Abstract
This work aims to investigate the utility of positron emission tomography/computed tomography (PET/CT) with fibroblast activation protein inhibitors (FAPI) in urological neoplasms, including prostate cancer, urothelial carcinoma, and renal cell carcinoma. Although the available data are very preliminary, FAPI PET showed potential for detecting primary prostate cancer with low prostate-specific membrane antigen expression, while prostate-specific membrane antigen PET/CT outperformed FAPI PET/CT in detecting biochemical recurrence. In urothelial carcinoma, FAPI PET/CT demonstrated increased detection rates compared with deoxy-2-[18F]fluoro-d-glucose PET/CT, in particular in small lymph node metastases, whose identification is still an unmet clinical need. Limited data are available for renal cell carcinoma. In conclusion, FAPI PET emerges as a promising imaging modality for urological neoplasms, in particular bladder cancer. Further research is warranted to establish its role in guiding therapeutic decisions and as a potential novel theranostic agent.
Key Points
| Although the evidence from the literature is very preliminary, fibroblast activation protein inhibitor (FAPI) positron emission tomography (PET) looks promising in urological cancers. FAPI deserves further investigation as a theranostic agent (labeled with 68Ga for imaging and 177Lu for radioligand therapy) in patients affected by urological cancers. |
| In bladder cancer, FAPI PET seems to enable the detection of metastatic lesions unrevealed at contrast-enhanced computed tomography and deoxy-2-[18F]fluoro-D-glucose PET, in particular small lymph nodes that are frequently missed at primary staging. |
| Less evidence in the literature is available in prostate cancer, with a conceivable synergic role to prostate-specific membrane antigen PET in the detection of primary tumors, and in renal cell carcinoma. |
Introduction
The tumor microenvironment has emerged as a significant factor in cancer research, playing a crucial role in immune evasion and cancer progression [1]. The tumor microenvironment is a dynamic field and includes various cellular and non-cellular elements. Notably, cancer-associated fibroblasts represent a predominant component in many neoplasms. Cancer-associated fibroblasts are activated in response to inflammation and tissue damage and foster tumor growth alongside cancer cells [2]. Of particular interest, fibroblast activation protein (FAP), a transmembrane serine protease typically expressed at low levels or undetectable in healthy tissues, is overexpressed by cancer-associated fibroblasts and, consequently, significantly upregulated in most cancers (i.e., colorectal, pancreatic, breast, and head and neck cancers and high-grade gliomas), presenting a potential target for theranostic approaches [2, 3]. In this paper, we provide an overview of the current literature data regarding positron emission tomography (PET) imaging with radiolabeled FAP inhibitors (FAPI) in urological malignancies.
[68Ga]Ga-FAPI PET in Prostate Cancer
Although radiolabeled prostate-specific membrane antigen (PSMA) PET is a well-established imaging method in prostate cancer (PCa), it is impaired by limitations both in terms of sensitivity—for some histotypes (i.e., neuroendocrine type) and in some settings of disease—and of specificity, i.e., in PSMA-avid non-prostate cancers [4]. Considering these limitations, a few researchers conducted preliminary explorations of the potential of FAPI PET in PCa [5, 6] (Table 1).
Table 1.
