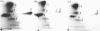Abstract
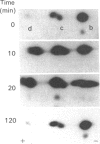
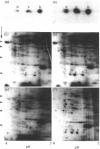
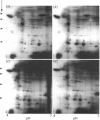
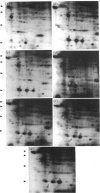
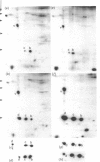
Interleukin 1 (IL1) increased phosphorylation of the small heat-shock protein (hsp 27) in MRC5 fibroblasts. The increase was maintained for at least 30 min, but levels had returned to pre-stimulation values by 2 h. When hsp 27 was metabolically labelled with [3H]leucine, about 15% was phosphorylated in resting confluent cells; this rose to 90% upon stimulation by IL1. Peptide maps of the three differently charged phosphorylated forms were consistent with their arising by phosphorylation of increasing numbers of serine residues. IL1 had the same effect on hsp 27 in pig articular chondrocytes, endothelial cells from human umbilical vein and an epidermoid carcinoma cell line (KB). Certain other agents were found selectively to increase phosphorylation of hsp 27 in MRC5 cells besides IL1 [and tumour necrosis factor (TNF)]. Platelet-derived growth factor had a similar effect to that of IL1; bradykinin, acid fibroblast growth factor and ATP caused an intermediate effect; phorbol myristate acetate (PMA) and 1-oleoyl-2-acetylglycerol had smaller effects. Dibutyryl cyclic AMP and forskolin had no effects on hsp 27 phosphorylation. When cells had been depleted of protein kinase C (PKC) by prolonged treatment with PMA, stimulation by IL1, TNF or bradykinin still increased hsp 27 phosphorylation. The stimulation by all three agents was also unaffected by the PKC inhibitor staurosporine. IL1, TNF and bradykinin each caused hsp 27 phosphorylation by a pathway independent of PKC. The results are consistent with IL1 activating a serine kinase which remains to be identified.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P. Tumor necrosis factor induces the rapid phosphorylation of the mammalian heat shock protein hsp28. Mol Cell Biol. 1990 Mar;10(3):1276–1280. doi: 10.1128/mcb.10.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. Down-modulation of epidermal growth factor receptor affinity in fibroblasts treated with interleukin 1 or tumor necrosis factor is associated with phosphorylation at a site other than threonine 654. J Biol Chem. 1990 Jan 5;265(1):235–240. [PubMed] [Google Scholar]
- Bird T. A., Saklatvala J. IL-1 and TNF transmodulate epidermal growth factor receptors by a protein kinase C-independent mechanism. J Immunol. 1989 Jan 1;142(1):126–133. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper S. W., Rocheleau T. A., Storm F. K. cDNA sequence of a human heat shock protein HSP27. Nucleic Acids Res. 1990 Nov 11;18(21):6457–6457. doi: 10.1093/nar/18.21.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. J. A study of the effects of catabolin on cyclic adenosine monophosphate biosynthesis and prostaglandin E2 secretion in pig articular chondrocytes. Br J Rheumatol. 1986 Nov;25(4):359–365. doi: 10.1093/rheumatology/25.4.359. [DOI] [PubMed] [Google Scholar]
- Chedid M., Shirakawa F., Naylor P., Mizel S. B. Signal transduction pathway for IL-1. Involvement of a pertussis toxin-sensitive GTP-binding protein in the activation of adenylate cyclase. J Immunol. 1989 Jun 15;142(12):4301–4306. [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters mediate phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Jul 10;259(13):8545–8549. [PubMed] [Google Scholar]
- Demolle D., Lecomte M., Boeynaems J. M. Pattern of protein phosphorylation in aortic endothelial cells. Modulation by adenine nucleotides and bradykinin. J Biol Chem. 1988 Dec 5;263(34):18459–18465. [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Didier M., Aussel C., Pelassy C., Fehlmann M. IL-1 signaling for IL-2 production in T cells involves a rise in phosphatidylserine synthesis. J Immunol. 1988 Nov 1;141(9):3078–3080. [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Goerig M., Grulich J., Rothe D., Gronwald R., Loth U., Schettler G., Kommerell B., Ross R. Human platelet-derived growth factor stimulates prostaglandin synthesis by activation and by rapid de novo synthesis of cyclooxygenase. J Clin Invest. 1985 Apr;75(4):1381–1387. doi: 10.1172/JCI111839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hepburn A., Demolle D., Boeynaems J. m., Fiers W., Dumont J. E. Rapid phosphorylation of a 27 kDa protein induced by tumor necrosis factor. FEBS Lett. 1988 Jan 25;227(2):175–178. doi: 10.1016/0014-5793(88)80892-5. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Kaur P., Saklatvala J. Interleukin 1 and tumour necrosis factor increase phosphorylation of fibroblast proteins. FEBS Lett. 1988 Dec 5;241(1-2):6–10. doi: 10.1016/0014-5793(88)81019-6. [DOI] [PubMed] [Google Scholar]
- Kaur P., Welch W. J., Saklatvala J. Interleukin 1 and tumour necrosis factor increase phosphorylation of the small heat shock protein. Effects in fibroblasts, Hep G2 and U937 cells. FEBS Lett. 1989 Dec 4;258(2):269–273. doi: 10.1016/0014-5793(89)81671-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. H., Bienkowski M. J., Gorman R. R. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989 Oct 15;264(29):17379–17383. [PubMed] [Google Scholar]
- Meichle A., Schütze S., Hensel G., Brunsing D., Krönke M. Protein kinase C-independent activation of nuclear factor kappa B by tumor necrosis factor. J Biol Chem. 1990 May 15;265(14):8339–8343. [PubMed] [Google Scholar]
- Mizel S. B. How does interleukin 1 activate cells? Cyclic AMP and interleukin 1 signal transduction. Immunol Today. 1990 Nov;11(11):390–391. doi: 10.1016/0167-5699(90)90154-2. [DOI] [PubMed] [Google Scholar]
- O'Neill L. A., Bird T. A., Saklatvala J. How does interleukin 1 activate cells? Interleukin 1 signal transduction. Immunol Today. 1990 Nov;11(11):392–394. doi: 10.1016/0167-5699(90)90155-3. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Meier K. E., Stanton T. H., Smith L. L., Bomsztyk K. Interferon-gamma and interleukin-1 alpha induce transient translocation of protein kinase C activity to membranes in a B lymphoid cell line. Evidence for a protein kinase C-independent pathway in lymphokine-induced cytoplasmic alkalinization. J Biol Chem. 1988 Sep 25;263(27):13786–13790. [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Robaye B., Hepburn A., Lecocq R., Fiers W., Boeynaems J. M., Dumont J. E. Tumor necrosis factor-alpha induces the phosphorylation of 28kDa stress proteins in endothelial cells: possible role in protection against cytotoxicity? Biochem Biophys Res Commun. 1989 Aug 30;163(1):301–308. doi: 10.1016/0006-291x(89)92135-9. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-dalton mammalian stress proteins. J Biol Chem. 1985 Mar 10;260(5):3058–3062. [PubMed] [Google Scholar]