliferation, metastasis, uncontrolled telomerase activity, and invasion.
Around 80–90% percent of cervical cancer cases are squamous arising from the transformation zone, where the exocervix joins the endocervix. The remainder are adenocarcinomas originating from endocervical glandular cells. Adenocarcinoma patients have poorer overall results and disease-free survival.28
Co-infection with Human Immunodeficiency Virus (HIV) significantly complicates the natural history and clinical outcomes of HPV infection.7 HIV risk is six times higher in southern Africa and three times higher in eastern Africa for young women compared to men.29 Women living with HIV have as much as a six-fold higher risk of developing cervical cancer relative to their counterparts without HIV.29 Similarly, an estimated 6% of new cervical cancer cases worldwide were diagnosed in women with HIV. In some countries, the proportion of women living with HIV among patients with cervical cancer was reported to be above 40%.29 HIV worsens dysplasia recurrence rates as well as cancer morbidity and mortality.30
HIV induces systemic loss of CD4 T lymphocytes, cells that coordinate immune response and fight infections. Lack of CD4 T lymphocytes compromises the host’s ability to mount an effective immune response against HPV, specifically within the dysregulated immune cells in the cervical mucosa. Women with HIV and a low CD4 T cell count have been shown to be more susceptible to acquiring an HPV infection.31
HIV is associated with skewed CD4/CD8 T-cell ratios, and the frequency of CD4 T cells is further decreased in women with a coexisting HIV and HPV infection. This immune system disruption may contribute to less effective HPV clearance in HIV+ women.32 Combined antiretroviral therapy users have lower rates of hrHPV, cervical dysplasia, and invasive cancer compared to their non-treated counterparts, highlighting the importance of well-rounded care in women living with HIV.33
In 2022, cervical cancer was the fourth most common malignancy in women globally, with approximately 660,000 new cases reported. Of the estimated 350,000 deaths resulting from cervical cancer that year, 94% occurred in low- and middle-income countries.1 Cervical cancer disproportionately impacts the poorest and most vulnerable women, particularly during their child-bearing years and periods of peak economic productivity. Traditional screening methods require specialized laboratory facilities, trained cytotechnologists, and a well-established healthcare delivery system—resources that are often scarce in low-income and rural settings.34 This disparity underscores the urgent need for scalable, cost-effective technologies that can be deployed in resource-limited environments to improve early detection and reduce mortality rates.
Primary Prevention
Primary prevention programs for cervical cancer focus on preventing initial HPV infections, with HPV vaccines being the most impactful strategy. While barrier methods such as condom use offer some protection, their efficacy is limited, making them challenging to implement widely.35
HPV Vaccines
Vaccines are highly effective at preventing HPV infection and pathogenesis. Trials leading to the approval of the bivalent vaccine (HPV 16 and 18) and quadrivalent vaccine (HPV 6, 11, 16, and 18) provide protection against both persistent cervical infections and dysplasia caused by HPV types 16 and 18 with a near 100% efficacy rate.36 In the efficacy trial that was the basis for nonavalent (9-strain) licensure, researchers demonstrated a 96.7% efficacy among 6,016 young women for preventing HPV 31-, 33-, 45-, 52-, 58-related late-stage cervical pre-cancer, cervical cancer, vaginal intraepithelial neoplasia 2/3, vulvar intraepithelial neoplasia 2/3, vulvar cancer, and vaginal cancer.37
In developed countries with high rates of vaccine uptake, rates of HPV infection have dipped dramatically. In a 13-year surveillance study, researchers found detection of 4-valent vaccine–type HPV decreased from 35% to 6.7% among vaccinated women, an over 80% decline.38 Similarly, 4-valent vaccine–type HPV detection decreased from 32.4% to 19.4% in unvaccinated women in a study of young women in the United States, indicating both high levels of vaccine efficacy and subsequent herd immunity.38 A similar study from 2019 showed that within 9–10 years of vaccine introduction, the prevalence of HPV types covered by the vaccines decreased 78% among 20–24 year-olds and 38% in 25–29 year-olds.39
In 2007, Australia implemented a comprehensive national vaccination program, achieving broad coverage. A model predicts that by 2028, Australia will have fewer than four new cervical cancer cases per 100,000 women, and by 2066 the annual incidence of cervical cancer will decrease and remain at fewer than one case per 100,000 women. Thus Australia is predicted to be one of the first countries to reach the elimination threshold of four new cases per 100,000 annually, effectively signaling the eradication of cervical cancer.40
Unfortunately, access to vaccines in resource-poor countries is limited. By 2022, 125 countries had introduced a HPV vaccine into their national immunization programs for women;9 however, only a third of the global population of girls were included in these national vaccine protocols.41 Women in populations with the highest incidence and mortality of disease remain largely unprotected, with only 2.7% of women in less developed regions completing the full series of vaccines.42 Even in areas with access, infrastructure is vulnerable to stress; WHO found that 3.5 million fewer women received a HPV vaccination in 2021 compared to 2019, as a result of the healthcare and educational barriers during the COVID-19 pandemic.43
Altering vaccine dosage and scheduling can increase preventative access without significantly altering immunogenicity outcomes. A single-dose of bivalent HPV vaccines was nearly as effective in preventing persistent oncogenic HPV infection compared to multi-dose regimens in a recent randomized controlled trial of 15 to 20-year-old women.44 A similar open-label study showed that over 97.5% of participants in the single-dose protocol group were seropositive 2 years after vaccination, with minimal clinical differences among dosage schedules.45 Modeling accounting for global resource and infrastructure limitations has predicted that a one-dose vaccination at 80% coverage over the next century could avert over 60 million cervical cancer cases.46
A model specific to Uganda predicted that after 40 years upfront investment costs of a national vaccination program are cheaper than the expenses associated with an alternate no-vaccine timeline. Economic investing would potentially be halved with a single-dose vaccination with modest cervical cancer incidence differences (15–16% of cervical cancer cases with one-dose vaccination versus 21% with two-dose vaccination).47 Other models make similar predictions, highlighting the cost-savings of a simpler vaccine delivery system and the circumnavigation of vaccine supply constraints.46
Given this data, the World Health Organization Strategic Advisory Group of Experts on Immunization (SAGE) recently altered its vaccine recommendations. New guidelines outline a one or two-dose schedule for girls and women 9–20 years old and two doses with a 6-month interval for women older than 21 years old.9 The transition away from the 2–3 dose model has the potential to improve coverage by offering a more efficient and cost-effective pathway to worldwide cervical cancer vaccination.
Simplified dosing schedules make vaccines more adaptable to the realities of resource-limited settings, facilitating easier storage, distribution, and administration in remote and underserved areas. This adaptability is crucial for reaching marginalized populations, where traditional multi-dose regimens may pose barriers to widespread coverage.48,49
Secondary Prevention
Secondary prevention of cervical cancer aims to detect and intervene in precancerous lesions early, thereby preventing their progression to invasive cancer.49 While cytology, or Pap smear, has been a traditional method for this purpose, challenges such as the need for skilled personnel and labor-intensive processing have led to the exploration of alternative approaches. Technologies like digital colposcopy and high-risk HPV testing offer improved sensitivity and accessibility, while innovative techniques such as self-swabbing programs and deep learning technologies further enhance screening capabilities. There is a growing desire for molecular tests that can accurately identify women with dysplasia at increased risk of progressing to cancer, enabling more precise risk stratification and targeted interventions.
Cytology/ Pap Smear
Cytologic examination of cervical cells, commonly known as Papanicolaou or “pap” smear, has been a cornerstone in cervical cancer screening for decades, primarily involving the microscopic examination of cervical cells to detect abnormalities indicative of precancerous or cancerous lesions.50 In the mid-1990s, the introduction of liquid-based cytology created a faster and more accurate alternative to the conventional Pap smear.44
Cytology has gradually fallen out of favor in developing regions for several reasons. Cytology-based screening requires access to skilled healthcare professionals (cytopathologists), which poses challenges in resource-limited settings. Processing cytology samples is labor-intensive, time-consuming, and requires skills that may further hinder its feasibility.34 Moreover, cytology has limitations in terms of sensitivity and specificity, particularly in detecting cervical adenocarcinoma and other glandular lesions, thereby compromising its effectiveness as a sole screening method.51 The limitations associated with cytology have spurred the exploration of alternative screening approaches, particularly for resource-limited settings.
