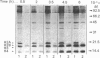
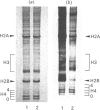
Abstract
ADP-ribosylation of core histones was investigated in isolated nuclei of Physarum polycephalum. Core histone species differed in the mode of modification. Whereas ADP-ribosylation of H2A and H2B is sensitive to inhibition by 3-methoxybenzamide, as with most other nuclear acceptor proteins, the modification of H3 and H4 is not inhibited. Cleavage experiments with hydroxylamine indicate a carboxylate ester type ADP-ribose-protein bond for H2A and H2B and arginine-linked ADP-ribose residues for H3 and H4. ADP-ribosylation preferentially occurs on acetylated histone subspecies, as shown for H4. These data are substantiated by the use of n-butyrate, which induces hyperacetylation of core histones; the butyrate-induced shift towards more acetylated H4 subspecies is accompanied by an increase of ADP-ribose incorporation into highly acetylated H4 subspecies.
Full text
PDF
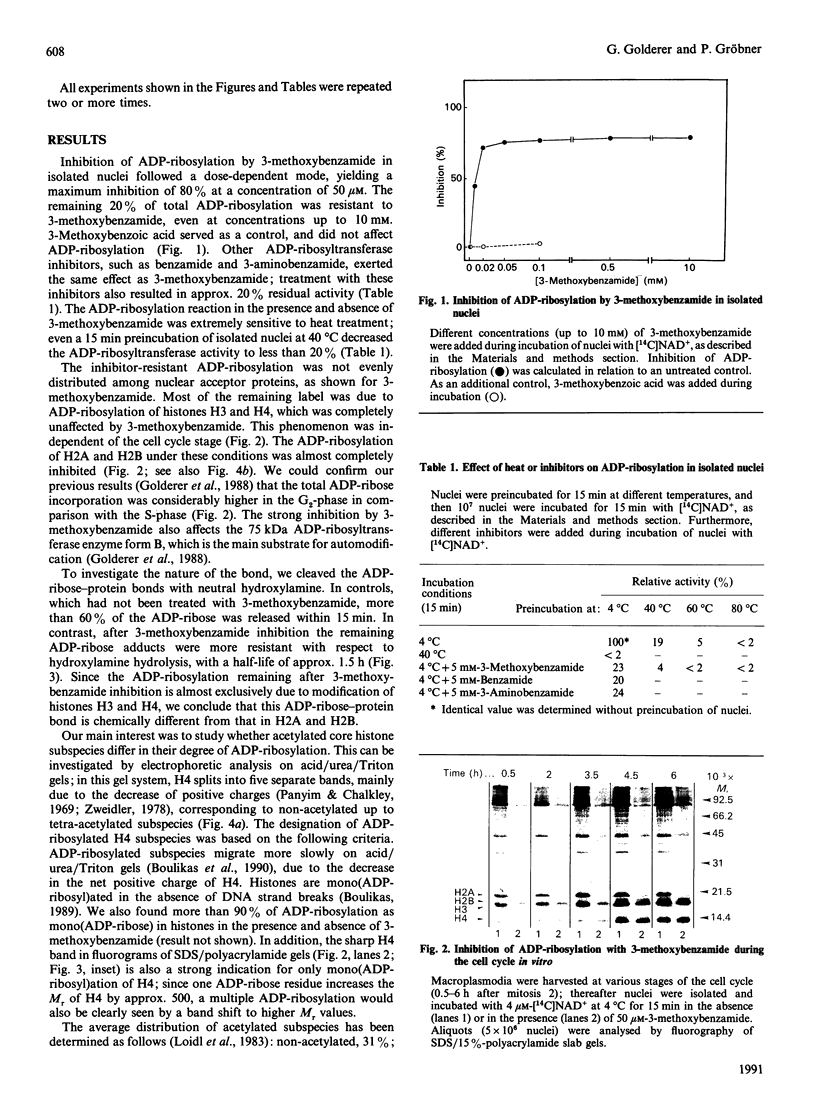
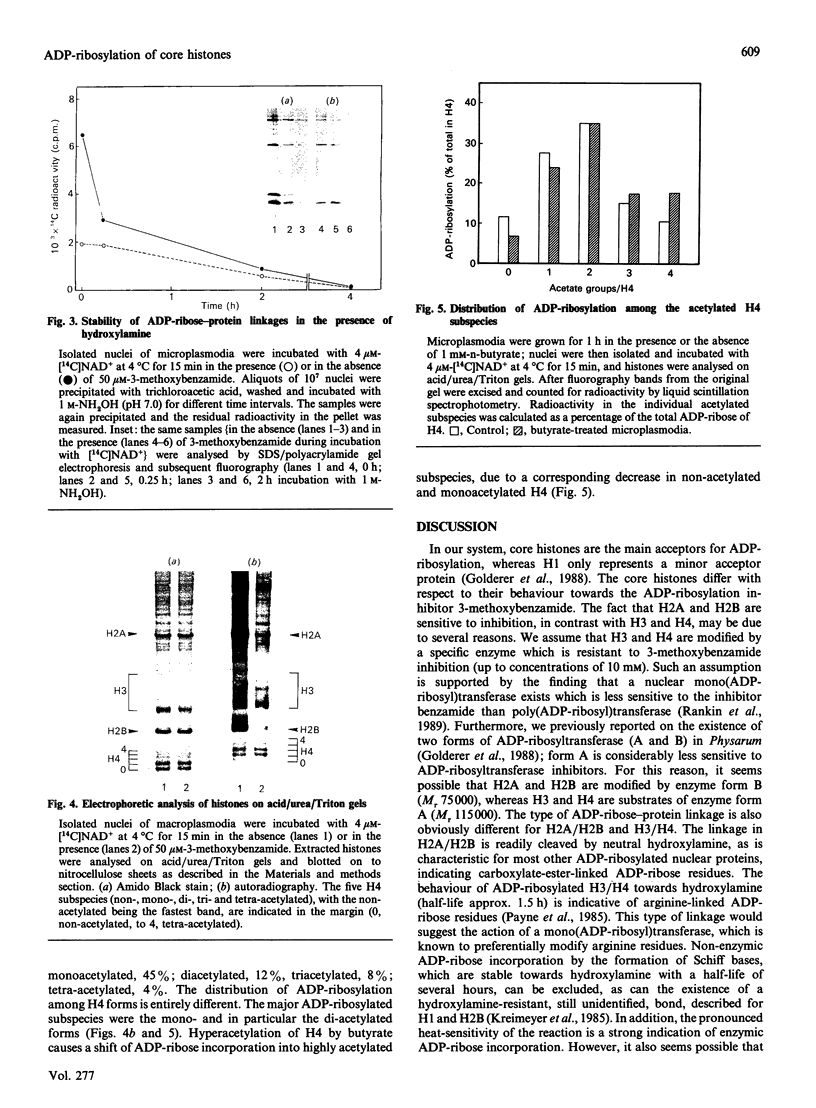

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulikas T. At least 60 ADP-ribosylated variant histones are present in nuclei from dimethylsulfate-treated and untreated cells. EMBO J. 1988 Jan;7(1):57–67. doi: 10.1002/j.1460-2075.1988.tb02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T., Bastin B., Boulikas P., Dupuis G. Increase in histone poly (ADP-ribosylation) in mitogen-activated lymphoid cells. Exp Cell Res. 1990 Mar;187(1):77–84. doi: 10.1016/0014-4827(90)90119-u. [DOI] [PubMed] [Google Scholar]
- Boulikas T. DNA strand breaks alter histone ADP-ribosylation. Proc Natl Acad Sci U S A. 1989 May;86(10):3499–3503. doi: 10.1073/pnas.86.