Original articles investigating the potential of FAPI PET in genitourinary tract neoplasms
| Disease | Author, ref. | Year | RP | Median RP dose (MBq) | Time between agent injection and acquisition (min) | PET acquisition time (min/bed) | No. of pts | Comparative imaging | Results |
|---|---|---|---|---|---|---|---|---|---|
| PCa | Ergül et al. [5] | 2024 | [68Ga]-FAP I-04 | NA | 60 | NA | 36 | [68Ga]-PSMA |
Staging group: in a mixed cohort of PSMA-positive and PSMA-negative tumors, FAPI PET detected more primary PCa than PSMA PET Biochemical recurrence group: PSMA showed uptakes in more patients than FAPI PET |
| RCC | Civan et al. [15] | 2023 | [68Ga]-FAPI-04 | 125 | 15 | 3–4 | 20 | [18F]FDG |
FAPI-TBR > FDG-TBR (5.6 vs 2.1 p = 0.001) |
| UC | Novruzov et al. [13] | 2022 |
[68Ga]-FAPI-04 [68Ga]-FAPI-46 |
148 | 60 | 3–5 | 8 | [18F]FDG | FAPI PET: 30% increased detection rate than FDG PET |
| Unterrainer et al. [12] | 2022 | [68Ga]-FAPI-46 | 210 ± 31 | 60 | 2.5 | 15 | ceCT | 26.7% of patients showed FAPI-positive secondary lesions missed on ceCT | |
| Koshkin et al. [14] | 2023 | [68Ga]-FAP-2286 | 219.04 ± 51.8 | 106 ± 26 | NA | 21 | Conventional imaging and [18F]FDG |
77.8% and 38.9% of FAPI-positive lesions, respectively, in the localized and metastatic cohorts were small lymph node and visceral micro-metastases (< 1 cm) opportunity to change clinical management in patients in which FAPI PET is not concordant with conventional imaging |
[18F]FDG deoxy-2-[18F]fluoro-d-glucose, ceCT contrast-enhanced CT, CT computed tomography, FAPI fibroblast activation protein inhibitors, min minutes, NA not available, PCa prostate cancer, PET positron emission tomography, PSMA prostate-specific membrane antigen, pts patients, RCC renal cell carcinoma, ref reference, RP radiopharmaceutical, TBR target to background ratio, UC urothelial carcinoma
In the only original article [5] currently available, 36 patients with PCa were imaged with both [68Ga]Ga-FAPI and [68Ga]Ga-PSMA PET/computed tomography (CT) within 1 week. The patients were divided into two groups: a staging group (n = 27) and a biochemical recurrence group (n = 9). Considering primary tumor detection within the staging group, an equal number of PSMA-positive and PSMA-negative patients were selected for evaluating the potential relevance of [68Ga]Ga-FAPI PET/CT. Overall, [68Ga]Ga-FAPI detected more primary prostate lesions than [68Ga]Ga-PSMA PET/CT [20 (74%) vs 14 (52%), respectively]. Of note, seven (54%) PSMA-negative PCa were FAPI positive with a higher uptake according to the International Society of Urological Pathology grade (mean maximum standardized uptake value [SUVmax]: 4.9 ± 3.2 vs 7.2 ± 3.8 for International Society of Urological Pathology grades 1–3 and 4–5, respectively; p = 0.03), while among PSMA-positive tumors, only one (7%) case showed no FAPI uptake. Moreover, FAPI uptake was most intense in patients with a SUVmax < 6.4 at [68Ga]Ga-PSMA PET/CT. Conversely, in patients investigated for the biochemical recurrence of PCa, [68Ga]Ga-PSMA PET/CT outperformed [68Ga]Ga-FAPI PET/CT, showing more sites of uptake in more patients and with a higher uptake intensity. In particular, [68Ga]Ga-FAPI PET/CT detected a local recurrence in two patients and multiple lymph node metastases in another patient with negative [68Ga]Ga-FAPI PET/CT. Considering lymph node and bone metastases, [68Ga]Ga-PSMA PET/CT showed a higher median SUVmax than [68Ga]Ga-FAPI PET/CT [8.5 (7.8–45.3) vs 4.5 (3.6–12.2), respectively].
All further evidence from the literature regarding FAPI PET in PCa is limited to case reports or case series. In the study by Kesch et al. [6], three patients (two patients with metastatic CRPC and one patient with neuroendocrine dedifferentiated PCa) underwent imaging with [68Ga]Ga-FAPI-04 PET/CT. All patients showed multiple lymph node, bone, and visceral metastases with an intense [68Ga]Ga-FAPI-04 uptake, subsequently confirmed by contrast-enhanced CT. According to the microarray analysis performed in the same study (in 94 patients with PCa with different stages of disease), FAP expression is detectable in all the different phases of PCa, although it increases as the natural history of PCa progresses.
In patients with advanced metastatic castration-resistant PCa, the disease is widely heterogeneous and the complementary value of deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) PET/CT and [68Ga]Ga-PSMA PET/CT is emerging in the literature [4]. Indeed, the evidence of mismatched FDG+/PSMA−lesions represents a strong negative prognostic factor in these patients, precluding an effective PSMA radioligand therapy (RLT) [7]. The additional relevance of [68Ga]Ga-FAPI PET/CT in metastatic castration-resistant PCa has been recently investigated in a few cases [8], with patients imaged with [68Ga]Ga-FAPI PET/CT in addition to [68Ga]Ga-PSMA and [18F]FDG PET/CT to evaluate the potential eligibility to [177Lu]Lu-PSMA RLT. In these patients, the evidence of a higher uptake intensity at [68Ga]Ga-FAPI PET/CT in comparison to [68Ga]Ga-PSMA could potentially candidate a patient to [177Lu]Lu-FAPI RLT, which is a treatment currently investigated in experimental clinical trials [9, 10].