Digital Colposcopy
Colposcopy has been used for decades for patients with abnormal primary screening results. This visual inspection procedure utilizes a microscope called a colposcope to magnify visualization of the cervix, ideally with a view of the squamocolumnar junction and the transformation zone. Generally, a solution of 3–5% acetic acid is applied to the cervix to induce acetowhite transformation of abnormal squamous cells.
Colposcopy has traditionally required significant training and equipment difficult to obtain in lower-resource settings. In recent years, digital optical technologies have advanced, spurring the development of digital colposcopes. These devices use compact, high-resolution cameras or smartphones to visualize the cervix, at times at higher magnifications than conventional colposcopes. Such highly portable alternatives also have the added benefit of easily creating high-quality digital images for electronic medical record keeping.52,53
Examples of such technologies include smartphone-based digital colposcopes such as MobileODT’s EVA and Gynius AB’d Gynocular.54,55 Generally, these devices pair digital colposcopy with a secure online data storage platform, enabling rapid digital record keeping and the fast export of images for review. In low-resource settings, digital colposcopes have the potential to remove the training, equipment, and cost barriers that can otherwise limit access to cervical health services.
High Risk HPV Screening
High-risk HPV (hrHPV) testing utilizes signal amplification techniques or nucleic acid amplification with polymerase chain reaction of vaginal swab specimens to detect DNA from oncogenic HPV types. Such tests determine if a patient has hrHPV and use strain typing to quantify the risk of cancer development and inform clinical decisions. In the primary screening context, a negative hrHPV result indicates a very low risk of future cervical cancer; a United States National Institutes of Health study of more than a million women found that a negative hrHPV test is a better predictor of cervical cancer risk than a negative cytology test.56,57
HrHPV testing is more sensitive and reproducible than the visual screening methods such as visualization with acetic acid (VIA) that have been utilized in low-resource settings.58,59 Additionally, data from clinical, cohort, and modeling studies illustrates primary hrHPV testing and co-testing detect more cases of high-grade CIN than cytology alone in average-risk patients aged 25–65 years.56,60
In recent years, major medical societies have updated guidelines to utilize HPV testing as either the primary method of cervical cancer screening or in tandem with established cytology guidelines.61 In 2020, the American Cancer Society recommended hrHPV testing as the preferred screening option for average-risk women aged 25–65.62 The following year, The American College of Obstetricians and Gynecologists, The Society of Gynecologic Oncology, and The American Society for Colposcopy and Cervical Pathology all endorsed guidelines utilizing cytology every three years for women aged 19–21, cytology alone every three years or hrHPV testing alone every five years or co-testing every five years for women aged 30–65.60 Co-testing remains the most prevalent screening method in USA hospitals. Notably, in 2021 the World Health Organization began recommending HPV DNA testing over cytology or visualization with acetic acid (VIA), starting at the age of 30 with a five to 10-year screening interval.1
In developing areas, the potential of hrHPV testing as a primary screening modality is particularly impressive. A modeling study of 78 low- and lower-middle-income countries found that screening with VIA or cytology every 3 years was less effective and less cost-effective than HPV screening every 5 years. Compared to hrHPV testing, VIA was predicted to generate more than double the number of pre-cancer treatments without consequent improvement in disease prevention, thereby highlighting the hrHPV testing’s efficiency and accuracy.63
A similar study modeling women living with HIV in Tanzania found that primary HPV screening every three years (even without colposcopy) would reduce age-standardized cervical cancer mortality rates by 72%.63 While the cost of HPV testing has historically proven to be prohibitive, newer technologies using isothermal amplification assays (as opposed to temperature-sensitive PCR-based assays) are significantly less expensive. Additionally, these newer systems can produce results in an hour or less with relatively compact and lightweight equipment which facilitates use in a mobile clinic setting.64
Assays modifying existing HPV testing technologies may further improve portability and efficiency of HPV testing. One such assay, the 13-type ScreenFireTM system utilizes risk-based extended HPV typing, separating HPV types into four channels by carcinogenicity: (a) HPV16, (b) HPV 18/45, (c) HPV 31/33/35/52/58, and (d) HPV 39/51/56/59/68.61 This risk stratification strategy can prioritize the HPV types by clinical significance and has been shown to be rapid, accurate, and cost-effective.65 A 2023 study of over 2,000 women found that time-to-positive test results using the ScreenFire system were shorter for women with HPV-16 related dysplasia. Additionally, women with high-grade dysplasia had assay results that turned positive faster than low-grade lesions.66 Time-to-positive result warrants further analysis as it could be used in combination with other triage strategies to stratify risk without increased cost.
HPV messenger RNA (mRNA) testing is another diagnostic method that detects hrHPV mRNA, particularly the E6 and E7 oncogenes. HrHPV RNA testing has similar cross-sectional sensitivity for detecting CIN2+ and CIN3+ lesions as DNA-based tests, with slightly higher specificity.67 However, the WHO guidelines suggest that HPV mRNA testing may be used as an alternative primary screening method for cervical cancer in the general population, with a conditional recommendation based on low-certainty evidence. The preferred method remains HPV DNA testing.1
HPV Self-Testing
A major benefit of hrHPV testing is simplified sample collection. Cytology requires a pelvic exam in a clinical setting with trained professionals to obtain a proper cellular sample. HrHPV testing allows for a patient to self-test with minimal instruction in less than five minutes by inserting a vaginal swab so that it brushes the cervix, then turning the swabbing handle two to three times. Upon completion, the sample is placed in a tube, and transported to a laboratory for testing.68 Multiple studies show that self-collected and physician-collected samples have comparable sensitivities and specificities for HPV typing.69–71 Self-swabbing also allows for sample collection in privacy.
Private self-swabbing is an excellent culturally sensitive alternative in areas where stigmatization, need for spousal permission, fear of exposure of genitalia to health care practitioners, and other factors inhibit screening acceptability.72,73 A large study of Argentine women found that offering self-collection of samples by community health workers during home visits increased screening uptake by four-fold compared to the control group which was told to attend a health clinic.74 A similar US-based study found that nearly 90% of women in rural Appalachia were more likely to self-collect a specimen for hrHPV testing compared to regular clinic-based cytology screening.75 Multiple studies across the globe show that self-sampling is not only acceptable, but preferable to both women and clinicians due to its relative ease of use and adaptability in areas with insufficient healthcare infrastructure.68,76
Self-collected HPV testing has the advantage of being far less expensive than cytology.77 Additionally, self-swabbing can be completed rapidly on a very large scale. A 2020 study in rural China used four HPV testing machines to screen 480–980 women per day using self-swab. All women with positive hrHPV test results were contacted and suspected lesions were treated on the same day.64
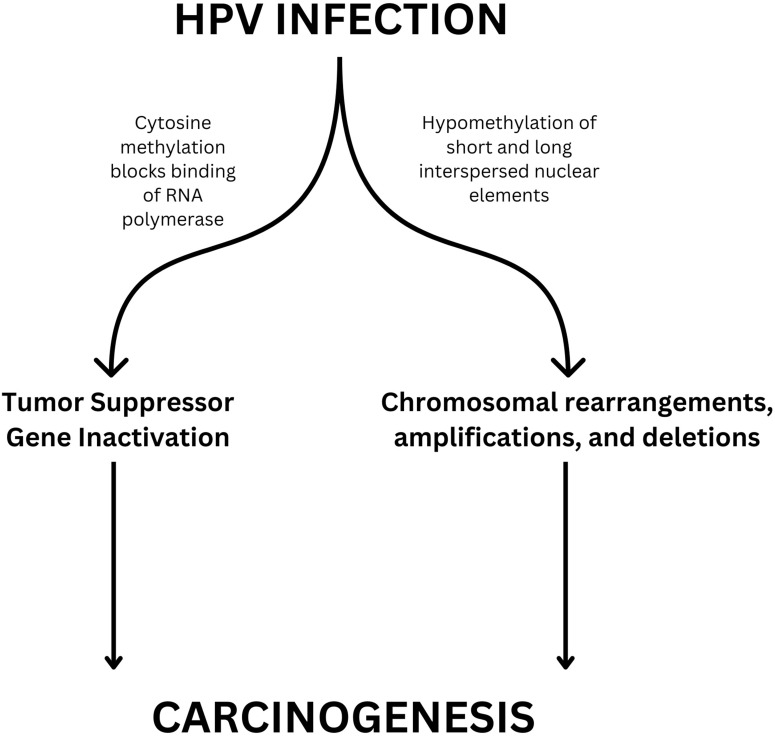
HPV DNA Methylation
In many cancers, alterations of DNA methylation patterns are a frequent and early marker of cancerous progression, with relative differences in methylation rates correlated with disease severity (Figure 2). Studies have shown that this relationship is evident across all carcinogenic HPV strains.78,79
Figure 2.