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golderer G., Schneider R., Auer B., Loidl P., Gröbner P. ADP-ribosylation in isolated nuclei of Physarum polycephalum. Biochem J. 1988 Aug 1;253(3):859–867. doi: 10.1042/bj2530859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröbner P., Loidl P. ADP-ribosyltransferase in isolated nuclei during the cell cycle of Physarum polycephalum. Biochem J. 1985 Nov 15;232(1):21–24. doi: 10.1042/bj2320021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M. J., Davie J. R. Distribution of methylated histones and histone methyltransferases in chicken erythrocyte chromatin. J Biol Chem. 1989 Nov 15;264(32):19208–19214. [PubMed] [Google Scholar]
- Kreimeyer A., Adamietz P., Hilz H. Alkylation-induced mono(ADP-ribosyl)-histones H1 and H2B. Hydroxylamine-resistant linkage in hepatoma cells. Biol Chem Hoppe Seyler. 1985 Jun;366(6):537–544. doi: 10.1515/bchm3.1985.366.1.537. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Loidl P., Gröbner P. Biosynthesis and posttranslational acetylation of histones during spherulation of Physarum polycephalum. Nucleic Acids Res. 1986 May 12;14(9):3745–3762. doi: 10.1093/nar/14.9.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Gröbner P. Histone synthesis during the cell cycle of Physarum polycephalum. Synthesis of different histone species is not under a common regulatory control. J Biol Chem. 1987 Jul 25;262(21):10195–10199. [PubMed] [Google Scholar]
- Loidl P., Gröbner P. Postsynthetic acetylation of histones during the cell cycle: a general function for the displacement of histones during chromatin rearrangements. Nucleic Acids Res. 1987 Oct 26;15(20):8351–8366. doi: 10.1093/nar/15.20.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Loidl A., Puschendorf B., Gröbner P. Lack of correlation between histone H4 acetylation and transcription during the Physarum cell cycle. 1983 Sep 29-Oct 5Nature. 305(5933):446–448. doi: 10.1038/305446a0. [DOI] [PubMed] [Google Scholar]
- Loidl P. Towards an understanding of the biological function of histone acetylation. FEBS Lett. 1988 Jan 25;227(2):91–95. doi: 10.1016/0014-5793(88)80874-3. [DOI] [PubMed] [Google Scholar]