[68Ga]Ga-FAPI PET in Urothelial Carcinoma
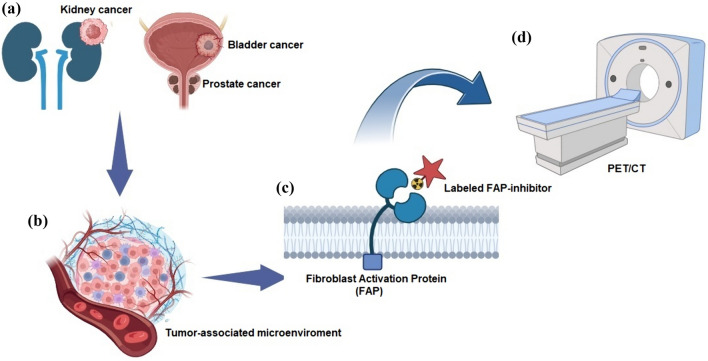
[18F]FDG PET imaging in non-prostate urological malignancies, such as urothelial carcinoma (UC) and renal cell carcinoma (RCC), has not seen the same advancements because of factors such as urinary excretion and low glucose metabolism limiting its utility [11]. Consequently, research is underway to identify alternative PET agents (Fig. 1).
Fig. 1.
Schematic of fibroblast activation protein (FAP)-targeted approaches in genitourinary tract neoplasms. (a) Renal (on the left), bladder, and prostatic tumors (on the right). Neoplasms share a tumor microenvironment (b) rich in cancer-associated fibroblasts. Fibroblast activation protein is a transmembrane serine protease expressed by cancer-associated fibroblasts, for which various radiolabeled ligands (c) have been developed, allowing positron emission tomography (PET) FAP-targeted imaging (d). CT computed tomography. Figure is the authors’ own work, created with Biorender.com
Three original articles are available regarding [68Ga]Ga-FAPI PET/CT in UC, mainly describing patients affected by bladder cancer (BC) (Table 1). Unterrainer et al. [12] compared the biodistribution and diagnostic information between contrast-enhanced CT and [68Ga]Ga-FAPI-46 PET/CT (median intercurrent time: 15.9 days) in 15 high-grade UC (three upper-tract UC and 12 bladder cancer). The study showed 59 FAPI-positive metastatic lesions, four more than contrast-enhanced CT (three lymph nodes and one peritoneal lesion). Nevertheless, [68Ga]Ga-FAPI-46 PET/CT also detected 15 non-oncological FAPI-positive lesions, while the evaluation of primary lesions was hindered by urinary activity.
In the study by Novruzov et al. [13], eight patients were imaged with both [18F]FDG and [68Ga]Ga-FAPI PET/CT (median intercurrent time: 5 days) for staging or restaging BC. Overall, [68Ga]Ga-FAPI detected 31 metastatic lesions, nine more than [18F]FDG (including eight lymph nodes and one peritoneal nodule), achieving a 30% increased detection rate. [68Ga]Ga-FAPI PET/CT had a statistically higher tumor-to-background ratio of metastatic lesions than [18F]FDG PET/CT (5.6 vs 1.95, respectively; p = 0.001).
In the study by Koshkin et al. [14], 21 patients with BC were imaged with [68Ga]Ga-FAPI PET/CT and divided into two cohorts according to disease extension: localized (n = 13) and metastatic (n = 8). Of note, most of the lesions detected by [68Ga]Ga-FAP-2286 PET/CT [14 out of 18 (77.8%) and 14 out of 36 (38.9%) in the localized and metastatic cohorts, respectively] were small lymph nodes with a short axis lower than 1 cm. In patients with both [68Ga]Ga-FAPI and [18F]FDG PET/CT, the average SUVmax was higher at [68Ga]Ga-FAPI PET/CT (9.9 ± 3.4 vs 4.2 ± 1.9, respectively). These data candidate [68Ga]Ga-FAPI as a potentially interesting agent for staging patients with BC.