Mechanism of Epigenetic Modifications in HPV-induced Carcinogenesis.
DNA methylation tests look for epigenetic changes to DNA from cervicovaginal cells that are caused by HPV. Many methylation sites have been identified that show either hyper- or hypo-methylation patterns in dysplastic and cancerous cells, with sites showing varied diagnostic accuracy.80,81
Gradissimo et al investigated the methylation levels in cervical cancer. Site-specific methylation percentages were measured and aggregated into a methylation score. The observed sensitivity and specificity were 74% and 89%, respectively, in detecting cervical adenocarcinoma, which is harder to detect with traditional cytology-based screening.82 These findings highlight the potential of DNA methylation tests as a potential biomarker, refining risk stratification approaches to cervical cancer screening.
While DNA methylation tests show promise for detecting early dysplasia, currently the practical application of such tests is limited due to their high cost, lack of international validation guidelines to ensure accurate and consistent testing, and the need for further study using larger sample populations.83
HPV E6/E7 DNA, mRNA, and Oncoprotein Testing
The viral oncoproteins E6 and E7 play a critical role in cervical carcinogenesis.84 In cervical samples, levels of HPV DNA, mRNA, and oncoproteins E6 and E7 are elevated, suggesting the potential for a novel biomarker.85–87A meta-analysis of 22 studies found that oncoprotein testing was significantly more specific than HPV DNA and mRNA testing (relative specificity of 1.34 and 1.66, respectively).88 Further study of the testing apparatus’s efficacy is needed, particularly in the case of HPV-positive cells that do not integrate HPV.88 However, the potential of a triage E6/E7 viral oncogene test is promising.
Dual Stains
Dual-stain cytology uses staining to detect cervical epithelial cells with overexpression of two proteins associated with cell proliferation: p16 and Ki-67.89 When both biomarkers are expressed in the same cervical epithelial cell they indicate cell transformation due to HPV infection and the potential presence of high-grade CIN lesions. Dual stains have superior risk stratification abilities, with negative results providing a longer window of reassurance.90–92
In 2020, the US FDA approved the CINtec PLUS Cytology test (Ventana Medical Systems Inc.) which uses qualitative immunocytochemical assays to detect p16/Ki-67 biomarkers. A 2023 meta-analysis of 24 studies found that dual-stain cytology was significantly more sensitive than traditional cytology (75.9% compared to 71.1%) and had higher specificity (79.7% compared to 64.3% for cytology).87 Similarly, dual immunostaining had a higher pooled diagnostic odds ratio compared to hrHPV testing and cytology.87 Dual stain tests were also more indicative of a 5-year risk of cervical cancer development than cytology-based tests.93
In 2024, the American Society of Colposcopy and Cervical Pathology (ASCCP) guidelines were updated to include dual stain as a screening tool. Colposcopy is recommended for individuals testing DS-positive, while those testing DS-negative should have a one-year follow-up with HPV-based testing, except when HPV16- or HPV18-positive results or high-grade cytology are present, which warrant immediate colposcopy. The guidelines also indicate that, compared to cytology, dual stain testing necessitates fewer colposcopies and enables earlier detection of high-grade dysplasia.94
Excitingly, new advances in the automated evaluation of dual-stained slides could alleviate some of the infrastructure limitations to dual-stain access. Deep-learning classifiers for dual-stained slides trained on biopsy data remove the subjective components of cervical cancer screening and may eliminate barriers to access for developing regions.95 One AI algorithm trained to evaluate dual-stain slides had equal sensitivity and substantially higher specificity compared with both cytology and manual dual stain. In practice, the AI-based dual stain evaluations reduced referral to colposcopy by one-third compared to Pap smear.96
In recent years, dual stains have been introduced as an alternative triage strategy in developing areas. A 2015 study in Kenya successfully screened over 500 women using p16/Ki-67 dual stain cytology.97 Later studies in comparable settings in China and Kenya utilized similar screening protocols successfully and on a larger scale.98–100 A model of Thai women found that a triage protocol of primary hrHPV testing and reflex dual stain cytology is less costly than pooled HPV tests with reflex liquid-based cytology triage or pooled HPV tests with dual stain.101 With the integration of AI-driven evaluation of dual-stained slides, the potential for cost-effective and accurate dual-stain testing in developing areas is increasingly feasible, transcending the barriers of specialized infrastructure and expertise.
Lab on Chip Assays
A 2022 pilot feasibility study published in Nature showed the potential of a point-of-care test that analyzed tumor-specific markers with nucleic acid amplification combined with testing for HPV DNA and RNA.102 This test utilizes lab-on-chip-assays, a device that integrates multiple tests onto a single chip. Additionally, this test uses an isothermal loop-mediated amplification (LAMP) assay to amplify HPV DNA and human telomerase reverse transcriptase (hTERT) mRNA. Other studies have shown that hTERT is overexpressed in dysplastic and cancerous lesions,103,104 and can serve as a tumor-specific marker. The described device detected hTERT mRNA in all malignant and none of the benign samples; concordant results were recorded between the HPV and hTERT LAMP reactions and conventional PCR. A separate study of LAMP technology for HPV detection demonstrated a sensitivity and specificity of 100% and 91.7%, respectively.102 Further research into the feasibility of clinical applications of this technology is needed before widespread implementation.99
Deep Learning Technologies
Artificial intelligence (AI) is increasingly being utilized in the analysis of Pap smears to enhance the accuracy and efficiency of cervical cancer screening. Traditionally, Pap smear interpretation relies on the manual examination of cervical cells by cytopathologists, a process that can be prone to human error and variability. AI systems, particularly those based on deep learning algorithms and convolutional neural networks, are trained on extensive datasets of cytological images to identify cellular abnormalities associated with pre-cancerous and cancerous conditions.
Recent studies have demonstrated the potential of artificial intelligence (AI) in improving the accuracy and efficiency of cervical cancer screening through Pap smear analysis. One such study focused on developing and validating an AI cervical cancer screening (AICCS) system designed to grade cervical cytology.105 The AICCS was trained and validated on various datasets, including retrospective, prospective, and randomized observational trial data, encompassing 16,056 participants. The system utilized two AI models: one for detecting cellular abnormalities at the patch level and another for classifying whole-slide images (WSIs). The AICCS system showed high accuracy across different datasets, with a prospective assessment revealing an area under the curve (AUC) of 0.947, sensitivity of 0.946, specificity of 0.890, and accuracy of 0.892. Notably, in randomized trials, cytopathologists assisted by AICCS demonstrated a 13.3% increase in sensitivity, along with higher AUC, specificity, and accuracy, compared to cytopathologists working without AI assistance.