- Malik N., Smulson M. A relationship between nuclear poly(adenosine diphosphate ribosylation) and acetylation posttranslational modifications. 1. Nucleosome studies. Biochemistry. 1984 Jul 31;23(16):3721–3725. doi: 10.1021/bi00311a023. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation of the nuclear histones from the Myxomycete, Physarum polycephalum. Arch Biochem Biophys. 1969 Nov;134(2):577–589. doi: 10.1016/0003-9861(69)90320-8. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Jacobson E. L., Moss J., Jacobson M. K. Modification of proteins by mono(ADP-ribosylation) in vivo. Biochemistry. 1985 Dec 17;24(26):7540–7549. doi: 10.1021/bi00347a006. [DOI] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Smolarz E., Gröbner P., Loidl P. Periodic fluctuations of nuclear high mobility group like proteins during the cell cycle of Physarum polycephalum. Biochemistry. 1988 May 31;27(11):4142–4147. doi: 10.1021/bi00411a036. [DOI] [PubMed] [Google Scholar]
- Sterner R., Allfrey V. G. Selective isolation of polypeptide chains bearing multiple types of postsynthetic modifications. Recovery of simultaneously acetylated and phosphorylated forms of histone H2A and high mobility group proteins 14 and 17. J Biol Chem. 1983 Oct 25;258(20):12135–12138. [PubMed] [Google Scholar]
- Tanigawa Y., Tsuchiya M., Imai Y., Shimoyama M. ADP-ribosylation regulates the phosphorylation of histones by the catalytic subunit of cyclic AMP-dependent protein kinase. FEBS Lett. 1983 Aug 22;160(1-2):217–220. doi: 10.1016/0014-5793(83)80970-3. [DOI] [PubMed] [Google Scholar]
- Wong M., Smulson M. A relationship between nuclear poly(adenosine diphosphate ribosylation) and acetylation posttranslational modifications. 2. Histone studies. Biochemistry. 1984 Jul 31;23(16):3726–3730. doi: 10.1021/bi00311a024. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]