[68Ga]Ga-FAPI PET in RCC
In RCC, available data on [68Ga]Ga-FAPI PET/CT are very few and preliminary. The only original article available about [68Ga]Ga-FAPI-04 PET/CT [15] compared the results of [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT (median intercurrent time: 7 days; interquartile range: 5–21 days) in a cohort of 18 patients, mainly affected by clear cell RCC (83.3%). FAPI-PET/CT was performed for primary staging in six patients and for restaging a known or suspected metastatic RCC in the remaining 12. Overall, FAPI-PET/CT showed significantly higher median target to background ratio (TBR) than [18F]FDG PET/CT [5.6 (2.2–7.8) vs 2.1 (0.8–4.3); p < 0.001], with a higher uptake intensity in particular in the 32 lung metastases detected (median SUVmax 3.8 vs 1.8, respectively; p = 0.02). In primary RCC lesions, the median SUVmax at [68Ga]Ga-FAPI PET/CT was significantly higher in advanced TNM-stage neoplasms [13.9 (range 4.9–26.1) vs 1.9 (range 1.5–2.3) in stage 4 and stage 1, respectively].
The remaining data from the literature on [68Ga]Ga-FAPI PET/CT in RCC are case reports. Two articles reported patients with chromophobe RCC imaged with [68Ga]Ga-FAPI PET/CT with a higher uptake and TBR at [68Ga]Ga-FAPI PET/CT than at [18F]FDG PET/CT [16, 17].
Conclusions
Based on these preliminary findings, radiolabeled FAPI PET deserves further investigation in genitourinary malignancies, with the potential to carve out a role as a complementary imaging modality. Notably, FAPI can also be radiolabeled with [177Lu]Lu, enabling a potential theranostic approach for genitourinary neoplasms overexpressing FAP.
In PCa, FAPI PET could be useful in detecting occult primary lesions, particularly when conventional imaging modalities such as multi-parametric magnetic resonance imaging and PSMA PET/CT or PET/magnetic resonance imaging yield negative or inconclusive results. Additionally, a high FAPI uptake correlates with tumor dedifferentiation. In metastatic castration-resistant PCa, especially in cases unresponsive to [177Lu]Lu-PSMA RLT, [68Ga]Ga-FAPI PET/CT may offer valuable insights. Prospective studies on the efficacy and safety of RLT with [177Lu]Lu-FAPI are mandatory to assess its potential role in patients with PCa.
In UC, FAPI holds promises in detecting metastatic lesions unrevealed at contrast-enhanced CT and FDG PET/CT, in particular small lymph node metastases [12–14]. Therefore, its potential is of particular interest at the initial staging, when undetected lymph node metastases are frequent and have a strong impact on patients’ prognosis. Data regarding [68Ga]Ga-FAPI PET/CT for restaging UC are still very scarce and deserve further investigations, in particular into guiding appropriate systemic/local therapies and as an imaging for assessing responses to systemic therapies.
Regarding RCC, FAPI’s role remains to be elucidated, particularly concerning the various histopathological RCC subtypes [18]. The attention of nuclear medicine research in this malignancy has recently focused mainly on radiolabeled PSMA ligands and [89Zr]-girentuximab PET/CT, although both agents are still not available in daily clinical practice [11]. The theranostic potential of FAPI makes it a candidate diagnostic and therapeutic option for this challenging cancer, to be investigated in prospective trials [19]. Given these prospects, it is imperative to stimulate the scientific community toward conducting spontaneous explorative studies as well as clinical trials that generate robust evidence, facilitating the swift integration of FAPI as a theranostic agent in genitourinary cancers.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Declarations
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Conflict of interest
Naima Ortolan, Luca Urso, Ilaria Zamberlan, Luca Filippi, Nicolò Maria Buffi, Corrado Cittanti, Licia Uccelli, Mirco Bartolomei, and Laura Evangelista have no conflicts of interest that are directly relevant to the content of this article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed.
Code availability
Not applicable.
Author contributions
Conceptualization: LUr and LE; writing first draft: NO, IZ, LUr, and LE; editing and revision: LF, CC, NMB, LUc, and MB. All authors read and approved the final version of the manuscript.