Similarly, another study validated the Genius™ Digital Diagnostics System, an AI-based system for screening Pap tests, by comparing its performance with traditional manual light microscopy slides.106 In this study, 319 retrospective cases were assessed by cytologists and cytopathologists using both digital and manual methods. The AI-based digital system showed higher concordance with the original diagnosis compared to manual review, with significant differences observed across exact Bethesda System diagnostic categories (62.1% vs 55.8%), condensed diagnostic categories (76.8% vs 71.5%), and condensed diagnoses based on clinical management (71.5% vs 65.2%). Additionally, the time required to evaluate cases was shorter for the digital system (mean 3.2 minutes) compared to manual review (mean 5.9 minutes), indicating improved efficiency.
Both studies underscore the advancements in AI-based systems for cervical cytology, highlighting their potential to improve diagnostic accuracy, enhance efficiency, and support clinical decision-making in cervical cancer screening. However, cytology still remains a high-cost and resource-intensive option for LMICs.
Deep learning‐based automated visual evaluation (AVE) integrated into portable colposcopes creates the potential for a low-cost device that can both photograph and diagnose a patient in real-time. AVE algorithms utilize sample photos from patient cervix exams to create parameters defining stages of carcinogenesis. For example, an AVE algorithm currently used in Desai et al adopts four stages: normal cervix, infection with high‐risk HPV, dysplasia, and invasive cervical cancer.107 Deep learning models analyze the image for identifying characteristics like texture, color, and edges, ultimately creating an “algorithm” with predictive power.
In its current form, AVE shows promise as a tool used in conjunction with other triage strategies, particularly in low and middle-resource countries. A 2023 study of over 8,000 women in Zambia developed and trained an AVE algorithm using cervical images and confirmatory histopathology. The AVE algorithm identified dysplasia with a sensitivity and specificity of 85% and 86%, respectively.108 Other deep-learning models using a multi-institution, multi-device, and multi-population datasets of over 9,000 women, when combined with HPV type, achieved an area under the receiver operating characteristics curve of 0.89 and minimal disagreement between image pairs across women.109
While promising, AVE technology faces multiple hurdles. First, thousands of representative images with confirmed pathology are required to calibrate an accurate and generalizable deep-learning model. The acquisition of such photos is difficult for several reasons: image quality discrepancy, “look-alike” confounding conditions such as cervicitis or cervical schistosomiasis or tuberculosis, and low point-prevalence of cervical pre-cancer in the general population.107 Similarly, the technical but subjective choices underpinning the deep learning algorithm may change the output.107,109 For example, simply determining if a photo is “usable” is a critical aspect of data collection and a significant challenge.110 Models that filter out blur, poor focus, poor light, noise, obscured view of the cervix due to mucus and/or blood, improper position, and over- or under-exposure among other factors are being developed to ensure that AI algorithms are trained using exclusively valid data. A three-tier image quality classification system has been effective in reducing misclassification by AVE achieving an area under the receiver operating characteristics curve of 0.92 (low quality, LQ vs rest) and 0.93 (high 166 quality, HQ vs rest).111
HPV- Automated Visual Evaluation (PAVE) Study
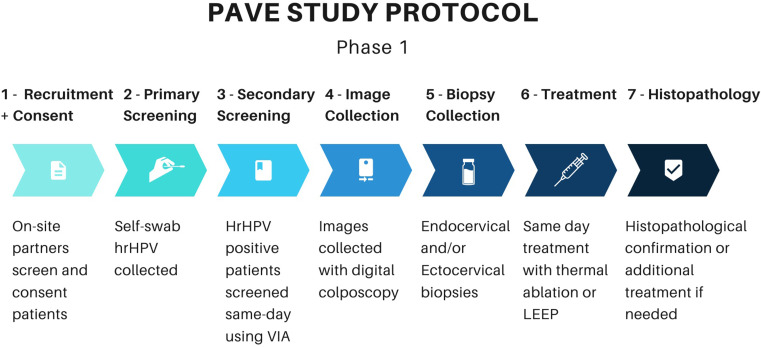
The integration of the aforementioned novel technologies is currently being studied through the American National Cancer Institute’s Papillomavirus-Automated Visual Evaluation (PAVE) Study. This multi-year international study aims to concurrently screen up to 100,000 women aged 25–49 and evaluate the feasibility of an AVE algorithm used to aid real-time clinical decision-making.112 At the time of writing, the two-phase study is still in its efficacy stage, which includes screening women with self-collected vaginal samples using hrHPV testing. HPV-positive women then proceed to pelvic exams, where cervical imaging and biopsies are taken. Images from this phase will be used to train the AVE algorithm and biopsy results will be used to corroborate image pathology. Study sites include Brazil, Cambodia, the Dominican Republic, El Salvador, Eswatini, Honduras, Malawi, Nigeria, and Tanzania. Site selection was determined by identifying pre-existing screening programs to incorporate into the study protocol.112
Obtained cervical images are being used to develop four AI algorithms (1) cervix detector, (2) image quality classifier, (3) disease classifier to identify dysplasia, and (4) treatability/ squamous-columnar junction (SCJ) classifier.106 The goal of the cervix detector is to aid providers in the identification of the cervix within the digital image. The image quality classifier is similar to the aforementioned quality-control algorithms and will flag unsuitable images that cannot be used to train AI. The disease classifier aims to classify cervical images as normal, indeterminate, or dysplastic with early data from algorithm prototypes showing promise across diverse geographics and demographics.113 However, further study of the repeatability, accuracy, portability, and calibration is needed before clinical application of this tool.114 The last algorithm will qualify the visualization of the SCJ, to aid in provider determination of treatment eligibility.
Phase two will integrate AVE results and HPV genotype data real-time into clinical decision-making. The AI algorithm will be installed into the portable digital colposcope and will assist providers in taking high-quality photos. The AI will then provide a disease classification score, assess treatment feasibility (in relation to SCJ visualization), and calculate a risk category using HPV genotype data.112
PAVE represents a paradigm shift–the leveraging of self-swabbing, hrHPV primary screening, and AI algorithms demonstrates how healthcare disparities can be bridged. The integration of these technologies not only streamlines the screening process but also addresses critical issues of accessibility and efficiency, particularly in resource-limited settings where the burden of cervical cancer is disproportionately high (Figure 3). This rapid, high-volume, low-cost triage strategy can feasibly become the model for cervical cancer screening worldwide.
Figure 3.
Phase 1 of PAVE Study Protocol.
Treatment
Cervical cancer treatment strategies are stratified based on the severity of the dysplasia or the presence of invasive cancer. For low-grade dysplasia (CIN 1), observation with regular cytological screening is the standard approach, as many cases regress spontaneously. High-grade dysplasia (CIN 2 and 3) typically requires more aggressive treatment, with the gold standard being excisional procedures such as Loop Electrosurgical Excision Procedure (LEEP) or cold knife conization to prevent progression to invasive carcinoma.1 In cases of invasive cervical cancer, treatment options include a combination of surgery, radiotherapy, and chemotherapy, tailored to the stage and spread of the disease. Radical hysterectomy with pelvic lymphadenectomy is considered the gold standard for early-stage disease, while advanced stages often necessitate concurrent chemoradiation.1
Emerging technologies are transforming the treatment landscape for cervical cancer, particularly with the development of therapeutic HPV vaccines. Currently in early clinical trials, these vaccines could serve as an additional tool to address existing gaps in cervical cancer programs.115 Unlike the prophylactic HPV vaccines that prevent new infections, therapeutic vaccines are being designed to eliminate or manage existing HPV infections, HPV-related precancerous conditions, or invasive cervical cancer. At time of writing, there are over 20 therapeutic HPV vaccines in development, with several already undergoing clinical trials.115
Current prophylactic HPV vaccines, which are highly effective, target the L1 protein, a structural component of the virus’s outer shell that facilitates its attachment to basal epithelial cells during infection. In contrast, therapeutic vaccine candidates, designed to treat more advanced stages of HPV-related diseases like precancerous lesions and invasive cervical cancer, primarily focus on the E1 and E2 proteins, which are essential for viral replication and transcription, and the E6 and E7 proteins, which drive abnormal cell proliferation.116
Several completed studies have demonstrated that therapeutic HPV vaccines can induce regression of high-grade cervical intraepithelial neoplasia (CIN2/3) to lower-grade lesions (CIN1) or even result in the complete absence of precancerous cells.115,117,118 While the differences between vaccinated individuals and those experiencing natural regression are modest, they are significant. A meta-analysis of controlled trials revealed that the regression rate following vaccination was approximately 50% higher than in placebo groups, providing strong evidence that therapeutic vaccines can stimulate immune responses capable of causing high-grade precancer regression.118
Lopinavir/ritonavir, marketed as Lopimune, is another potential treatment that combines two protease inhibitors used in the management of HIV infection.119 Lopinavir functions by inhibiting the HIV protease enzyme, a critical component in viral maturation and replication. Ritonavir serves as a pharmacokinetic enhancer, inhibiting cytochrome P450 3A4 (CYP3A4) enzymes and thus increasing lopinavir’s plasma concentration and half-life.120 Although primarily indicated for HIV treatment, lopinavir/ritonavir has been evaluated in clinical trials for off-label use in other viral infections.121
A 2016 study evaluated the use of Lopimune as a treatment for HPV-related high-grade squamous intraepithelial lesions.122 At the 12-week post-treatment mark, cytological analysis showed that 14 of 22 women had no evidence of dysplasia, while 4 of 22 women displayed only low-grade lesions. Overall, 81.8% of the women showed a positive treatment response, with 77.8% confirmed by histological examination. These results were further supported by colposcopic imaging, which demonstrated the regression of cervical lesions, offering promising proof-of-concept data for the therapeutic application of Lopimune in HPV-related precancerous cervical disease.122
RNA interference (RNAi) using short hairpin RNA (shRNA) could represent another promising approach for cervical cancer treatment. Sato et al showed that cervical cancer cells transduced with an adeno-associated virus vector containing HPV-16 E6/E7-targeting shRNA demonstrated a reduction in E6 and E7 mRNA levels. This reduction could potentially increase p53, p21, and pRb expression, leading to apoptosis in dysplastic. Such outcomes suggest that RNAi might be a valuable strategy in targeting HPV-related cervical cancer.123
Conclusion
Efforts to prevent cervical cancer globally must account for the unique challenges in resource-limited settings, including limitations on monetary and physical resources, trained providers, and acceptability to patients.34
Enhanced HPV vaccines signify a pivotal stride towards preventing the spread of HPV, with the recent paradigm shift towards one or two-dose schedules offering the promise of broader accessibility.124 By requiring fewer doses, the vaccines become more adaptable to the realities of resource-limited settings, where traditional multi-dose regimens may pose barriers to widespread coverage.48,125 Since the WHO recommended a single-dose regimen in 2022, over 30 countries (including developed countries like Australia and the United Kingdom) have adopted this protocol.126
High-risk human papillomavirus (hrHPV) testing offers a cheaper, faster, and more accurate alternative to traditional cytology-based methods. In addition to hrHPV testing, the introduction of self-swabbing for sample collection offers a simplified and cost-effective triage approach that improves patient acceptability of screening.34
Genomic testing, including DNA methylation assays and E6/E7 oncoprotein testing, may become commonly used for refining risk stratification, exhibiting potential in detecting early markers of cancerous progression. The intersection of AI and cervical cancer screening holds significant promise, with deep learning-based automated visual evaluation showcasing the capacity for real-time, automated diagnosis. Implementing on-site cervical cancer screening in developing areas by local practitioners with limited training, coupled with instant diagnostic capabilities, establishes an efficient tiered system, reducing the reliance on highly skilled physicians. This approach not only streamlines the screening process but also operates as a self-refining system—increased data collection through more photos enhances diagnostic accuracy over time.112
A critical consideration in the future implications of cervical cancer research is the pursuit of global health equity. Efforts to streamline vaccine delivery, develop cost-effective screening methods, and leverage AI technologies for diagnosis should be aligned with a commitment to reducing health disparities and improving outcomes for all women, regardless of their geographical location.
Funding for these global initiatives comes from both private and public sources. In 2024, a collection of governments, international nonprofits, and donors pledged nearly $600 million to expand vaccine access and screening coverage globally.127 New funding includes $180 million from the Bill & Melinda Gates Foundation and $400 million from the World Bank, securing country commitments from Indonesia, Ethiopia, Democratic Republic of Congo, and Nigeria.128 Other initiatives, including PAVE, are funded through the National Cancer Institute as well as the Cancer Cures Moonshot Initiative.112 Continued research into diverse funding streams, such as public-private partnerships and institutional commitments, will be crucial for sustaining momentum in global health initiatives.
Addressing co-infection with HIV is imperative in the comprehensive approach to preventing cervical cancer, particularly in regions where HIV prevalence is high among young women and adolescents. Women living with HIV are not only at increased risk of Human Papillomavirus (HPV) infection but also face accelerated progression to cervical cancer and poorer treatment outcomes.
Integration of HIV screening and treatment services with cervical cancer prevention programs can ensure early detection and management of co-infections. Additionally, efforts to improve access to antiretroviral therapy (ART) and promote adherence among women living with HIV can help mitigate the immunosuppressive effects of the virus, thereby reducing the risk of HPV-related cervical lesions progressing to cancer.
Ultimately, a framework that adopts not one, but multiple triage and screening technologies is necessary to screen the more than 1.6 billion women worldwide who have never been screened for cervical cancer.129 There is nothing simple about this goal, however, the technologies outlined above provide a path toward this future.
Abbreviations
HPV, human papillomavirus; CD, cervical dysplasia; CIN, cervical intraepithelial neoplasia.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Organization GWH. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. Published online 2021. doi:Licence: CC BY-NC-SA 3.0 IGO.
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013;2(1):35. doi: 10.1186/2046-4053-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccarella S, Laversanne M, Ferlay J, Bray F. Cervical cancer in Africa, Latin America and the Caribbean and Asia: regional inequalities and changing trends. Int J Cancer. 2017;141:1997–2001. doi: 10.1002/ijc.30901 [DOI] [PubMed] [Google Scholar]
- 5.Petersen Z, Jaca A, Ginindza TG, et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: a systematic review. BMC Womens Health. 2022;22(1):486. doi: 10.1186/s12905-022-02043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervical Cancer Fact Sheet. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer. Accessed October 15, 2024.