References
- 1.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. 2021;62:160–7. [DOI] [PubMed] [Google Scholar]
- 3.Chandekar KR, Prashanth A, Vinjamuri S, Kumar R. FAPI PET/CT imaging: an updated review. Diagnostics. 2023;13:2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urso L, Filippi L, Castello A, Marzola MC, Bartolomei M, Cittanti C, et al. PSMA PET/CT in castration-resistant prostate cancer: myth or reality? J Clin Med. 2023;12:7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ergül N, Çermik TF, Alçin G, Arslan E, Erol Fenercioǧlu Ö, Beyhan E, et al. Contribution of 68Ga-DOTA-FAPI-04 PET/CT to prostate cancer imaging: complementary role in PSMA-negative cases. Clin Nucl Med. 2024;49:E105–10. [DOI] [PubMed] [Google Scholar]
- 6.Kesch C, Yirga L, Dendl K, Handke A, Darr C, Krafft U, et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur J Nucl Med Mol Imaging. 2021;49:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalski K, Ruf J, Goetz C, Seitz AK, Buck AK, Lapa C, et al. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur J Nucl Med Mol Imaging. 2021;48:2024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isik EG, Has-Simsek D, Sanli O, Sanli Y, Kuyumcu S. Fibroblast activation protein-targeted PET imaging of metastatic castration-resistant prostate cancer compared with 68Ga-PSMA and 18F-FDG PET/CT. Clin Nucl Med. 2022;47:e54–5. [DOI] [PubMed] [Google Scholar]
- 9.Fendler WP, Pabst KM, Kessler L, Costa PF, Ferdinandus J, Weber M, et al. Safety and efficacy of 90Y-FAPI-46 radioligand therapy in patients with advanced sarcoma and other cancer entities. Clin Cancer Res. 2022;28:4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assadi M, Rekabpour SJ, Jafari E, Divband GA, Nikkholgh B, Amini H, et al. Feasibility and therapeutic potential of 177Lu-fibroblast activation protein inhibitor-46 for patients with relapsed or refractory cancers: a preliminary study. Clin Nucl Med. 2021;46:E523–30. [DOI] [PubMed] [Google Scholar]
- 11.Urso L, Bauckneht M, Albano D, Chondrogiannis S, Grassetto G, Lanfranchi F, et al. The evolution of PET imaging in renal, bladder, upper urinary tract urothelial, testicular and penile carcinoma: today’s impact, tomorrow’s potential. Expert Rev Med Devices. 2024;21:55–72. [DOI] [PubMed] [Google Scholar]
- 12.Unterrainer LM, Lindner S, Eismann L, Casuscelli J, Gildehaus FJ, Bui VN, et al. Feasibility of [68Ga]Ga-FAPI-46 PET/CT for detection of nodal and hematogenous spread in high-grade urothelial carcinoma. Eur J Nucl Med Mol Imaging. 2022;49:3571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novruzov E, Dendl K, Ndlovu H, Choyke PL, Dabir M, Beu M, et al. Head-to-head intra-individual comparison of [68Ga]-FAPI and [18F]-FDG PET/CT in patients with bladder cancer. Mol Imaging Biol. 2022;24:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshkin VS, Kumar V, Kline B, Escobar D, Aslam M, Cooperberg MR, et al. Initial experience with 68Ga-FAP-2286 PET imaging in patients with urothelial cancer. J Nucl Med. 2024;65:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Civan C, Kuyumcu S, Has Simsek D, Sanli O, Isik EG, Ozkan ZG, et al. The role of [68 Ga]Ga-FAPI-04 PET/CT in renal cell carcinoma: a preliminary study. Eur J Nucl Med Mol Imaging. 2024;51:852–61. [DOI] [PubMed] [Google Scholar]
- 16.Xie F, Fu L, Zhou W. Superiority of 68Ga-FAPI-04 in delineation of soft tissue and liver metastases in chromophobe renal cell carcinoma for restaging. Clin Nucl Med. 2022;47:E758–9. [DOI] [PubMed] [Google Scholar]
- 17.Pang Y, Wei J, Shang Q, Zhao L, Chen H. 68Ga-fibroblast activation protein inhibitor, a promising radiopharmaceutical in PET/CT to detect the primary and metastatic lesions of chromophobe renal cell carcinoma. Clin Nucl Med. 2021;46:177–9. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Zhou X, Huang G, Liu J. Bisphosphatase 1 expression reduces 18 F-FDG uptake in clear cell renal cell carcinoma. Res Article Fructose. 2019;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udovicich C, Perera M, Hofman MS, Siva S. FAPI PET/CT: a new kid on the block for RCC. Eur J Nucl Med Mol Imaging. 2024;51:862–3. [DOI] [PubMed] [Google Scholar]