- 7.Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep. 2015;12(1):6–15. doi: 10.1007/s11904-014-0254-4 [DOI] [PubMed] [Google Scholar]
- 8.Burk RD, Kelly P, Feldman J, et al. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex Transm Dis. 1996;23(4):333–341. doi: 10.1097/00007435-199607000-00013 [DOI] [PubMed] [Google Scholar]
- 9.Santé WHO= O mondiale de la. Weekly epidemiological record, 2022, vol. 97, 50 [full issue]. Wkly Epidemiol Rec Relevé Épidémiologique Hebd. 2022;97(50):645–672. [Google Scholar]
- 10.Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Silviade S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 12.Human Papillomavirus. Data on human papillomavirus reported by researchers at national cancer institute (human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions). Cancer Vaccine Week.Published online August 31, 2015:7. https://search.proquest.com/docview/1707631269. [Google Scholar]
- 13.Vaccarella S, Franceschi S, Snijders PJF, et al. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2010;19(2):503–510. doi: 10.1158/1055-9965.EPI-09-0983 [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Katki HA, Hildesheim A, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203(7):910–920. doi: 10.1093/infdis/jiq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson EL, Vogel RI, Bliss RL, Downs LS. Multiple-type human papillomavirus (HPV) infections: a cross-sectional analysis of the prevalence of specific types in 309,000 women referred for HPV testing at the time of cervical cytology. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2013;23(7):1295–1302. doi: 10.1097/IGC.0b013e31829e9fb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Park NJY, Jeong JY, Park JY. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13(7):1342. doi: 10.3390/v13071342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1796–1807. doi: 10.1086/428850 [DOI] [PubMed] [Google Scholar]
- 18.Roles of extended human papillomavirus genotyping and multiple infections in early detection of cervical precancer and cancer and HPV vaccination | BMC Cancer | Full Text. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-021-09126-3. Accessed September 3, 2024. [DOI] [PMC free article] [PubMed]
- 19.Li M, Du X, Lu M, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91(3):473–481. doi: 10.1002/jmv.25331 [DOI] [PubMed] [Google Scholar]
- 20.Pal A, Kundu R. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 2020;10:3116. doi: 10.3389/fmicb.2019.03116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017;13(4):e1006211. doi: 10.1371/journal.ppat.1006211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(S1):2–23. doi: 10.1002/rmv.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herfs M, Yamamoto Y, Laury A, et al. discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci. 2012;109(26):10516–10521. doi: 10.1073/pnas.1202684109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(1):S12–S17. doi: 10.1016/j.ygyno.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci. 2017;131(17):2201–2221. doi: 10.1042/CS20160786 [DOI] [PubMed] [Google Scholar]
- 26.McBride AA. The papillomavirus E2 proteins. Virology. 2013;445(1):57–79. doi: 10.1016/j.virol.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56(20):4620–4624. [PubMed] [Google Scholar]
- 28.Hu K, Wang W, Liu X, Meng Q, Zhang F. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat Oncol Lond Engl. 2018;13(1):249. doi: 10.1186/s13014-018-1197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9(2):e161–e169. doi: 10.1016/S2214-109X(20)30459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol. 2016;34(31):3749–3757. doi: 10.1200/JCO.2016.67.9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palefsky JM, Holly EA, Ralston ML, Costa MD, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)—positive and high-risk HIV-negative women. J Infect Dis. 2001;183(3):383–391. doi: 10.1086/318071 [DOI] [PubMed] [Google Scholar]
- 32.Mbuya W, Mcharo R, Mhizde J, et al. Depletion and activation of mucosal CD4 T cells in HIV infected women with HPV-associated lesions of the cervix uteri. PLoS One. 2020;15(10):e0240154. doi: 10.1371/journal.pone.0240154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45–e58. doi: 10.1016/S2352-3018(17)30149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bedell SL, Goldstein LS, Goldstein AR, Goldstein AT. Cervical cancer screening: past, present, and future. Sex Med Rev. 2020;8(1):28–37. doi: 10.1016/j.sxmr.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 35.Lam JUH, Rebolj M, Dugué PA, Bonde J, von Euler-Chelpin M, Lynge E. Condom use in prevention of human papillomavirus infections and cervical neoplasia: systematic review of longitudinal studies. J Med Screen. 2014;21(1):38–50. doi: 10.1177/0969141314522454 [DOI] [PubMed] [Google Scholar]
- 36.National cancer institute- HPV vaccination progress report. Available from: https://progressreport.cancer.gov/prevention/hpv_immunization. Accessed October 15, 2024.
- 37.Package insert - GARDASIL 9. Available from: https://www.fda.gov/media/90064/download. Accessed October 15, 2024.
- 38.Spinner C, Ding L, Bernstein DI, et al. Human papillomavirus vaccine effectiveness and herd protection in young women. Pediatrics. 2019;143(2):e20181902. doi: 10.1542/peds.2018-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markowitz LE, Naleway AL, Lewis RM, et al. Declines in HPV vaccine type prevalence in women screened for cervical cancer in the United States: evidence of direct and herd effects of vaccination. Vaccine. 2019;37(29):3918–3924. doi: 10.1016/j.vaccine.2019.04.099 [DOI] [PubMed] [Google Scholar]
- 40.Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4(1):e19–e27. doi: 10.1016/S2468-2667(18)30183-X [DOI] [PubMed] [Google Scholar]
- 41.Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus vaccination in the European Union/European economic area and globally: a moral dilemma. Eurosurveillance. 2021;26(50):2001659. doi: 10.2807/1560-7917.ES.2021.26.50.2001659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 43.American cancer society T. 2021 hPV VACs impact report. Canva. Available from: https://www.canva.com/design/DAE7n8PEHpE/t9vHRG34Ze501edgE4iwDw/view. Accessed April 6, 2024.
- 44.Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. Lancet Glob Health. 2022;10(10):e1473–e1484. doi: 10.1016/S2214-109X(22)00309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnabas RV, Brown ER, Onono MA, et al. Durability of single-dose HPV vaccination in young Kenyan women: randomized controlled trial 3-year results. Nat Med. 2023;29(12):3224–3232. doi: 10.1038/s41591-023-02658-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prem K, Choi YH, Bénard É, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modelling analysis. medRxiv. 2021:2021.02.08.21251186. doi: 10.1101/2021.02.08.21251186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burger EA, Campos NG, Sy S, Regan C, Kim JJ. Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country. Vaccine. 2018;36(32):4823–4829. doi: 10.1016/j.vaccine.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kepka D, Christini K, McGough E, et al. Successful multi-level HPV vaccination intervention at a rural healthcare center in the era of COVID-19. Front Digit Health. 2021;3:719138. doi: 10.3389/fdgth.2021.719138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeronimo J, Castle PE, Temin S, Shastri SS. Secondary prevention of cervical cancer: American society of clinical oncology resource-stratified clinical practice guideline summary. J Oncol Pract. 2017;13(2):129–133. doi: 10.1200/JOP.2016.017889 [DOI] [PubMed] [Google Scholar]
- 50.Safaeian M, Solomon D. Cervical cancer prevention - cervical screening: science in evolution. Obstet Gynecol Clin North Am. 2007;34(4):739–ix. doi: 10.1016/j.ogc.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyrgiou M, Arbyn M, Bergeron C, et al. Cervical screening: ESGO-EFC position paper of the European Society of Gynaecologic Oncology (ESGO) and the European Federation of Colposcopy (EFC). Br J Cancer. 2020;123(4):510–517. doi: 10.1038/s41416-020-0920-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendez MJ, Xue P, Qiao Y. Cervical cancer elimination in the era of COVID-19: the potential role of Artificial Intelligence (AI)-guided digital colposcope cloud platform. Eur J Gynaecol Oncol. 2022;43(1):160. doi: 10.31083/j.ejgo4301019 [DOI] [Google Scholar]
- 53.Liu AH, Gold MA, Schiffman M, et al. Comparison of colposcopic impression based on live colposcopy and evaluation of static digital images. J Low Genit Tract Dis. 2016;20(2):154–161. doi: 10.1097/LGT.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mink J, Peterson C. MobileODT: a case study of a novel approach to an mHealth-based model of sustainable impact. mHealth. 2016;2:12. doi: 10.21037/mhealth.2016.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evaluation of stationary colposcope and the gynocular, by the Swede score systematic colposcopic system in VIA positive women: a crossover randomized trial | International Journal of Gynecologic Cancer. Available from: https://ijgc.bmj.com/content/24/2/339.abstract. Accessed April 7, 2024. [DOI] [PMC free article] [PubMed]
- 56.Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320(7):687–705. doi: 10.1001/jama.2018.10400 [DOI] [PubMed] [Google Scholar]
- 57.Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106(8):dju153. doi: 10.1093/jnci/dju153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ajenifuja KO, Gage JC, Adepiti AC, et al. A population-based study of visual inspection with acetic acid (via) for cervical screening in rural Nigeria. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2013;23(3):507–512. doi: 10.1097/IGC.0b013e318280f395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fokom Domgue J, Valea FA. Is it relevant to keep advocating visual inspection of the cervix with acetic acid for primary cervical cancer screening in limited-resource settings? J Glob Oncol. 2018;(4):1–5. doi: 10.1200/JGO.17.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Updated cervical cancer screening guidelines. July 5, 2023. Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines. Accessed October 15, 2025.
- 61.Swid MA, Monaco SE. Should screening for cervical cancer go to primary human papillomavirus testing and eliminate cytology? Mod Pathol. 2022;35(7):858–864. doi: 10.1038/s41379-022-01052-4 [DOI] [PubMed] [Google Scholar]
- 62.Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321–346. doi: 10.3322/caac.21628 [DOI] [PubMed] [Google Scholar]
- 63.Simms KT, Keane A, Nguyen DTN, et al. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat Med. 2023;29(12):3050–3058. doi: 10.1038/s41591-023-02600-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein A, Goldstein LS, Lipson R, et al. Assessing the feasibility of a rapid, high-volume cervical cancer screening programme using HPV self-sampling and digital colposcopy in rural regions of Yunnan, China. BMJ Open. 2020;10(3):e035153. doi: 10.1136/bmjopen-2019-035153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desai KT, Adepiti CA, Schiffman M, et al. Redesign of a rapid, low‐cost HPV typing assay to support risk‐based cervical screening and management. Int J Cancer. 2022;151(7):1142–1149. doi: 10.1002/ijc.34151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inturrisi F, de Sanjosé S, Desai KT, et al. A rapid HPV typing assay to support global cervical cancer screening and risk‐based management: a cross‐sectional study. Int J Cancer. 2023;154(2):241–250. doi: 10.1002/ijc.34698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: a systematic review and meta-analysis - The Lancet Oncology. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(22)00294-7/fulltext. Accessed September 3, 2024. [DOI] [PubMed]
- 68.Defo VF, Domgue JF. Why consider self-sampling for cervical cancer screening in low- and middle-income countries? AMA J Ethics. 2020;22(2):116. doi: 10.1001/amajethics.2020.116 [DOI] [PubMed] [Google Scholar]
- 69.Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ Online. 2018;363:k4823. doi: 10.1136/bmj.k4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20(2):229–238. doi: 10.1016/S1470-2045(18)30763-0 [DOI] [PubMed] [Google Scholar]
- 71.Ogilvie GS, Patrick DM, Schulzer M, et al. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81(3):207–212. doi: 10.1136/sti.2004.011858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandt T, Wubneh SB, Handebo S, et al. Genital self-sampling for HPV-based cervical cancer screening: a qualitative study of preferences and barriers in rural Ethiopia. BMC Public Health. 2019;19:1026. doi: 10.1186/s12889-019-7354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women. Can Fam Physician. 2017;63(8):597–601. [PMC free article] [PubMed] [Google Scholar]
- 74.Arrossi S, Thouyaret L, Herrero R, et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015;3(2):e85–94. doi: 10.1016/S2214-109X(14)70354-7 [DOI] [PubMed] [Google Scholar]
- 75.Crosby RA, Hagensee ME, Vanderpool R, et al. Community-based screening for cervical cancer: a feasibility study of rural Appalachian women. Sex Transm Dis. 2015;42(11):607. doi: 10.1097/OLQ.0000000000000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldstein A, Plafker B, Stamper S, et al. Patient satisfaction with human papillomavirus self-sampling in a cohort of ethnically diverse and rural women in Yunnan Province, China. J Low Genit Tract Dis. 2020;24(4):349–352. doi: 10.1097/LGT.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malone C, Barnabas RV, Buist DSM, Tiro JA, Winer RL. Cost-effectiveness studies of HPV self-sampling: a systematic review. Prev Med. 2020;132:105953. doi: 10.1016/j.ypmed.2019.105953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marongiu L, Godi A, Parry JV, Beddows S. Human papillomavirus 16, 18, 31 and 45 viral load, integration and methylation status stratified by cervical disease stage. BMC Cancer. 2014;14(1):384. doi: 10.1186/1471-2407-14-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke MA, Gradissimo A, Schiffman M, et al. Human papillomavirus DNA methylation as a biomarker for cervical precancer: consistency across 12 genotypes and potential impact on management of HPV-positive women. Clin Cancer Res. 2018;24(9):2194–2202. doi: 10.1158/1078-0432.CCR-17-3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu H, Zhu H, Tian M, Wang D, He J, Xu T. DNA methylation and hydroxymethylation in cervical cancer: diagnosis, prognosis and treatment. Front Genet. 2020;11:347. doi: 10.3389/fgene.2020.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dovnik A, Poljak M. The role of methylation of host and/or human papillomavirus (HPV) DNA in management of cervical intraepithelial neoplasia grade 2 (CIN2) lesions. Int J Mol Sci. 2023;24(7):6479. doi: 10.3390/ijms24076479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gradissimo A, Clarke MA, Xue X, et al. A novel human papillomavirus and host DNA methylation score and detection of cervical adenocarcinoma. JNCI J Natl Cancer Inst. Published online July 19, 2023. doi: 10.1093/jnci/djad134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burdier FR, Waheed DN, Nedjai B, et al. DNA methylation as a triage tool for cervical cancer screening – a meeting report. Prev Med Rep. 2024;41:102678. doi: 10.1016/j.pmedr.2024.102678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng Q, Wang L, Zuo L, et al. HPV E6/E7: insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024;31(1):9–17. doi: 10.1038/s41417-023-00682-3 [DOI] [PubMed] [Google Scholar]
- 85.Rezhake R, Hu SY, Zhao S, et al. Eight-type human papillomavirus E6/E7 oncoprotein detection as a novel and promising triage strategy for managing HPV-positive women. Int J Cancer. 2019;144(1):34–42. doi: 10.1002/ijc.31633 [DOI] [PubMed] [Google Scholar]
- 86.Rosty C, Sheffer M, Tsafrir D, et al. Identification of a proliferation gene cluster associated with HPV E6/E7 expression level and viral DNA load in invasive cervical carcinoma. Oncogene. 2005;24(47):7094–7104. doi: 10.1038/sj.onc.1208854 [DOI] [PubMed] [Google Scholar]
- 87.Yao YL, Tian QF, Cheng B, Cheng YF, Ye J, Lu WG. Human papillomavirus (HPV) E6/E7 mRNA detection in cervical exfoliated cells: a potential triage for HPV-positive women. J Zhejiang Univ Sci B. 2017;18(3):256–262. doi: 10.1631/jzus.B1600288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Downham L, Jaafar I, Rol ML, et al. Accuracy of HPV E6/E7 oncoprotein tests to detect high-grade cervical lesions: a systematic literature review and meta-analysis. Br J Cancer. 2024;130(4):517–525. doi: 10.1038/s41416-023-02490-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol. 2011;121(3):505–509. doi: 10.1016/j.ygyno.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 90.Wentzensen N, Clarke MA, Bremer R, et al. Clinical evaluation of human papillomavirus screening with p16/Ki-67 dual stain triage in a large organized cervical cancer screening program. Arch Intern Med. 2019;179(7):881–888. doi: 10.1001/jamainternmed.2019.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Chen C, Liu L, et al. Evaluation of p16/Ki-67 dual-stain as triage test for high-risk HPV-positive women: a hospital-based cross-sectional study. Cancer Cytopathol. 2022;130(12):955–963. doi: 10.1002/cncy.22628 [DOI] [PubMed] [Google Scholar]
- 92.Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-positive women. JNCI J Natl Cancer Inst. 2015;107(12):djv257. doi: 10.1093/jnci/djv257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarke MA, Cheung LC, Castle PE, et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 2019; 5(2):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clarke MA, Wentzensen N, Perkins RB, et al. Recommendations for use of p16/Ki67 dual stain for management of individuals testing positive for human papillomavirus. J Low Genit Tract Dis. 2024;28(2):124–130. doi: 10.1097/LGT.0000000000000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang P, Li X, Shen H, et al. A systematic review of deep learning-based cervical cytology screening: from cell identification to whole slide image analysis. Artif Intell Rev. 2023;56(2):2687–2758. doi: 10.1007/s10462-023-10588-z [DOI] [Google Scholar]
- 96.Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning–based automation of dual stain cytology in cervical cancer screening. JNCI J Natl Cancer Inst. 2021;113(1):72–79. doi: 10.1093/jnci/djaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngugi CW, Schmidt D, Wanyoro K, et al. p16INK4a/Ki-67 dual stain cytology for cervical cancer screening in Thika district, Kenya. Infect Agent Cancer. 2015;10(1). doi: 10.1186/s13027-015-0020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orang’o EO, Were E, Rode O, et al. Novel concepts in cervical cancer screening: a comparison of VIA, HPV DNA test and p16INK4a/Ki-67 dual stain cytology in Western Kenya. Infect Agent Cancer. 2020;15(1):1–57. doi: 10.1186/s13027-020-00323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Y, Hong Z, Gu L, et al. Evaluation of p16/Ki-67 dual-stained cytology in triaging HPV-positive women during cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2020;29(6):1246–1252. doi: 10.1158/1055-9965.EPI-19-1180 [DOI] [PubMed] [Google Scholar]
- 100.Han Q, Guo H, Geng L, Wang Y. p16/Ki-67 dual-stained cytology used for triage in cervical cancer opportunistic screening. Chin J Cancer Res. 2020;32(2):208–217. doi: 10.21147/j.issn.1000-9604.2020.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tantitamit T, Khemapech N, Havanond P, Termrungruanglert W. Cost-effectiveness of primary HPV screening strategies and triage with cytology or dual stain for cervical cancer. Cancer Control. 2020;27(1):1073274820922540. doi: 10.1177/1073274820922540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wormald BW, Moser N, deSouza NM, et al. Lab-on-chip assay of tumour markers and human papilloma virus for cervical cancer detection at the point-of-care. Sci Rep. 2022;12(1):8750. doi: 10.1038/s41598-022-12557-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu H, Liu S, Wang H, et al. Genomic amplification of the human telomerase gene (hTERC) associated with human papillomavirus is related to the progression of uterine cervical dysplasia to invasive cancer. Diagn Pathol. 2012;7:147. doi: 10.1186/1746-1596-7-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang K, Wang RL, Liu JJ, et al. The prognostic significance of hTERT overexpression in cancers. Medicine. 2018;97(35):e11794. doi: 10.1097/MD.0000000000011794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Artificial intelligence enables precision diagnosis of cervical cytology grades and cervical cancer | nature communications. Available from: https://www.nature.com/articles/s41467-024-48705-3. Accessed September 3, 2024. [DOI] [PMC free article] [PubMed]
- 106.Validation of AI-assisted ThinPrep® Pap test screening using the GeniusTM digital diagnostics system - ScienceDirect. Available from: https://www.sciencedirect.com/science/article/pii/S2153353924000300#s0060. Accessed September 3, 2024. [DOI] [PMC free article] [PubMed]
- 107.Desai KT, Befano B, Xue Z, et al. The development of “automated visual evaluation” for cervical cancer screening: the promise and challenges in adapting deep-learning for clinical testing: interdisciplinary principles of automated visual evaluation in cervical screening. Int J Cancer. 2022;150(5):741–752. doi: 10.1002/ijc.33879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu L, Mwanahamuntu MH, Sahasrabuddhe VV, et al. Validation of automated visual evaluation (AVE) on smartphone images for cervical cancer screening in a prospective study in Zambia. MedRxiv Prepr Serv Health Sci. Published online July 23, 2023. https://www.ncbi.nlm.nih.gov/pubmed/37560093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soenksen LR, Kassis T, Conover ST, et al. Using deep learning for dermatologist-level detection of suspicious pigmented skin lesions from wide-field images. Sci Transl Med. 2021;13(581):eabb3652. doi: 10.1126/scitranslmed.abb3652 [DOI] [PubMed] [Google Scholar]
- 110.Xue Z, Angara S, Guo P, et al. Image quality classification for automated visual evaluation of cervical precancer. In: Vol 13559. Medical Image Learning with Limited and Noisy Data. Springer Nature Switzerland; 2022:206–217. doi: 10.1007/978-3-031-16760-7_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed SR, Befano B, Egemen D, et al. Generalizable deep neural networks for image quality classification of cervical images. Published online 2024.
- 112.de Sanjosé S, Perkins RB, Campos NG, et al. Design of the HPV-Automated Visual Evaluation (PAVE) Study: Validating a Novel Cervical Screening Strategy. Cold Spring Harbor Laboratory; 2023. doi: 10.1101/2023.08.30.23294826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahmed, SR, Egemen, D, Befano, B et al. Assessing generalizability of an AI-based visual test for cervical cancer screening. PLOS Digit Health. 2024;3(10) e0000364. doi: 10.1371/journal.pdig.0000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Egemen D, Perkins RB, Cheung LC, et al. Artificial intelligence-based image analysis in clinical testing: lessons from cervical cancer screening. JNCI J Natl Cancer Inst. 2024;116(1):26–33. doi: 10.1093/jnci/djad202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.WHO preferred product characteristics for therapeutic HPV vaccines. Available from: https://www.who.int/publications/i/item/9789240092174. Accessed September 11, 2024.
- 116.Ashique S, Hussain A, Fatima N, Altamimi MA. HPV pathogenesis, various types of vaccines, safety concern, prophylactic and therapeutic applications to control cervical cancer, and future perspective. VirusDisease. 2023;34(2):172–190. doi: 10.1007/s13337-023-00824-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mo Y, Ma J, Zhang H, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Front Cell Infect Microbiol. 2022;12:909223. doi: 10.3389/fcimb.2022.909223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ibrahim Khalil A, Zhang L, Muwonge R, Sauvaget C, Basu P. Efficacy and safety of therapeutic HPV vaccines to treat CIN 2/CIN 3 lesions: a systematic review and meta-analysis of Phase II/III clinical trials. BMJ Open. 2023;13(10):e069616. doi: 10.1136/bmjopen-2022-069616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004 [DOI] [PubMed] [Google Scholar]
- 120.Loos NHC, Beijnen JH, Schinkel AH. The mechanism-based inactivation of CYP3A4 by Ritonavir: what mechanism? Int J Mol Sci. 2022;23(17):9866. doi: 10.3390/ijms23179866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang Y, Huang X, Luo Y, et al. Assessing the efficacy of lopinavir/ritonavir-based preferred and alternative second-line regimens in HIV-infected patients: a meta-analysis of key evidence to support WHO recommendations. Front Pharmacol. 2018:9. doi: 10.3389/fphar.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hampson L, Maranga IO, Masinde MS, et al. A single-arm, proof-of-concept trial of lopimune (lopinavir/ritonavir) as a treatment for HPV-related pre-invasive cervical disease. PLoS One. 2016;11(1):e0147917. doi: 10.1371/journal.pone.0147917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sato N, Saga Y, Uchibori R, et al. Eradication of cervical cancer in vivo by an AAV vector that encodes shRNA targeting human papillomavirus type 16 E6/E7. Int J Oncol. 2018;52(3):687–696. doi: 10.3892/ijo.2018.4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.WHO updates recommendations on HPV vaccination schedule. PharmaBiz. Published online December 22, 2022.
- 125.Head KJ, Vanderpool RC, Mills LA. Health care providers’ perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs Boston Mass. 2013;30(4):351–360. doi: 10.1111/phn.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.World Health Organization Interactive HPV Map. Available from: https://app.powerbi.com/view?r=eyJrIjoiNDIxZTFkZGUtMDQ1Ny00MDZkLThiZDktYWFlYTdkOGU2NDcwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9. Accessed October 15, 2024.
- 127.Wave of New Commitments Marks Historic Step Towards the Elimination of Cervical Cancer. Bill & Melinda gates foundation. Available from: https://www.gatesfoundation.org/ideas/media-center/press-releases/2024/03/cervical-cancer-global-forum. Accessed April 11, 2024.
- 128.Global Cervical Cancer Elimination Forum Commitments. Available from: https://www.who.int/initiatives/cervical-cancer-elimination-initiative/cervical-cancer-forum/commitments. Accessed April 11, 2024. [DOI] [PMC free article] [PubMed]
- 129.Bruni L, Serrano B, Roura E, et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob Health. 2022;10(8):e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]